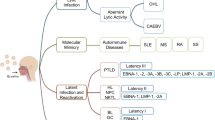
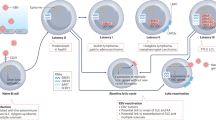
Abstract
Sixty years after its discovery as the first human tumour virus, Epstein–Barr virus (EBV)-specific therapies and vaccines have entered clinical trials. These might not only be applicable for EBV-associated malignancies, where the virus was originally discovered, but also to immunopathologies, including the autoimmune disease multiple sclerosis, which might be triggered in susceptible individuals by primary EBV infection. This Review discusses the surprisingly large spectrum of diseases that EBV seems to cause, as well as which of these might be treated by the therapeutic approaches that are currently being developed or are already clinically applied. New pharmacological inhibitors, antibody therapies, adoptive T cell therapies and active vaccinations are beginning to offer possibilities to target the various EBV infection programmes that are associated with different diseases. These novel developments might allow us to specifically target EBV rather than its host cells in virus-associated pathologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Epstein, M. A., Henle, G., Achong, B. G. & Barr, Y. M. Morphological and biological studies on a virus in cultured lymphoblasts from Burkitt’s lymphoma. J. Exp. Med. 121, 761–770 (1964).
Epstein, M. A., Achong, B. G. & Barr, Y. M. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet 1, 702–703 (1964).
Farrell, P. J. Epstein–Barr virus and cancer. Annu. Rev. Pathol. 14, 29–53 (2019).
Damania, B., Kenney, S. C. & Raab-Traub, N. Epstein–Barr virus: biology and clinical disease. Cell 185, 3652–3670 (2022).
Thorley-Lawson, D. A. & Gross, A. Persistence of the Epstein–Barr virus and the origins of associated lymphomas. N. Engl. J. Med. 350, 1328–1337 (2004).
Münz, C. Latency and lytic replication in the oncogenesis of the Epstein–Barr virus. Nat. Rev. Microbiol. 17, 691–700 (2019).
Ressing, M. E. et al. Immune evasion by Epstein–Barr virus. Curr. Top. Microbiol. Immunol. 391, 355–381 (2015).
Albanese, M., Tagawa, T. & Hammerschmidt, W. Strategies of Epstein–Barr virus to evade innate antiviral immunity of its human host. Front. Microbiol. 13, 955603 (2022).
de Martel, C., Georges, D., Bray, F., Ferlay, J. & Clifford, G. M. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob. Health 8, e180–e190 (2020).
Wong, Y., Meehan, M. T., Burrows, S. R., Doolan, D. L. & Miles, J. J. Estimating the global burden of Epstein–Barr virus-related cancers. J. Cancer Res. Clin. Oncol. 148, 31–46 (2022).
Shannon-Lowe, C. & Rickinson, A. The global landscape of EBV-associated tumors. Front. Oncol. 9, 713 (2019).
Bjornevik, K. et al. Longitudinal analysis reveals high prevalence of Epstein–Barr virus associated with multiple sclerosis. Science 375, 296–301 (2022).
Bjornevik, K., Münz, C., Cohen, J. I. & Ascherio, A. Epstein–Barr virus as a leading cause of multiple sclerosis: mechanisms and implications. Nat. Rev. Neurol. 19, 160–171 (2023).
Cohen, J. I. Therapeutic vaccines for herpesviruses. J. Clin. Invest. 134, e179483 (2024).
Muller, F. et al. CD19 CAR T-cell therapy in autoimmune disease — a case series with follow-up. N. Engl. J. Med. 390, 687–700 (2024).
Bonifacius, A. et al. Patient-tailored adoptive immunotherapy with EBV-specific T cells from related and unrelated donors. J. Clin. Invest. 133, e163548 (2023).
Tugizov, S. M., Herrera, R. & Palefsky, J. M. Epstein–Barr virus transcytosis through polarized oral epithelial cells. J. Virol. 87, 8179–8194 (2013).
Tugizov, S. M., Berline, J. W. & Palefsky, J. M. Epstein–Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat. Med. 9, 307–314 (2003).
Pich, D. et al. First days in the life of naive human B lymphocytes infected with Epstein–Barr virus. mBio 10, e01723-19 (2019).
Mrozek-Gorska, P. et al. Epstein–Barr virus reprograms human B lymphocytes immediately in the prelatent phase of infection. Proc. Natl Acad. Sci. USA 116, 16046–16055 (2019).
Altmann, M. & Hammerschmidt, W. Epstein–Barr virus provides a new paradigm: a requirement for the immediate inhibition of apoptosis. PLoS Biol. 3, e404 (2005).
Alfieri, C., Birkenbach, M. & Kieff, E. Early events in Epstein–Barr virus infection of human B lymphocytes. Virology 181, 595–608 (1991).
