Abstract
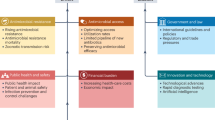
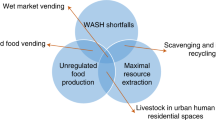
Antimicrobial resistance (AMR) is a global health challenge that threatens humans, animals and the environment. Evidence is emerging for a role of healthcare infrastructure, environments and patient pathways in promoting and maintaining AMR via direct and indirect mechanisms. Advances in vaccination and monoclonal antibody therapies together with integrated surveillance, rapid diagnostics, targeted antimicrobial therapy and infection control measures offer opportunities to address healthcare-associated AMR risks more effectively. Additionally, innovations in artificial intelligence, data linkage and intelligent systems can be used to better predict and reduce AMR and improve healthcare resilience. In this Review, we examine the mechanisms by which healthcare functions as a driver, reservoir and amplifier of AMR, contextualized within a One Health framework. We also explore the opportunities and innovative solutions that can be used to combat AMR throughout the patient journey. We provide a perspective on the current evidence for the effectiveness of interventions designed to mitigate healthcare-associated AMR and promote healthcare resilience within high-income and resource-limited settings, as well as the challenges associated with their implementation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
World Health Organization. Global Action Plan on Antimicrobial Resistance (WHO, 2015).
Holmes, A. H. et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 387, 176–187 (2016).
Gorrie, C. L. et al. Antimicrobial-resistant Klebsiella pneumoniae carriage and infection in specialized geriatric care wards linked to acquisition in the referring hospital. Clin. Infect. Dis. 67, 161–170 (2018).
Tinelli, M. et al. Dynamics of carbapenemase-producing Enterobacterales intestinal colonisation in the elderly population after hospital discharge, Italy, 2018-2020. Int. J. Antimicrob. Agents 59, 106594 (2022).
Zhang, S., Huang, J., Zhao, Z., Cao, Y. & Li, B. Hospital wastewater as a reservoir for antibiotic resistance genes: a meta-analysis. Front. Public Health 8, 574968 (2020).
World Health Organization. Global Research Agenda for Antimicrobial Resistance in Human Health: Policy Brief (WHO, 2023).
Barber, R. M. et al. Healthcare Access and Quality Index based on mortality from causes amenable to personal health care in 195 countries and territories, 1990–2015: a novel analysis from the Global Burden of Disease Study 2015. Lancet 390, 231–266 (2017).
Duval, A. et al. Close proximity interactions support transmission of ESBL-K. pneumoniae but not ESBL-E. coli in healthcare settings. PLoS Comput. Biol. 15, e1006496 (2019).
Temime, L. et al. Peripatetic health-care workers as potential superspreaders. Proc. Natl Acad. Sci. USA 106, 18420–18425 (2009).
Eyre, D. W. et al. A Candida auris outbreak and its control in an intensive care setting. N. Engl. J. Med. 379, 1322–1331 (2018).
Holmes, A. et al. Infection Prevention and Control: Lessons from Acute Care in England (The Health Foundation, 2015).
Nanayakkara, A. K. et al. Antibiotic resistance in the patient with cancer: escalating challenges and paths forward. CA Cancer J. Clin. 71, 488–504 (2021).
Trecarichi, E. M. & Tumbarello, M. Antimicrobial-resistant Gram-negative bacteria in febrile neutropenic patients with cancer: current epidemiology and clinical impact. Curr. Opin. Infect. Dis. 27, 200–210 (2014).
Stecher, B., Maier, L. & Hardt, W.-D. ‘Blooming’ in the gut: how dysbiosis might contribute to pathogen evolution. Nat. Rev. Microbiol. 11, 277–284 (2013).
Lerner, A. et al. Spread of KPC-producing carbapenem-resistant Enterobacteriaceae: the importance of super-spreaders and rectal KPC concentration. Clin. Microbiol. Infect. 21, 470.e1–7 (2015).
Lewis, J. M. et al. Colonization dynamics of extended-spectrum beta-lactamase-producing Enterobacterales in the gut of Malawian adults. Nat. Microbiol. 7, 1593–1604 (2022).
Faith, J. J. et al. The long-term stability of the human gut microbiota. Science 341, 1237439 (2013).
Donskey, C. J. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin. Infect. Dis. 39, 219–226 (2004).
Yassour, M. et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl. Med. 8, 343ra81 (2016).
Donskey, C. J. et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N. Engl. J. Med. 343, 1925–1932 (2000).
Shen, Z. et al. Emerging carriage of NDM-5 and MCR-1 in Escherichia coli from healthy people in multiple regions in China: a cross sectional observational study. EClinicalMedicine 6, 11–20 (2018).
Sands, K. et al. Characterization of antimicrobial-resistant Gram-negative bacteria that cause neonatal sepsis in seven low- and middle-income countries. Nat. Microbiol. 6, 512–523 (2021).
Gorrie, C. L. et al. Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin. Infect. Dis. 65, 208–215 (2017).
