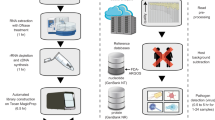
Abstract
Metagenomics is becoming more widely used for diagnosis of viral infections and surveillance of viruses. Its pathogen-agnostic approach makes metagenomics useful for unknown and novel infection diagnosis, outbreak investigation, and new and emerging pathogen surveillance. New metagenomics methods, such as the use of rapid sequencing technologies and approaches that can selectively enrich for a wide range of viruses, are expanding the range of clinical and public health scenarios in which metagenomics can be used. Following the COVID-19 pandemic, there is increasing interest in viral surveillance worldwide, using clinical samples, potential zoonotic reservoirs and environmental sources, such as wastewater. Validation and accreditation of metagenomics protocols to ensure quality, together with further innovation in methods, will be necessary to bring metagenomics into routine service in clinical and public health laboratories.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Smyrlaki, I. et al. Massive and rapid COVID-19 testing is feasible by extraction-free SARS-CoV-2 RT-PCR. Nat. Commun. 11, 4812 (2020).
Bayart, J.-L. et al. Clinical performance evaluation of the Fluorecare® SARS-CoV-2 & influenza A/B & RSV rapid antigen combo test in symptomatic individuals. J. Clin. Virol. 161, 105419 (2023).
Ferrani, S. et al. Diagnostic accuracy of a rapid antigen triple test (SARS-CoV-2, respiratory syncytial virus, and influenza) using anterior nasal swabs versus multiplex RT-PCR in children in an emergency department. Infect. Dis. Now 53, 104769 (2023).
Eigner, U. et al. Clinical evaluation of multiplex RT-PCR assays for the detection of influenza A/B and respiratory syncytial virus using a high throughput system. J. Virol. Methods 269, 49–54 (2019).
Enne, V. I. et al. Multicentre evaluation of two multiplex PCR platforms for the rapid microbiological investigation of nosocomial pneumonia in UK ICUs: the INHALE WP1 study. Thorax 77, 1220–1228 (2022).
Shoar, S. & Musher, D. M. Etiology of community-acquired pneumonia in adults: a systematic review. Pneumonia 12, 11 (2020).
Venkatesan, A. et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the International Encephalitis Consortium. Clin. Infect. Dis. 57, 1114–1128 (2013).
Zhu, N. et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382, 727–733 (2020).
Jacob, S. T. et al. Ebola virus disease. Nat. Rev. Dis. Primers 6, 14 (2020).
Pierson, T. C. & Diamond, M. S. The emergence of Zika virus and its new clinical syndromes. Nature 560, 573–581 (2018).
Castañeda-Mogollón, D. et al. A metagenomics workflow for SARS-CoV-2 identification, co-pathogen detection, and overall diversity. J. Clin. Virol. 145, 105025 (2021).
Isidro, J. et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat. Med. 28, 1569–1572 (2022).
Proctor, L. M. et al. The integrative human microbiome project. Nature 569, 641–648 (2019).
Fourgeaud, J. et al. Performance of clinical metagenomics in France: a prospective observational study. Lancet Microbe 5, e52–e61 (2024). This work evaluates the performance of metagenomics as a diagnostic test in a French hospital.
Hogan, C. A. et al. Clinical impact of metagenomic next-generation sequencing of plasma cell-free DNA for the diagnosis of infectious diseases: a multicenter retrospective cohort study. Clin. Infect. Dis. 72, 239–245 (2021).
Wallen, Z. D. et al. Metagenomics of Parkinson’s disease implicates the gut microbiome in multiple disease mechanisms. Nat. Commun. 13, 6958 (2022).
Servellita, V. et al. Adeno-associated virus type 2 in US children with acute severe hepatitis. Nature 617, 574–580 (2023).
Morfopoulou, S. et al. Genomic investigations of unexplained acute hepatitis in children. Nature 617, 564–573 (2023).
Ho, A. et al. Adeno-associated virus 2 infection in children with non-A–E hepatitis. Nature 617, 555–563 (2023). Together with Servellita et al. (2023) and Morfopoulou et al. (2023), this study describes the metagenomic identification of AAV2 in cases from the 2022 outbreak of acute hepatitis in children.
Li, P. et al. Rapid identification and metagenomics analysis of the adenovirus type 55 outbreak in Hubei using real-time and high-throughput sequencing platforms. Infect. Genet. Evol. 93, 104939 (2021).
Liu, D. et al. Fingerprinting of human noroviruses co-infections in a possible foodborne outbreak by metagenomics. Int. J. Food Microbiol. 333, 108787 (2020).
Orf, G. S. et al. Purifying selection decreases the potential for Bangui orthobunyavirus outbreaks in humans. Virus Evol. 9, vead018 (2023).
Ergunay, K. et al. The expanding range of emerging tick-borne viruses in Eastern Europe and the Black Sea region. Sci. Rep. 13, 19824 (2023).
Chiu, C. Y. et al. Two human cases of fatal meningoencephalitis associated with Potosi and Lone Star virus infections, United States, 2020–2023. Emerg. Infect. Dis. 31, 215–221 (2025).
Yang, R. et al. Human infection of avian influenza A H3N8 virus and the viral origins: a descriptive study. Lancet Microbe 3, e824–e834 (2022).
Chiu, C. Y. & Miller, S. A. Clinical metagenomics. Nat. Rev. Genet. 20, 341–355 (2019).