Kempkes, B. & Ling, P. D. EBNA2 and its coactivator EBNA-LP. Curr. Top. Microbiol. Immunol. 391, 35–59 (2015).
Allday, M. J., Bazot, Q. & White, R. E. The EBNA3 family: two oncoproteins and a tumour suppressor that are central to the biology of EBV in B cells. Curr. Top. Microbiol. Immunol. 391, 61–117 (2015).
Babcock, J. G., Hochberg, D. & Thorley-Lawson, A. D. The expression pattern of Epstein–Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 13, 497–506 (2000).
SoRelle, E. D. et al. Single-cell RNA-seq reveals transcriptomic heterogeneity mediated by host–pathogen dynamics in lymphoblastoid cell lines. eLife 10, e62586 (2021).
Thorley-Lawson, D. A. Epstein–Barr virus: exploiting the immune system. Nat. Rev. Immunol. 1, 75–82 (2001).
Hochberg, D. et al. Demonstration of the Burkitt’s lymphoma Epstein–Barr virus phenotype in dividing latently infected memory cells in vivo. Proc. Natl Acad. Sci. USA 101, 239–244 (2004).
Babcock, G. J., Decker, L. L., Volk, M. & Thorley-Lawson, D. A. EBV persistence in memory B cells in vivo. Immunity 9, 395–404 (1998).
Laichalk, L. L. & Thorley-Lawson, D. A. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein–Barr virus in vivo. J. Virol. 79, 1296–1307 (2005).
Reusch, J. A., Nawandar, D. M., Wright, K. L., Kenney, S. C. & Mertz, J. E. Cellular differentiation regulator BLIMP1 induces Epstein–Barr virus lytic reactivation in epithelial and B cells by activating transcription from both the R and Z promoters. J. Virol. 89, 1731–1743 (2015).
Woellmer, A., Arteaga-Salas, J. M. & Hammerschmidt, W. BZLF1 governs CpG-methylated chromatin of Epstein–Barr virus reversing epigenetic repression. PLoS Pathog. 8, e1002902 (2012).
Dunmire, S. K., Grimm, J. M., Schmeling, D. O., Balfour, H. H. Jr. & Hogquist, K. A. The incubation period of primary Epstein–Barr virus infection: viral dynamics and immunologic events. PLoS Pathog. 11, e1005286 (2015).
Ali, A. et al. Rta is the principal activator of Epstein–Barr virus epithelial lytic transcription. PLoS Pathog. 18, e1010886 (2022).
Van Sciver, N. et al. Hippo signaling effectors YAP and TAZ induce Epstein–Barr virus (EBV) lytic reactivation through TEADs in epithelial cells. PLoS Pathog. 17, e1009783 (2021).
Taylor, G. S., Long, H. M., Brooks, J. M., Rickinson, A. B. & Hislop, A. D. The immunology of Epstein–Barr virus-induced disease. Annu. Rev. Immunol. 33, 787–821 (2015).
Damania, B. & Münz, C. Immunodeficiencies that predispose to pathologies by human oncogenic gamma-herpesviruses. FEMS Microbiol. Rev. 43, 181–192 (2019).
Caduff, N. et al. Immunosuppressive FK506 treatment leads to more frequent EBV-associated lymphoproliferative disease in humanized mice. PLoS Pathog. 16, e1008477 (2020).
McHugh, D. et al. EBV renders B cells susceptible to HIV-1 in humanized mice. Life Sci. Alliance 3, e202000640 (2020).
Tangye, S. G. & Latour, S. Primary immunodeficiencies reveal the molecular requirements for effective host defense against EBV infection. Blood 135, 644–655 (2020).
Martin, E. et al. Role of IL-27 in Epstein–Barr virus infection revealed by IL-27RA deficiency. Nature 628, 620–629 (2024).
Appay, V. et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8, 379–385 (2002).
Schmidt, F. et al. In-depth analysis of human virus-specific CD8+ T cells delineates unique phenotypic signatures for T cell specificity prediction. Cell Rep. 42, 113250 (2023).
Deng, Y. et al. CD27 is required for protective lytic EBV antigen specific CD8+ T cell expansion. Blood 137, 3225–3236 (2021).
Kirchmeier, D. et al. Epstein Barr virus infection induces tissue-resident memory T cells in mucosal lymphoid tissues. JCI Insight 9, e173489 (2024).
Azzi, T. et al. Role for early-differentiated natural killer cells in infectious mononucleosis. Blood 124, 2533–2543 (2014).
Chijioke, O. et al. Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein–Barr virus infection. Cell Rep. 5, 1489–1498 (2013).
Caduff, N. et al. KSHV infection drives poorly cytotoxic CD56 negative natural killer cell differentiation in vivo upon KSHV/EBV dual infection. Cell Rep. 35, 109056 (2021).