Vincent, J.-L. Nosocomial infections in adult intensive-care units. Lancet 361, 2068–2077 (2003).
Pulcini, C. et al. Developing core elements and checklist items for global hospital antimicrobial stewardship programmes: a consensus approach. Clin. Microbiol. Infect. 25, 20–25 (2019).
Kajova, M., Khawaja, T. & Kantele, A. European hospitals as source of multidrug-resistant bacteria: analysis of travellers screened in Finland after hospitalization abroad. J. Travel Med. 29, taac022 (2022).
Bezabih, Y. M. et al. Comparison of the global prevalence and trend of human intestinal carriage of ESBL-producing Escherichia coli between healthcare and community settings: a systematic review and meta-analysis. JAC Antimicrob. Resist. 4, dlac048 (2022).
Ita, T. et al. Prevalence of colonization with multidrug-resistant bacteria in communities and hospitals in Kenya. Sci. Rep. 12, 22290 (2022).
O’Hara, L. M. et al. Optimizing contact precautions to curb the spread of antibiotic-resistant bacteria in hospitals: a multicenter cohort study to identify patient characteristics and healthcare personnel interactions associated with transmission of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 69, S171–S177 (2019).
Pittet, D. et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect. Dis. 6, 641–652 (2006).
Birgand, G. et al. Innovation for infection prevention and control-revisiting Pasteur’s vision. Lancet 400, 2250–2260 (2022).
Mitchell, B. G., Dancer, S. J., Anderson, M. & Dehn, E. Risk of organism acquisition from prior room occupants: a systematic review and meta-analysis. J. Hosp. Infect. 91, 211–217 (2015).
Chng, K. R. et al. Cartography of opportunistic pathogens and antibiotic resistance genes in a tertiary hospital environment. Nat. Med. 26, 941–951 (2020).
Lowe, C. et al. Outbreak of extended-spectrum β-lactamase-producing Klebsiella oxytoca infections associated with contaminated handwashing sinks. Emerg. Infect. Dis. 18, 1242–1247 (2012).
Anantharajah, A. et al. Long-term intensive care unit outbreak of carbapenemase-producing organisms associated with contaminated sink drains. J. Hosp. Infect. 143, 38–47 (2024).
Chia, P. Y. et al. The role of hospital environment in transmissions of multidrug-resistant gram-negative organisms. Antimicrob. Resist. Infect. Control 9, 29 (2020).
Chiarello, L. A. & Tapper, M. L. Healthcare settings as amplifiers of infectious disease. Emerg. Infect. Dis. 10, 2048–2049 (2004).
Tomczyk, S. et al. The first WHO global survey on infection prevention and control in health-care facilities. Lancet Infect. Dis. 22, 845–856 (2022).
World Health Organization. Global Report on Infection Prevention and Control: Executive Summary (WHO, 2022).
Plachouras, D. et al. Antimicrobial use in European acute care hospitals: results from the second point prevalence survey (PPS) of healthcare-associated infections and antimicrobial use, 2016 to 2017. Eurosurveillance 23, 1800393 (2018).
Nathwani, D., Raman, G., Sulham, K., Gavaghan, M. & Menon, V. Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 3, 32 (2014).
Cosgrove, S. E. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin. Infect. Dis. 42, S82–89 (2006).
Rawson, T. M. et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 71, 2459–2468 (2020).
Almagor, J. et al. The impact of antibiotic use on transmission of resistant bacteria in hospitals: insights from an agent-based model. PLoS ONE 13, e0197111 (2018).
Scott, P. et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin. Infect. Dis. 44, 1577–1584 (2007).
Merrick, R. et al. Transferring inpatients between wards drives large nosocomial COVID-19 outbreaks, Wales, United Kingdom, 2020-22: a matched case-control study using routine and enhanced surveillance data. J. Hosp. Infect. 145, 1–10 (2023).
Thornley, T., Ashiru-Oredope, D., Normington, A., Beech, E. & Howard, P. Antibiotic prescribing for residents in long-term-care facilities across the UK. J. Antimicrob. Chemother. 74, 1447–1451 (2019).
McKinnell, J. A. et al. The SHIELD orange county project: multidrug-resistant organism prevalence in 21 nursing homes and long-term acute care facilities in Southern California. Clin. Infect. Dis. 69, 1566–1573 (2019).
Adams, E. et al. Candida auris in healthcare facilities, New York, USA, 2013-2017. Emerg. Infect. Dis. 24, 1816–1824 (2018).
Desai, A. N., Mohareb, A. M., Hauser, N. & Abbara, A. Antimicrobial resistance and human mobility. Infect. Drug Resist. 15, 127–133 (2022).
Fayad, A. A. et al. Antimicrobial resistance and the Iraq wars: armed conflict as an underinvestigated pathway with growing significance. BMJ Glob. Health 7, e010863 (2023).
World Health Organization. Building climate-resilient health systems. WHO https://www.who.int/teams/environment-climate-change-and-health/climate-change-and-health/country-support/building-climate-resilient-health-systems (2024).