Zhang, J. et al. LMP1 and EBNA2 constitute a minimal set of EBV genes for transformation of human B cells. Front. Immunol. 14, 1331730 (2023).
Lopez, C. et al. Burkitt lymphoma. Nat. Rev. Dis. Primers 8, 78 (2022).
Robbiani, D. F. et al. Plasmodium infection promotes genomic instability and AID-dependent B cell lymphoma. Cell 162, 727–737 (2015).
Whittle, H. C. et al. T-cell control of Epstein–Barr virus-infected B cells is lost during P. falciparum malaria. Nature 312, 449–450 (1984).
Moormann, A. M. et al. Children with endemic Burkitt lymphoma are deficient in EBNA1-specific IFN-γ T cell responses. Int. J. Cancer 124, 1721–1726 (2009).
Moormann, A. M. et al. Exposure to holoendemic malaria results in elevated Epstein–Barr virus loads in children. J. Infect. Dis. 191, 1233–1238 (2005).
Connors, J. M. et al. Hodgkin lymphoma. Nat. Rev. Dis. Primers 6, 61 (2020).
Hjalgrim, H. et al. Characteristics of Hodgkin’s lymphoma after infectious mononucleosis. N. Engl. J. Med. 349, 1324–1332 (2003).
Callan, M. F. et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein–Barr virus In vivo. J. Exp. Med. 187, 1395–1402 (1998).
Callan, M. F. et al. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat. Med. 2, 906–911 (1996).
Cesarman, E., Chadburn, A. & Rubinstein, P. G. KSHV/HHV8-mediated hematologic diseases. Blood 139, 1013–1025 (2022).
Wong, K. C. W. et al. Nasopharyngeal carcinoma: an evolving paradigm. Nat. Rev. Clin. Oncol. 18, 679–695 (2021).
Xu, M. et al. Genome sequencing analysis identifies Epstein–Barr virus subtypes associated with high risk of nasopharyngeal carcinoma. Nat. Genet. 51, 1131–1136 (2019).
Li, Z. et al. Epstein–Barr virus ncRNA from a nasopharyngeal carcinoma induces an inflammatory response that promotes virus production. Nat. Microbiol. 4, 2475–2486 (2019).
Kimura, H., de Leval, L., Cai, Q. & Kim, W. S. EBV-associated NK and T-cell lymphoid neoplasms. Curr. Opin. Oncol. 34, 422–431 (2022).
Vij, M. et al. CARMIL2 immunodeficiency with Epstein Barr virus associated smooth muscle tumor (EBV-SMT). Report of a case with comprehensive review of literature. Fetal Pediatr. Pathol. 41, 1023–1034 (2022).
Shaw, R. K. et al. Bilateral adrenal EBV-associated smooth muscle tumors in a child with a natural killer cell deficiency. Blood 119, 4009–4012 (2012).
Fournier, B. et al. Inherited TNFSF9 deficiency causes broad Epstein–Barr virus infection with EBV+ smooth muscle tumors. J. Exp. Med. 219, e20211682 (2022).
Collins, P. J. et al. Characterizing EBV-associated lymphoproliferative diseases and the role of myeloid-derived suppressor cells. Blood 137, 203–215 (2021).
Fournier, B. et al. Rapid identification and characterization of infected cells in blood during chronic active Epstein–Barr virus infection. J. Exp. Med. 217, e20192262 (2020).
Coleman, C. B. et al. Epstein–Barr virus type 2 infects T cells and induces B cell lymphomagenesis in humanized mice. J. Virol. 92, e00813-18 (2018).
Wongwiwat, W. et al. Epstein–Barr virus genome deletions in Epstein–Barr virus-positive T/NK cell lymphoproliferative diseases. J. Virol. 96, e0039422 (2022).
Dunmire, S. K., Verghese, P. S. & Balfour, H. H. Jr. Primary Epstein–Barr virus infection. J. Clin. Virol. 102, 84–92 (2018).
Panikkar, A. et al. Cytokine-mediated loss of blood dendritic cells during Epstein–Barr virus-associated acute infectious mononucleosis: implication for immune dysregulation. J. Infect. Dis. 212, 1957–1961 (2015).
Jayasooriya, S. et al. Early virological and immunological events in asymptomatic Epstein–Barr virus infection in African children. PLoS Pathog. 11, e1004746 (2015).
Gujer, C. et al. Plasmacytoid dendritic cells respond to Epstein–Barr virus infection with a distinct type I interferon subtype profile. Blood Adv. 3, 1129–1144 (2019).
Severa, M. et al. EBV stimulates TLR- and autophagy-dependent pathways and impairs maturation in plasmacytoid dendritic cells: implications for viral immune escape. Eur. J. Immunol. 43, 147–158 (2013).
Fiola, S., Gosselin, D., Takada, K. & Gosselin, J. TLR9 contributes to the recognition of EBV by primary monocytes and plasmacytoid dendritic cells. J. Immunol. 185, 3620–3631 (2010).