Gilbert, J. A. & Stephens, B. Microbiology of the built environment. Nat. Rev. Microbiol. 16, 661–670 (2018).
Boiocchi, F., Davies, M. P. & Hilton, A. C. An examination of flying insects in seven hospitals in the United Kingdom and carriage of bacteria by true flies (Diptera: Calliphoridae, Dolichopodidae, Fanniidae, Muscidae, Phoridae, Psychodidae, Sphaeroceridae). J. Med. Entomol. 56, 1684–1697 (2019).
Berendonk, T. U. et al. Tackling antibiotic resistance: the environmental framework. Nat. Rev. Microbiol. 13, 310–317 (2015).
Stanton, I. C., Bethel, A., Leonard, A. F. C., Gaze, W. H. & Garside, R. Existing evidence on antibiotic resistance exposure and transmission to humans from the environment: a systematic map. Environ. Evid. 11, 8 (2022).
Kennedy, D. A. & Read, A. F. Why does drug resistance readily evolve but vaccine resistance does not? Proc. R. Soc. B Biol. Sci. 284, 20162562 (2017).
Micoli, F., Bagnoli, F., Rappuoli, R. & Serruto, D. The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Microbiol. 19, 287–302 (2021).
Atkins, K. E. & Lipsitch, M. Can antibiotic resistance be reduced by vaccinating against respiratory disease? Lancet Respir. Med. 6, 820–821 (2018).
Langford, B. J. et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin. Microbiol. Infect. 27, 520–531 (2021).
Taylor, S. et al. Modelling estimates of the burden of respiratory syncytial virus infection in children in the UK. BMJ Open 6, e009337 (2016).
Knight, G. M., Clarkson, M. & de Silva, T. I. Potential impact of influenza vaccine roll-out on antibiotic use in Africa. J. Antimicrob. Chemother. 73, 2197–2200 (2018).
Hopkins, H. et al. Impact of introduction of rapid diagnostic tests for malaria on antibiotic prescribing: analysis of observational and randomised studies in public and private healthcare settings. BMJ 356, j1054 (2017).
Andrews, J. R. et al. Typhoid conjugate vaccines: a new tool in the fight against antimicrobial resistance. Lancet Infect. Dis. 19, e26–e30 (2019).
Singleton, D. et al. Cross-sectional health centre and community-based evaluation of the impact of pneumococcal and malaria vaccination on antibiotic prescription and usage, febrile illness and antimicrobial resistance in young children in Malawi: the IVAR study protocol. BMJ Open 13, e069560 (2023).
Kurauchi, A., Struchiner, C. J., Wilder-Smith, A. & Massad, E. Modelling the effect of a dengue vaccine on reducing the evolution of resistance against antibiotic due to misuse in dengue cases. Theor. Biol. Med. Model. 17, 7 (2020).
Troeger, C. et al. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 17, 909–948 (2017).
Feikin, D. R. et al. Efficacy of pentavalent rotavirus vaccine in a high HIV prevalence population in Kenya. Vaccine 30, A52–60 (2012).
Lewnard, J. A., Lo, N. C., Arinaminpathy, N., Frost, I. & Laxminarayan, R. Childhood vaccines and antibiotic use in low- and middle-income countries. Nature 581, 94–99 (2020).
Ngabo, F. et al. Effect of pentavalent rotavirus vaccine introduction on hospital admissions for diarrhoea and rotavirus in children in Rwanda: a time-series analysis. Lancet Glob. Health 4, e129–e136 (2016).
Vasileiou, E. et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet 397, 1646–1657 (2021).
Eythorsson, E. et al. Impact of the 10-valent pneumococcal conjugate vaccine on antimicrobial prescriptions in young children: a whole population study. BMC Infect. Dis. 18, 505 (2018).
Cohen, R., Cohen, J. F., Chalumeau, M. & Levy, C. Impact of pneumococcal conjugate vaccines for children in high- and non-high-income countries. Expert Rev. Vaccines 16, 625–640 (2017).
Swarthout, T. D. et al. High residual carriage of vaccine-serotype Streptococcus pneumoniae after introduction of pneumococcal conjugate vaccine in Malawi. Nat. Commun. 11, 2222 (2020).
Costanzo, V. & Roviello, G. N. The potential role of vaccines in preventing antimicrobial resistance (AMR): an update and future perspectives. Vaccines 11, 333 (2023).
Frost, I. et al. The role of bacterial vaccines in the fight against antimicrobial resistance: an analysis of the preclinical and clinical development pipeline. Lancet Microbe 4, e113–e125 (2023).
Kitchin, N. et al. A phase 2 study evaluating the safety, tolerability, and immunogenicity of two 3-dose regimens of a Clostridium difficile vaccine in healthy US adults aged 65 to 85 years. Clin. Infect. Dis. 70, 1–10 (2020).
Knisely, J. M., Liu, B., Ranallo, R. T. & Zou, L. Vaccines for healthcare-associated infections: promise and challenge. Clin. Infect. Dis. 63, 657–662 (2016).
Martinot, M., Mohseni-Zadeh, M., Marion, L. & Roncalez, D. COVID-19 vaccine: missed opportunities and hospital vaccine implementation. Clin. Microbiol. Infect. 28, 1174–1175 (2022).
Gurtman, A. et al. The development of a Staphylococcus aureus four antigen vaccine for use prior to elective orthopedic surgery. Hum. Vaccines Immunother. 15, 358–370 (2018).
COVIDSurg Collaborative & GlobalSurg Collaborative.SARS-CoV-2 vaccination modelling for safe surgery to save lives: data from an international prospective cohort study. Br. J. Surg. 108, 1056–1063 (2021).
Liu, W.-C. et al. Effect of influenza vaccination against postoperative pneumonia and mortality for geriatric patients receiving major surgery: a nationwide matched study. J. Infect. Dis. 217, 816–826 (2018).
Glasbey, J. C. et al. Elective surgery system strengthening: development, measurement, and validation of the surgical preparedness index across 1632 hospitals in 119 countries. Lancet 400, 1607–1617 (2022).
Knight, G. M. et al. Antimicrobial resistance and COVID-19: intersections and implications. eLife 10, e64139 (2021).
van der Plaat, D. A. et al. Risks of COVID-19 by occupation in NHS workers in England. Occup. Environ. Med. 79, 176–183 (2022).
Shah, A. S. V. et al. Effect of vaccination on transmission of SARS-CoV-2. N. Engl. J. Med. 385, 1718–1720 (2021).
Verelst, F., Beutels, P., Hens, N. & Willem, L. Workplace influenza vaccination to reduce employee absenteeism: an economic analysis from the employers’ perspective. Vaccine 39, 2005–2015 (2021).
Theuretzbacher, U., Outterson, K., Engel, A. & Karlén, A. The global preclinical antibacterial pipeline. Nat. Rev. Microbiol. 18, 275–285 (2020).
Troisi, M. et al. A new dawn for monoclonal antibodies against antimicrobial resistant bacteria. Front. Microbiol. 13, 1080059 (2022).
Theuretzbacher, U. & Piddock, L. J. V. Non-traditional antibacterial therapeutic options and challenges. Cell Host Microbe 26, 61–72 (2019).
Wilcox, M. H. et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N. Engl. J. Med. 376, 305–317 (2017).
Sun, M. et al. Monoclonal antibody for the prevention of respiratory syncytial virus in infants and children: a systematic review and network meta-analysis. JAMA Netw. Open 6, e230023 (2023).
Zurawski, D. V. & McLendon, M. K. Monoclonal antibodies as an antibacterial approach against bacterial pathogens. Antibiotics 9, 155 (2020).
Baker, S., Kellam, P., Krishna, A. & Reece, S. Protecting intubated patients from the threat of antimicrobial resistant infections with monoclonal antibodies. Lancet Microbe 1, e191–e192 (2020).
Kreitmann, L. et al. Next-generation molecular diagnostics: leveraging digital technologies to enhance multiplexing in real-time PCR. TrAC Trends Anal. Chem. 160, 116963 (2023).
Roy, S. et al. Recent developments towards portable point-of-care diagnostic devices for pathogen detection. Sens. Diagn. 1, 87–105 (2022).
Vasala, A., Hytönen, V. P. & Laitinen, O. H. Modern tools for rapid diagnostics of antimicrobial resistance. Front. Cell. Infect. Microbiol. 10, 308 (2020).
European Centre for Disease Prevention & Control. A Scoping Review of Point-of-Care Testing Devices for Infectious Disease Surveillance, Prevention and Control (ECDC, 2022).
Tolley, A., Bansal, A., Murerwa, R. & Howard Dicks, J. Cost-effectiveness of point-of-care diagnostics for AMR: a systematic review. J. Antimicrob. Chemother. 79, 1248–1269 (2024).
Barlam, T. F. et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 62, e51–e77 (2016).
National Institute for Health and Care Excellence (NICE). Pneumonia in adults: diagnosis and management (NICE, 2023).
Au Yeung, V. et al. Differences in antibiotic and antiviral use in people with confirmed influenza: a retrospective comparison of rapid influenza PCR and multiplex respiratory virus PCR tests. BMC Infect. Dis. 21, 321 (2021).
Yamin, D. et al. Current and future technologies for the detection of antibiotic-resistant bacteria. Diagnostics 13, 3246 (2023).
Kose, E. et al. The effect of rapid antigen detection test on antibiotic prescription decision of clinicians and reducing antibiotic costs in children with acute pharyngitis. J. Trop. Pediatr. 62, 308–315 (2016).
Camprubí-Ferrer, D. et al. Rapid diagnostic tests for dengue would reduce hospitalizations, healthcare costs and antibiotic prescriptions in Spain: a cost-effectiveness analysis. Enferm. Infecc. Microbiol. Clin. Engl. Ed https://doi.org/10.1016/j.eimce.2022.12.016 (2023).