Williams, H. et al. The immune response to primary EBV infection: a role for natural killer cells. Br. J. Haematol. 129, 266–274 (2005).
Balfour, H. H. Jr. et al. Behavioral, virologic, and immunologic factors associated with acquisition and severity of primary Epstein–Barr virus infection in university students. J. Infect. Dis. 207, 80–88 (2013).
Pappworth, I. Y., Wang, E. C. & Rowe, M. The switch from latent to productive infection in Epstein–Barr virus-infected B cells is associated with sensitization to NK cell killing. J. Virol. 81, 474–482 (2007).
Hochberg, D. et al. Acute infection with Epstein–Barr virus targets and overwhelms the peripheral memory B-cell compartment with resting, latently infected cells. J. Virol. 78, 5194–5204 (2004).
Thacker, E. L., Mirzaei, F. & Ascherio, A. Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann. Neurol. 59, 499–503 (2006).
Olsson, T., Barcellos, L. F. & Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 13, 25–36 (2017).
Lünemann, J. D. et al. Elevated Epstein–Barr virus-encoded nuclear antigen-1 immune responses predict conversion to multiple sclerosis. Ann. Neurol. 67, 159–169 (2010).
Kvistad, S. et al. Antibodies to Epstein–Barr virus and MRI disease activity in multiple sclerosis. Mult. Scler. 20, 1833–1840 (2014).
Cepok, S. et al. Identification of Epstein–Barr virus proteins as putative targets of the immune response in multiple sclerosis. J. Clin. Invest. 115, 1352–1360 (2005).
Lanz, T. V. et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 603, 321–327 (2022).
Tengvall, K. et al. Molecular mimicry between Anoctamin 2 and Epstein–Barr virus nuclear antigen 1 associates with multiple sclerosis risk. Proc. Natl Acad. Sci. USA 116, 16955–16960 (2019).
Thomas, O. G. et al. Cross-reactive EBNA1 immunity targets alpha-crystallin B and is associated with multiple sclerosis. Sci. Adv. 9, eadg3032 (2023).
Vietzen, H. et al. Ineffective control of Epstein-Barr-virus-induced autoimmunity increases the risk for multiple sclerosis. Cell 186, 5705–5718.e13 (2023).
Wilkinson, N. M., Chen, H. C., Lechner, M. G. & Su, M. A. Sex differences in immunity. Annu. Rev. Immunol. 40, 75–94 (2022).
Lünemann, J. D. et al. Increased frequency and broadened specificity of latent EBV nuclear antigen-1-specific T cells in multiple sclerosis. Brain 129, 1493–1506 (2006).
Lünemann, J. D. et al. EBNA1-specific T cells from patients with multiple sclerosis cross react with myelin antigens and co-produce IFN-γ and IL-2. J. Exp. Med. 205, 1763–1773 (2008).
Thomas, O. G. et al. Heightened Epstein–Barr virus immunity and potential cross-reactivities in multiple sclerosis. PLoS Pathog. 20, e1012177 (2024).
Jilek, S. et al. Strong EBV-specific CD8+ T-cell response in patients with early multiple sclerosis. Brain 131, 1712–1721 (2008).
Serafini, B., Rosicarelli, B., Veroni, C., Mazzola, G. A. & Aloisi, F. Epstein–Barr virus-specific CD8 T cells selectively infiltrate the brain in multiple sclerosis and interact locally with virus-infected cells: clue for a virus-driven immunopathological mechanism. J. Virol. 93, e00980-19 (2019).
Schneider-Hohendorf, T. et al. Broader Epstein–Barr virus-specific T cell receptor repertoire in patients with multiple sclerosis. J. Exp. Med. 219, e20220650 (2022).
Gottlieb, A., Pham, H. P. T., Saltarrelli, J. G. & Lindsey, J. W. Expanded T lymphocytes in the cerebrospinal fluid of multiple sclerosis patients are specific for Epstein–Barr-virus-infected B cells. Proc. Natl Acad. Sci. USA 121, e2315857121 (2024).
Serafini, B. et al. Dysregulated Epstein–Barr virus infection in the multiple sclerosis brain. J. Exp. Med. 204, 2899–2912 (2007).
Zdimerova, H. et al. Attenuated immune control of Epstein–Barr virus in humanized mice is associated with the multiple sclerosis risk factor HLA-DR15. Eur. J. Immunol. 51, 64–75 (2021).
Serafini, B., Rosicarelli, B., Veroni, C. & Aloisi, F. Tissue-resident memory T cells in the multiple sclerosis brain and their relationship to Epstein–Barr virus infected B cells. J. Neuroimmunol. 376, 578036 (2023).
Tosato, G. et al. Abnormally elevated frequency of Epstein–Barr virus-infected B cells in the blood of patients with rheumatoid arthritis. J. Clin. Invest. 73, 1789–1795 (1984).
Tsokos, G. C., Magrath, I. T. & Balow, J. E. Epstein–Barr virus induces normal B cell responses but defective suppressor T cell responses in patients with systemic lupus erythematosus. J. Immunol. 131, 1797–1801 (1983).
AlDabbagh, M. A. et al. The role of antiviral prophylaxis for the prevention of Epstein–Barr virus-associated posttransplant lymphoproliferative disease in solid organ transplant recipients: a systematic review. Am. J. Transpl. 17, 770–781 (2017).
Dugan, J. P. et al. Complete and durable responses in primary central nervous system post-transplant lymphoproliferative disorder with zidovudine, ganciclovir, rituximab and dexamethasone. Clin. Cancer Res. 24, 3273–3281 (2018).
Haverkos, B. et al. Targeted therapy with nanatinostat and valganciclovir in recurrent EBV-positive lymphoid malignancies: a phase 1b/2 study. Blood Adv. 7, 6339–6350 (2023).
Drosu, N. C., Edelman, E. R. & Housman, D. E. Tenofovir prodrugs potently inhibit Epstein–Barr virus lytic DNA replication by targeting the viral DNA polymerase. Proc. Natl Acad. Sci. USA 117, 12368–12374 (2020).
Torkildsen, O., Myhr, K. M., Brugger-Synnes, P. & Bjornevik, K. Antiviral therapy with tenofovir in MS. Mult. Scler. Relat. Disord. 83, 105436 (2024).
Frappier, L. Ebna1. Curr. Top. Microbiol. Immunol. 391, 3–34 (2015).
Humme, S. et al. The EBV nuclear antigen 1 (EBNA1) enhances B cell immortalization several thousandfold. Proc. Natl Acad. Sci. USA 100, 10989–10994 (2003).
Thompson, S., Messick, T., Schultz, D. C., Reichman, M. & Lieberman, P. M. Development of a high-throughput screen for inhibitors of Epstein–Barr virus EBNA1. J. Biomol. Screen. 15, 1107–1115 (2010).
Li, N. et al. Discovery of selective inhibitors against EBNA1 via high throughput in silico virtual screening. PLoS ONE 5, e10126 (2010).
Bochkarev, A. et al. Crystal structure of the DNA-binding domain of the Epstein–Barr virus origin-binding protein, EBNA1, bound to DNA. Cell 84, 791–800 (1996).
Messick, T. E. et al. Structure-based design of small-molecule inhibitors of EBNA1 DNA binding blocks Epstein–Barr virus latent infection and tumor growth. Sci. Transl Med. 11, eaau5612 (2019).
Soldan, S. S. et al. EBNA1 inhibitors have potent and selective antitumor activity in xenograft models of Epstein–Barr virus-associated gastric cancer. Gastric Cancer 24, 1076–1088 (2021).
Monaco, M. C. G. et al. EBNA1 inhibitors block proliferation of spontaneous lymphoblastoid cell lines from patients with multiple sclerosis and healthy controls. Neurol. Neuroimmunol. Neuroinflamm. 10, e200149 (2023).
Colevas, A. D. et al. First-in-human clinical trial of a small molecule EBNA1 inhibitor, VK-2019, in patients with Epstein–Barr positive nasopharyngeal cancer, with pharmacokinetic and pharmacodynamic studies. Clin. Cancer Res. 31, 815–823 (2025).
Kang, M. S. et al. Roscovitine inhibits EBNA1 serine 393 phosphorylation, nuclear localization, transcription, and episome maintenance. J. Virol. 85, 2859–2868 (2011).
Kieser, A. & Sterz, K. R. The latent membrane protein 1 (LMP1). Curr. Top. Microbiol. Immunol. 391, 119–149 (2015).
Kang, M. S. & Kieff, E. Epstein–Barr virus latent genes. Exp. Mol. Med. 47, e131 (2015).
Giehler, F. et al. Epstein–Barr virus-driven B cell lymphoma mediated by a direct LMP1-TRAF6 complex. Nat. Commun. 15, 414 (2024).
Muller-Durovic, B. et al. A metabolic dependency of EBV can be targeted to hinder B cell transformation. Science 385, eadk4898 (2024).
Mellman, I., Chen, D. S., Powles, T. & Turley, S. J. The cancer–immunity cycle: indication, genotype, and immunotype. Immunity 56, 2188–2205 (2023).
Dharnidharka, V. R. et al. Post-transplant lymphoproliferative disorders. Nat. Rev. Dis. Primers 2, 15088 (2016).
Curtis, R. E. et al. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood 94, 2208–2216 (1999).
Styczynski, J. et al. Response to rituximab-based therapy and risk factor analysis in Epstein Barr virus-related lymphoproliferative disorder after hematopoietic stem cell transplant in children and adults: a study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Clin. Infect. Dis. 57, 794–802 (2013).