Zhan, Y., Wang, Y., Zhang, W., Ying, B. & Wang, C. Diagnostic accuracy of the artificial intelligence methods in medical imaging for pulmonary tuberculosis: a systematic review and meta-analysis. J. Clin. Med. 12, 303 (2022).
Miesler, T., Wimschneider, C., Brem, A. & Meinel, L. Frugal innovation for point-of-care diagnostics controlling outbreaks and epidemics. ACS Biomater. Sci. Eng. 6, 2709–2725 (2020).
Vos, L. M. et al. Rapid molecular tests for influenza, respiratory syncytial virus, and other respiratory viruses: a systematic review of diagnostic accuracy and clinical impact studies. Clin. Infect. Dis. 69, 1243–1253 (2019).
Peri, A. M. et al. Rapid diagnostic tests and antimicrobial stewardship programs for the management of bloodstream infection: what is their relative contribution to improving clinical outcomes? A systematic review and network meta-analysis. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciae234 (2024).
Liu, Z., Banaei, N. & Ren, K. Microfluidics for combating antimicrobial resistance. Trends Biotechnol. 35, 1129–1139 (2017).
Kaprou, G. D., Bergšpica, I., Alexa, E. A., Alvarez-Ordóñez, A. & Prieto, M. Rapid methods for antimicrobial resistance diagnostics. Antibiotics 10, 209 (2021).
Helou, R. I. et al. Use of stewardship smartphone applications by physicians and prescribing of antimicrobials in hospitals: a systematic review. PLoS One 15, e0239751 (2020).
Watkins, R. R. Antibiotic stewardship in the era of precision medicine. JAC Antimicrob. Resist. 4, dlac066 (2022).
Vasikasin, V., Rawson, T. M., Holmes, A. H. & Otter, J. Can precision antibiotic prescribing help prevent the spread of carbapenem-resistant organisms in the hospital setting? JAC Antimicrob. Resist. 5, dlad036 (2023).
Roberts, J. A. et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect. Dis. 14, 498–509 (2014).
Roberts, J. A. et al. Challenges and potential solutions — individualised antibiotic dosing at the bedside for critically Ill patients: a structured review. Lancet Infect. Dis. 14, 498–509 (2014).
Rawson, T. M. et al. Delivering precision antimicrobial therapy through closed-loop control systems. J. Antimicrob. Chemother. 73, 835–843 (2018).
Ioannou, P. et al. Indications for medical antibiotic prophylaxis and potential targets for antimicrobial stewardship intervention: a narrative review. Clin. Microbiol. Infect. 28, 362–370 (2022).
Laxminarayan, R. et al. Antibiotic resistance — the need for global solutions. Lancet Infect. Dis. 13, 1057–1098 (2013).
Drain, P. K. et al. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect. Dis. 14, 239–249 (2014).
Miller, M. B. et al. Clinical utility of advanced microbiology testing tools. J. Clin. Microbiol. 57, e00495-19 (2019).
Ming, D. et al. Connectivity of rapid-testing diagnostics and surveillance of infectious diseases. Bull. World Health Organ. 97, 242–244 (2019).
Keenan, K. et al. Unravelling patient pathways in the context of antibacterial resistance in East Africa. BMC Infect. Dis. 23, 414 (2023).
McLeod, M. et al. A whole-health–economy approach to antimicrobial stewardship: analysis of current models and future direction. PLoS Med. 16, e1002774 (2019).
Pilmis, B., Le Monnier, A. & Zahar, J.-R. Gut microbiota, antibiotic therapy and antimicrobial resistance: a narrative review. Microorganisms 8, 269 (2020).
Yeh, Y.-C., Huang, T.-H., Yang, S.-C., Chen, C.-C. & Fang, J.-Y. Nano-based drug delivery or targeting to eradicate bacteria for infection mitigation: a review of recent advances. Front. Chem. 8, 286 (2020).
Brochado, A. R. et al. Species-specific activity of antibacterial drug combinations. Nature 559, 259–263 (2018).
Grall, N. et al. Oral DAV131, a charcoal-based adsorbent, inhibits intestinal colonization by β-lactam-resistant Klebsiella pneumoniae in cefotaxime-treated mice. Antimicrob. Agents Chemother. 57, 5423–5425 (2013).
Kokai-Kun, J. F. et al. Ribaxamase, an orally administered β-lactamase, diminishes changes to acquired antimicrobial resistance of the gut resistome in patients treated with ceftriaxone. Infect. Drug Resist. 13, 2521–2535 (2020).
Suez, J. et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 174, 1406–1423.e16 (2018).
Huttner, B. D. et al. A 5-day course of oral antibiotics followed by faecal transplantation to eradicate carriage of multidrug-resistant Enterobacteriaceae: a randomized clinical trial. Clin. Microbiol. Infect. 25, 830–838 (2019).