Salles, G. et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv. Ther. 34, 2232–2273 (2017).
Naismith, R. T. et al. Rituximab add-on therapy for breakthrough relapsing multiple sclerosis: a 52-week phase II trial. Neurology 74, 1860–1867 (2010).
Hawker, K. et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann. Neurol. 66, 460–471 (2009).
Hauser, S. L. et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 358, 676–688 (2008).
Montalban, X. et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 376, 209–220 (2017).
Hauser, S. L. et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N. Engl. J. Med. 376, 221–234 (2017).
Mackensen, A. et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. Med. 28, 2124–2132 (2022).
Cencioni, M. T., Mattoscio, M., Magliozzi, R., Bar-Or, A. & Muraro, P. A. B cells in multiple sclerosis — from targeted depletion to immune reconstitution therapies. Nat. Rev. Neurol. 17, 399–414 (2021).
Studer, V., Rossi, S., Motta, C., Buttari, F. & Centonze, D. Peripheral B cell depletion and central proinflammatory cytokine reduction following repeated intrathecal administration of rituximab in progressive multiple sclerosis. J. Neuroimmunol. 276, 229–231 (2014).
Piccio, L. et al. Changes in B- and T-lymphocyte and chemokine levels with rituximab treatment in multiple sclerosis. Arch. Neurol. 67, 707–714 (2010).
Langer-Gould, A., Li, B. H., Smith, J. B. & Xu, S. Multiple sclerosis, rituximab, hypogammaglobulinemia, and risk of infections. Neurol. Neuroimmunol. Neuroinflamm 11, e200211 (2024).
Deutsch, Y. E., Tadmor, T., Podack, E. R. & Rosenblatt, J. D. CD30: an important new target in hematologic malignancies. Leuk. Lymphoma 52, 1641–1654 (2011).
Younes, A. et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J. Clin. Oncol. 30, 2183–2189 (2012).
Moskowitz, C. H. et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 385, 1853–1862 (2015).
Jacobsen, E. D. et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood 125, 1394–1402 (2015).
Bartlett, N. L. et al. Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J. Hematol. Oncol. 7, 24 (2014).
Pro, B. et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J. Clin. Oncol. 30, 2190–2196 (2012).
Prince, H. M. et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet 390, 555–566 (2017).
Kim, H. K. et al. Complete remission in CD30-positive refractory extranodal NK/T-cell lymphoma with brentuximab vedotin. Blood Res. 50, 254–256 (2015).
Poon, L. M. & Kwong, Y. L. Complete remission of refractory disseminated NK/T cell lymphoma with brentuximab vedotin and bendamustine. Ann. Hematol. 95, 847–849 (2016).
Ansell, S. M. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 372, 311–319 (2015).
Fang, W. et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 19, 1338–1350 (2018).
Jiang, W. et al. Enhancing efficacy and reducing toxicity: therapeutic optimization in locoregionally advanced nasopharyngeal carcinoma. Cell Rep. Med. 5, 101594 (2024).
Chatterjee, B. et al. CD8+ T cells retain protective functions despite sustained inhibitory receptor expression during Epstein–Barr virus infection in vivo. PLoS Pathog. 15, e1007748 (2019).
Volk, V. et al. PD-1 blockade aggravates Epstein–Barr virus+ post-transplant lymphoproliferative disorder in humanized mice resulting in central nervous system involvement and CD4+ T cell dysregulations. Front. Oncol. 10, 614876 (2020).
Johnson, D. B. et al. A case report of clonal EBV-like memory CD4+ T cell activation in fatal checkpoint inhibitor-induced encephalitis. Nat. Med. 25, 1243–1250 (2019).
Matsumura, T. et al. Lymphoproliferative disorder in an esophageal cancer patient treated with pembrolizumab. Intern. Med. https://doi.org/10.2169/internalmedicine.3743-24 (2024).
Heslop, H. E., Brenner, M. K. & Rooney, C. M. Donor T cells to treat EBV-associated lymphoma. N. Engl. J. Med. 331, 679–680 (1994).
Papadopoulos, E. B. et al. Infusions of donor leukocytes to treat Epstein–Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N. Engl. J. Med. 330, 1185–1191 (1994).
Leen, A. M., Rooney, C. M. & Foster, A. E. Improving T cell therapy for cancer. Annu. Rev. Immunol. 25, 243–265 (2007).
Hanley, P. J. et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood 114, 1958–1967 (2009).
Papadopoulou, A. et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci. Transl Med. 6, 242ra283 (2014).
Bollard, C. M. et al. The generation and characterization of LMP2-specific CTLs for use as adoptive transfer from patients with relapsed EBV-positive Hodgkin disease. J. Immunother. 27, 317–327 (2004).
Bollard, C. M. et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein–Barr virus latent membrane proteins. J. Clin. Oncol. 32, 798–808 (2014).
Icheva, V. et al. Adoptive transfer of Epstein–Barr virus (EBV) nuclear antigen 1-specific T cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J. Clin. Oncol. 31, 39–48 (2013).
Moss, D. J., Khanna, R. & Gandhi, M. The use of T-cell directed cellular therapies in Australia. Cytotherapy 9, 222–224 (2007).
Pender, M. P. et al. Epstein–Barr virus-specific adoptive immunotherapy for progressive multiple sclerosis. Mult. Scler. 20, 1541–1544 (2014).
Pender, M. P. et al. Epstein–Barr virus-specific T cell therapy for progressive multiple sclerosis. JCI Insight 3, e124717 (2018).
Leen, A. M. et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 121, 5113–5123 (2013).
Doubrovina, E. et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood 119, 2644–2656 (2012).
O’Reilly, R. J., Prockop, S., Hasan, A. & Doubrovina, E. Therapeutic advantages provided by banked virus-specific T-cells of defined HLA-restriction. Bone Marrow Transpl. 54, 759–764 (2019).
Haque, T. et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood 110, 1123–1131 (2007).
Haque, T. et al. Treatment of Epstein–Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet 360, 436–442 (2002).
Sinha, D. et al. ‘Off-the-shelf’ allogeneic antigen-specific adoptive T-cell therapy for the treatment of multiple EBV-associated malignancies. J. Immunother. Cancer 9, e001608 (2021).
Bollard, C. M. et al. Tumor-specific T-cells engineered to overcome tumor immune evasion induce clinical responses in patients with relapsed hodgkin lymphoma. J. Clin. Oncol. 36, 1128–1139 (2018).
Smith, C. et al. Complete response to PD-1 blockade following EBV-specific T-cell therapy in metastatic nasopharyngeal carcinoma. npj Precis. Oncol. 5, 24 (2021).
Gandhi, M. K. et al. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood 108, 2280–2289 (2006).
Paliannina, D. et al. Stem cell memory EBV-specific T cells control EBV tumor growth and persist in vivo. Sci. Adv. 10, eado2048 (2024).
Hui, E. P. et al. Phase I trial of recombinant modified vaccinia ankara encoding Epstein–Barr viral tumor antigens in nasopharyngeal carcinoma patients. Cancer Res. 73, 1676–1688 (2013).
Taylor, G. S. et al. A recombinant modified vaccinia ankara vaccine encoding Epstein–Barr virus (EBV) target antigens: a phase I trial in UK patients with EBV-positive cancer. Clin. Cancer Res. 20, 5009–5022 (2014).
Rühl, J. et al. Heterologous prime-boost vaccination protects from EBV antigen expressing lymphomas. J. Clin. Invest. 129, 2071–2087 (2019).
Paludan, C. et al. EBNA1 specific CD4+ Th1 cells kill Burkitt’s lymphoma cells. J. Immunol. 169, 1593–1603 (2002).
Gurer, C. et al. Targeting the nuclear antigen 1 of Epstein–Barr virus to the human endocytic receptor DEC-205 stimulates protective T-cell responses. Blood 112, 1231–1239 (2008).
Meixlsperger, S. et al. CD141+ dendritic cells produce prominent amounts of IFN-α after dsRNA recognition and can be targeted via DEC-205 in humanized mice. Blood 121, 5034–5044 (2013).
Läderach, F. et al. MHC class II-deficient mice allow functional human CD4+ T-cell development. Eur. J. Immunol. 53, e2250313 (2023).
van Zyl, D. G. et al. Immunogenic particles with a broad antigenic spectrum stimulate cytolytic T cells and offer increased protection against EBV infection ex vivo and in mice. PLoS Pathog. 14, e1007464 (2018).
Dasari, V. et al. Lymph node targeted multi-epitope subunit vaccine promotes effective immunity to EBV in HLA-expressing mice. Nat. Commun. 14, 4371 (2023).
Moutschen, M. et al. Phase I/II studies to evaluate safety and immunogenicity of a recombinant gp350 Epstein–Barr virus vaccine in healthy adults. Vaccine 25, 4697–4705 (2007).
Sokal, E. M. et al. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein–Barr virus vaccine in healthy young adults. J. Infect. Dis. 196, 1749–1753 (2007).
Cui, X. et al. A novel tetrameric gp350 1-470 as a potential Epstein–Barr virus vaccine. Vaccine 31, 3039–3045 (2013).
Kanekiyo, M. et al. Rational design of an Epstein–Barr virus vaccine targeting the receptor-binding site. Cell 162, 1090–1100 (2015).
Ogembo, J. G. et al. A chimeric EBV gp350/220-based VLP replicates the virion B-cell attachment mechanism and elicits long-lasting neutralizing antibodies in mice. J. Transl. Med. 13, 50 (2015).