Piewngam, P. et al. Probiotic for pathogen-specific Staphylococcus aureus decolonisation in Thailand: a phase 2, double-blind, randomised, placebo-controlled trial. Lancet Microbe 4, e75–e83 (2023).
de Smet, A. M. G. A. et al. Selective digestive tract decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. Lancet Infect. Dis. 11, 372–380 (2011).
Tacconelli, E. et al. ESCMID-EUCIC clinical guidelines on decolonization of multidrug-resistant Gram-negative bacteria carriers. Clin. Microbiol. Infect. 25, 807–817 (2019).
Poirel, L. et al. A phage-based decolonisation strategy against pan-resistant enterobacterial strains. Lancet Infect. Dis. 20, 525–526 (2020).
Fassarella, M. et al. Gut microbiome stability and resilience: elucidating the response to perturbations in order to modulate gut health. Gut 70, 595–605 (2021).
Lax, S. et al. Bacterial colonization and succession in a newly opened hospital. Sci. Transl. Med. 9, eaah6500 (2017).
Klassert, T. E. et al. Bacterial colonization dynamics and antibiotic resistance gene dissemination in the hospital environment after first patient occupancy: a longitudinal metagenetic study. Microbiome 9, 169 (2021).
Sukhum, K. V. et al. Antibiotic-resistant organisms establish reservoirs in new hospital built environments and are related to patient blood infection isolates. Commun. Med. 2, 62 (2022).
Marimuthu, K. et al. Whole genome sequencing reveals hidden transmission of carbapenemase-producing Enterobacterales. Nat. Commun. 13, 3052 (2022).
Buckner, M. M. C., Ciusa, M. L. & Piddock, L. J. V. Strategies to combat antimicrobial resistance: anti-plasmid and plasmid curing. FEMS Microbiol. Rev. 42, 781–804 (2018).
Lepainteur, M. et al. Evaluation of excreta management in a large French multi-hospital institution. J. Hosp. Infect. 91, 346–350 (2015).
Mitchell, B. G., Dancer, S. J., Shaban, R. Z. & Graves, N. Moving forward with hospital cleaning. Am. J. Infect. Control 41, 1138–1139 (2013).
Muller, M. P. et al. Antimicrobial surfaces to prevent healthcare-associated infections: a systematic review. J. Hosp. Infect. 92, 7–13 (2016).
Goto, M. et al. Effectiveness of ultraviolet-C disinfection on hospital-onset gram-negative rod bloodstream infection: a nationwide stepped-wedge time-series analysis. Clin. Infect. Dis. 76, 291–298 (2023).
Marra, A. R., Schweizer, M. L. & Edmond, M. B. No-touch disinfection methods to decrease multidrug-resistant organism infections: a systematic review and meta-analysis. Infect. Control Hosp. Epidemiol. 39, 20–31 (2018).
Dancer, S. J. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin. Microbiol. Rev. 27, 665–690 (2014).
Jarlier, V. et al. Curbing methicillin-resistant Staphylococcus aureus in 38 French hospitals through a 15-year institutional control program. Arch. Intern. Med. 170, 552–559 (2010).
French, K. Ten articles on hand hygiene innovation that have been reported in the Journal of Hospital Infection. J. Hosp. Infect. 100, 242–243 (2018).
Miller Loren, G. et al. Decolonization in nursing homes to prevent infection and hospitalization. N. Engl. J. Med. 389, 1766–1777 (2023).
Buxser, S. Has resistance to chlorhexidine increased among clinically-relevant bacteria? A systematic review of time course and subpopulation data. PLoS ONE 16, e0256336 (2021).
Eyre, D. W. Infection prevention and control insights from a decade of pathogen whole-genome sequencing. J. Hosp. Infect. 122, 180–186 (2022).
Forde, B. M. et al. Clinical implementation of routine whole-genome sequencing for hospital infection control of multi-drug resistant pathogens. Clin. Infect. Dis. 76, e1277–e1284 (2022).
Pehrsson, E. C. et al. Interconnected microbiomes and resistomes in low-income human habitats. Nature 533, 212–216 (2016).
Ruppé, E. et al. High rate of acquisition but short duration of carriage of multidrug-resistant Enterobacteriaceae after travel to the tropics. Clin. Infect. Dis. 61, 593–600 (2015).
Martischang, R. et al. Household carriage and acquisition of extended-spectrum β-lactamase-producing Enterobacteriaceae: a systematic review. Infect. Control Hosp. Epidemiol. 41, 286–294 (2020).
Jamal, A. J. et al. Household transmission of carbapenemase-producing Enterobacterales in Ontario, Canada. Clin. Infect. Dis. 73, e4607–e4615 (2021).
Platteel, T. N. et al. Predicting carriage with extended-spectrum beta-lactamase-producing bacteria at hospital admission: a cross-sectional study. Clin. Microbiol. Infect. 21, 141–146 (2015).
Chau, K. K. et al. Systematic review of wastewater surveillance of antimicrobial resistance in human populations. Environ. Int. 162, 107171 (2022).