Bu, W. et al. Immunization with components of the viral fusion apparatus elicits antibodies that neutralize Epstein–Barr virus in B cells and epithelial cells. Immunity 50, 1305–1316.e1306 (2019).
Cui, X. et al. Rabbits immunized with Epstein–Barr virus gH/gL or gB recombinant proteins elicit higher serum virus neutralizing activity than gp350. Vaccine 34, 4050–4055 (2016).
Wei, C. J. et al. A bivalent Epstein–Barr virus vaccine induces neutralizing antibodies that block infection and confer immunity in humanized mice. Sci. Transl Med. 14, eabf3685 (2022).
Cui, X. et al. Immunization with Epstein–Barr virus core fusion machinery envelope proteins elicit high titers of neutralizing activities and protect humanized mice from lethal dose EBV challenge. Vaccines 9, 285 (2021).
Sun, C. et al. A gB nanoparticle vaccine elicits a protective neutralizing antibody response against EBV. Cell Host Microbe 31, 1882–1897.e10 (2023).
Zhong, L. et al. A cocktail nanovaccine targeting key entry glycoproteins elicits high neutralizing antibody levels against EBV infection. Nat. Commun. 15, 5310 (2024).
Escalante, G. M. et al. Multivalent MVA-vectored vaccine elicits EBV neutralizing antibodies in rhesus macaques that reduce EBV infection in humanized mice. Front. Immunol. 15, 1445209 (2024).
Shannon-Lowe, C. D., Neuhierl, B., Baldwin, G., Rickinson, A. B. & Delecluse, H. J. Resting B cells as a transfer vehicle for Epstein–Barr virus infection of epithelial cells. Proc. Natl Acad. Sci. USA 103, 7065–7070 (2006).
Shannon-Lowe, C. & Rowe, M. Epstein–Barr virus infection of polarized epithelial cells via the basolateral surface by memory B cell-mediated transfer infection. PLoS Pathog. 7, e1001338 (2011).
Connolly, S. A., Jardetzky, T. S. & Longnecker, R. The structural basis of herpesvirus entry. Nat. Rev. Microbiol. 19, 110–121 (2021).
Borza, C. M. & Hutt-Fletcher, L. M. Alternate replication in B cells and epithelial cells switches tropism of Epstein–Barr virus. Nat. Med. 8, 594–599 (2002).
Chen, J. et al. Ephrin receptor A2 is a functional entry receptor for Epstein–Barr virus. Nat. Microbiol. 3, 172–180 (2018).
Zhang, H. et al. Ephrin receptor A2 is an epithelial cell receptor for Epstein–Barr virus entry. Nat. Microbiol. 3, 1–8 (2018).
Sommermann, T. et al. Functional interplay of Epstein–Barr virus oncoproteins in a mouse model of B cell lymphomagenesis. Proc. Natl Acad. Sci. USA 117, 14421–14432 (2020).
Bristol, J. A. et al. A cancer-associated Epstein–Barr virus BZLF1 promoter variant enhances lytic infection. PLoS Pathog. 14, e1007179 (2018).
Okuno, Y. et al. Defective Epstein–Barr virus in chronic active infection and haematological malignancy. Nat. Microbiol. 4, 404–413 (2019).
Ma, S. D. et al. A new model of Epstein–Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas. J. Virol. 85, 165–177 (2011).
Antsiferova, O. et al. Adoptive transfer of EBV specific CD8+ T cell clones can transiently control EBV infection in humanized mice. PLoS Pathog. 10, e1004333 (2014).
Ma, S. D. et al. An Epstein–Barr virus (EBV) mutant with enhanced BZLF1 expression causes lymphomas with abortive lytic EBV infection in a humanized mouse model. J. Virol. 86, 7976–7987 (2012).
McHugh, D. et al. Persistent KSHV infection increases EBV-associated tumor formation in vivo via enhanced EBV lytic gene expression. Cell Host Microbe 22, 61–73 (2017).
Acknowledgements
Research in the author’s laboratory is in part financially supported by the Swiss National Science Foundation (310030_204470/1 and CRSII_222718_10000065), Cancer Research Switzerland (KFS-5896-08-2023-R), the Swiss MS Society (2023-17), the Swiss State Secretariat for Education, Research and Innovation (SERI) for EU Horizon BEHIND-MS, the Sobek Foundation, the Swiss Vaccine Research Institute, the Vontobel Foundation, Roche and Pfizer.
Author information
Authors and Affiliations
Contributions
C.M. wrote and edited this manuscript.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Paul Farrell, Benjamin Gewurz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Münz, C. Epstein–Barr virus pathogenesis and emerging control strategies. Nat Rev Microbiol 23, 667–679 (2025). https://doi.org/10.1038/s41579-025-01181-y
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41579-025-01181-y