Flach, C.-F., Hutinel, M., Razavi, M., Åhrén, C. & Larsson, D. G. J. Monitoring of hospital sewage shows both promise and limitations as an early-warning system for carbapenemase-producing Enterobacterales in a low-prevalence setting. Water Res. 200, 117261 (2021).
Newton, R. J. et al. Sewage reflects the microbiomes of human populations. mBio 6, e02574-14 (2015).
Nekkab, N., Astagneau, P., Temime, L. & Crépey, P. Spread of hospital-acquired infections: a comparison of healthcare networks. PLoS Comput. Biol. 13, e1005666 (2017).
Slayton, R. B. et al. Vital signs: estimated effects of a coordinated approach for action to reduce antibiotic-resistant infections in health care facilities — United States. MMWR Morb. Mortal. Wkly Rep. 64, 826–831 (2015).
Pita, R., Mendonça, E., Reis, S., Barreto, M. & Denaxas, S. in Big Data Analytics and Knowledge Discovery (eds Bellatreche, L. & Chakravarthy, S.) 214–227 (Springer, 2017).
Batenburg, D., Verheij, T., Van’t Veen, A. & van der Velden, A. Practice-level association between antibiotic prescribing and resistance: an observational study in primary care. Antibiotics 9, 470 (2020).
Argimón, S. et al. Integrating whole-genome sequencing within the National Antimicrobial Resistance Surveillance Program in the Philippines. Nat. Commun. 11, 2719 (2020).
Cocker, D. et al. Investigating One Health risks for human colonisation with extended spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Malawian households: a longitudinal cohort study. Lancet Microbe 4, e534–e543 (2023).
Hashim, R. et al. Tricycle Project — One Health approach: whole genome sequencing (WGS) of extended-spectrum beta-lactamase (ESBL) producing Eschericia (E.) coli derived from human, food chain and environment. Int. J. Infect. Dis. 116, S105–S106 (2022).
Burton, J. K. et al. Developing a minimum data set for older adult care homes in the UK: exploring the concept and defining early core principles. Lancet Healthy Longev. 3, e186–e193 (2022).
Ahmad, R., Gordon, A. C., Aylin, P., Redhead, J. & Holmes, A. Effective knowledge mobilisation: creating environments for quick generation, dissemination, and use of evidence. BMJ 379, e070195 (2022).
Tamuhla, T., Lulamba, E. T., Mutemaringa, T. & Tiffin, N. Multiple modes of data sharing can facilitate secondary use of sensitive health data for research. BMJ Glob. Health 8, e013092 (2023).
Asnicar, F., Thomas, A. M., Passerini, A., Waldron, L. & Segata, N. Machine learning for microbiologists. Nat. Rev. Microbiol. 22, 191–205 (2023).
Peiffer-Smadja, N. et al. Machine learning for clinical decision support in infectious diseases: a narrative review of current applications. Clin. Microbiol. Infect. 26, 584–595 (2020).
Pryor, R., Godbout, E. J. & Bearman, G. Precision infection prevention: the next frontier in patient safety. J. Hosp. Infect. 105, 232–233 (2020).
Roth, J. A., Battegay, M., Juchler, F., Vogt, J. E. & Widmer, A. F. Introduction to machine learning in digital healthcare epidemiology. Infect. Control Hosp. Epidemiol. 39, 1457–1462 (2018).
Fitzpatrick, F., Doherty, A. & Lacey, G. Using artificial intelligence in infection prevention. Curr. Treat. Options Infect. Dis. 12, 135–144 (2020).
Myall, A. et al. Prediction of hospital-onset COVID-19 infections using dynamic networks of patient contact: an international retrospective cohort study. Lancet Digit. Health 4, e573–e583 (2022).
Panchavati, S. et al. A comparative analysis of machine learning approaches to predict C. difficile infection in hospitalized patients. Am. J. Infect. Control 50, 250–257 (2022).
Flores-Balado, Á. et al. Using artificial intelligence to reduce orthopedic surgical site infection surveillance workload: algorithm design, validation, and implementation in 4 Spanish hospitals. Am. J. Infect. Control 51, 1225–1229 (2023).
Wheeler, N. E. et al. Innovations in genomic antimicrobial resistance surveillance. Lancet Microbe 4, e1063–e1070 (2023).
Brownstein, J. S., Rader, B., Astley, C. M. & Tian, H. Advances in artificial intelligence for infectious-disease surveillance. N. Engl. J. Med. 388, 1597–1607 (2023).
Arango-Argoty, G. et al. DeepARG: a deep learning approach for predicting antibiotic resistance genes from metagenomic data. Microbiome 6, 23 (2018).
Sundermann, A. J. et al. Automated data mining of the electronic health record for investigation of healthcare-associated outbreaks. Infect. Control Hosp. Epidemiol. 40, 314–319 (2019).
Sundermann, A. J. et al. Whole-genome sequencing surveillance and machine learning of the electronic health record for enhanced healthcare outbreak detection. Clin. Infect. Dis. 75, 476–482 (2022).
Sundermann, A. J. et al. Outbreak of Pseudomonas aeruginosa infections from a contaminated gastroscope detected by whole genome sequencing surveillance. Clin. Infect. Dis. 73, e638–e642 (2020).
Kim, J. I. et al. Machine learning for antimicrobial resistance prediction: current practice, limitations, and clinical perspective. Clin. Microbiol. Rev. 35, e0017921 (2022).
Weis, C. et al. Direct antimicrobial resistance prediction from clinical MALDI-TOF mass spectra using machine learning. Nat. Med. 28, 164–174 (2022).
Pascucci, M. et al. AI-based mobile application to fight antibiotic resistance. Nat. Commun. 12, 1173 (2021).
Tsoukalas, A., Albertson, T. & Tagkopoulos, I. From data to optimal decision making: a data-driven, probabilistic machine learning approach to decision support for patients with sepsis. JMIR Med. Inform. 3, e11 (2015).
Shen, Y. et al. An ontology-driven clinical decision support system (IDDAP) for infectious disease diagnosis and antibiotic prescription. Artif. Intell. Med. 86, 20–32 (2018).
Beaudoin, M., Kabanza, F., Nault, V. & Valiquette, L. Evaluation of a machine learning capability for a clinical decision support system to enhance antimicrobial stewardship programs. Artif. Intell. Med. 68, 29–36 (2016).
Pryor, R. J., Vokes, R., Anderson, D. & Bearman, G. Virtual infection prevention — the next frontier. Infect. Control Hosp. Epidemiol. 42, 1374–1375 (2021).
Rawson, T. M., Ahmad, R., Toumazou, C., Georgiou, P. & Holmes, A. H. Artificial intelligence can improve decision-making in infection management. Nat. Hum. Behav. 3, 543–545 (2019).
Wu, S., Tannous, E., Haldane, V., Ellen, M. E. & Wei, X. Barriers and facilitators of implementing interventions to improve appropriate antibiotic use in low- and middle-income countries: a systematic review based on the Consolidated Framework for Implementation Research. Implement. Sci. 17, 30 (2022).
Charani, E. et al. Optimising antimicrobial use in humans — review of current evidence and an interdisciplinary consensus on key priorities for research. Lancet Reg. Health Eur. 7, 100161 (2021).
Okeke, I. N. et al. Leapfrogging laboratories: the promise and pitfalls of high-tech solutions for antimicrobial resistance surveillance in low-income settings. BMJ Glob. Health 5, e003622 (2020).
Verberk, J. D. M. et al. Automated surveillance systems for healthcare-associated infections: results from a European survey and experiences from real-life utilization. J. Hosp. Infect. 122, 35–43 (2022).
Ahmad, R. et al. Strengthening strategic management approaches to address antimicrobial resistance in global human health: a scoping review. BMJ Glob. Health 4, e001730 (2019).
AMR Policy Accelerator. Global Strategy Lab https://amrpolicy.org/ (2024).
Birgand, G. et al. Comparison of governance approaches for the control of antimicrobial resistance: analysis of three European countries. Antimicrob. Resist. Infect. Control. 7, 28 (2018).
Acknowledgements
The authors gratefully acknowledge the David Price Evans Endowment fund and thank the members of the Centre for Antimicrobial Optimisation Network (CAMO-Net) for the helpful discussions. CAMO-Net is a global research partnership funded by the Wellcome Trust (grant ref. 226691/Z/22/Z). Research in the laboratories of the authors was also partially funded by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Healthcare Associated Infections and Antimicrobial Resistance at Imperial College London in partnership with the UK Health Security Agency (previously PHE) in collaboration with Imperial Healthcare Partners, University of Cambridge and University of Warwick. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, the Department of Health and Social Care or the UK Health Security Agency. G.B. contributed to this work as part of the COMBINE research programme, which benefited from a government grant managed by the Agence Nationale de la Recherche under the Programme France 2030 with the reference ANR 22 PAMR 0003.
Author information
Authors and Affiliations
Contributions
D.C., G.B., J.R.-M., N.Z., R.A. and A.H. contributed to the discussion, research, writing, and reviewing and editing of this manuscript. K.J. and A.S.L. contributed to discussion, writing, and reviewing and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Alessandro Cassini, Nicholas Turner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cocker, D., Birgand, G., Zhu, N. et al. Healthcare as a driver, reservoir and amplifier of antimicrobial resistance: opportunities for interventions. Nat Rev Microbiol 22, 636–649 (2024). https://doi.org/10.1038/s41579-024-01076-4
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41579-024-01076-4
This article is cited by
-
The global resistance problem and the clinical antibacterial pipeline
Nature Reviews Microbiology (2025)
-
Drug-induced differential culturability in diverse strains of Mycobacterium tuberculosis
Scientific Reports (2025)
-
Addressing the challenge of antimicrobial resistance
Communications Medicine (2025)
-
Antibiotics re-booted—time to kick back against drug resistance
npj Antimicrobials and Resistance (2025)
-
Machine learning in point-of-care testing: innovations, challenges, and opportunities
Nature Communications (2025)