Penner, J. et al. Translating metagenomics into clinical practice for complex paediatric neurological presentations. J. Infect. 87, 451–458 (2023).
Arroyo Mühr, L. S., Dillner, J., Ure, A. E., Sundström, K. & Hultin, E. Comparison of DNA and RNA sequencing of total nucleic acids from human cervix for metagenomics. Sci. Rep. 11, 18852 (2021).
Kalantar, K. L. et al. Integrated host-microbe plasma metagenomics for sepsis diagnosis in a prospective cohort of critically ill adults. Nat. Microbiol. 7, 1805–1816 (2022).
Mick, E. et al. Integrated host/microbe metagenomics enables accurate lower respiratory tract infection diagnosis in critically ill children. J. Clin. Invest. 133, e165904 (2023).
Baud, D., Gubler, D. J., Schaub, B., Lanteri, M. C. & Musso, D. An update on Zika virus infection. Lancet 390, 2099–2109 (2017).
Wahl, A., Huptas, C. & Neuhaus, K. Comparison of rRNA depletion methods for efficient bacterial mRNA sequencing. Sci. Rep. 12, 5765 (2022).
Atkinson, L. et al. Untargeted metagenomics protocol for the diagnosis of infection from CSF and tissue from sterile sites. Heliyon 9, e19854 (2023).
Alcolea-Medina, A. et al. Unified metagenomic method for rapid detection of microorganisms in clinical samples. Commun. Med. 4, 135 (2024). This paper describes a rapid clinical metagenomics workflow for diagnosis of respiratory infections in patients in intensive care units.
Charalampous, T. et al. Nanopore metagenomics enables rapid clinical diagnosis of bacterial lower respiratory infection. Nat. Biotechnol. 37, 783–792 (2019).
Schuele, L., Cassidy, H., Peker, N., Rossen, J. W. A. & Couto, N. Future potential of metagenomics in microbiology laboratories. Expert Rev. Mol. Diagn. 21, 1273–1285 (2021).
Pichler, I. et al. Rapid and sensitive single-sample viral metagenomics using Nanopore Flongle sequencing. J. Virol. Methods 320, 114784 (2023).
Kohl, C. et al. Protocol for metagenomic virus detection in clinical specimens1. Emerg. Infect. Dis. 21, 48–57 (2015).
Wylie, T. N., Wylie, K. M., Herter, B. N. & Storch, G. A. Enhanced virome sequencing using targeted sequence capture. Genome Res. 25, 1910–1920 (2015).
Bonsall, D. et al. ve-SEQ: robust, unbiased enrichment for streamlined detection and whole-genome sequencing of HCV and other highly diverse pathogens. F1000Res 4, 1062 (2015).
Buddle, S. et al. Evaluating metagenomics and targeted approaches for diagnosis and surveillance of viruses. Genome Med. 16, 111 (2024). This study provides a detailed comparison of different sequencing approaches and bioinformatics tools for the detection of viral pathogens.
Briese, T. et al. Virome capture sequencing enables sensitive viral diagnosis and comprehensive virome analysis. mBio 6, e01491-15 (2015).
Pyöriä, L. et al. Unmasking the tissue-resident eukaryotic DNA virome in humans. Nucleic Acids Res. 51, 3223–3239 (2023).
Kapoor, V. et al. Validation of the VirCapSeq-VERT system for differential diagnosis, detection, and surveillance of viral infections. J. Clin. Microbiol. 62, e0061223 (2024).
Kapel, N. et al. Evaluation of sequence hybridization for respiratory viruses using the twist bioscience respiratory virus research panel and the OneCodex respiratory virus sequence analysis workflow. Microb. Genom. 9, 001103 (2023).
Lin, G.-L. et al. Targeted metagenomics reveals association between severity and pathogen co-detection in infants with respiratory syncytial virus. Nat. Commun. 15, 2379 (2024).
Bermudez, T., Schmitz, J. E., Boswell, M. & Humphries, R. Novel technologies for the diagnosis of urinary tract infections. J. Clin. Microbiol. 63, e0030624 (2025).
Deng, X. et al. Metagenomic sequencing with spiked primer enrichment for viral diagnostics and genomic surveillance. Nat. Microbiol. 5, 443–454 (2020).
Lopez-Labrador, F. X. et al. Multicenter benchmarking of short and long read wet lab protocols for clinical viral metagenomics. J. Clin. Virol. 173, 105695 (2024).
Wilson, M. R. et al. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N. Engl. J. Med. 380, 2327–2340 (2019).
Kantor, R. S. & Jiang, M. Considerations and opportunities for probe capture enrichment sequencing of emerging viruses from wastewater. Environ. Sci. Technol. 58, 8161–8168 (2024).
Cook, R. et al. The long and short of it: benchmarking viromics using Illumina, Nanopore and PacBio sequencing technologies. Microb. Genom. 10, 001198 (2024).
Zaragoza-Solas, A., Haro-Moreno, J. M., Rodriguez-Valera, F. & López-Pérez, M. Long-read metagenomics improves the recovery of viral diversity from complex natural marine samples. mSystems 7, e00192-22 (2022).
Mishra, D., Satpathy, G., Chawla, R., Paliwal, D. & Panda, S. K. Targeted metagenomics using next generation sequencing in laboratory diagnosis of culture negative endophthalmitis. Heliyon 7, e06780 (2021).
Meslier, V. et al. Benchmarking second and third-generation sequencing platforms for microbial metagenomics. Sci. Data 9, 694 (2022).
de Vries, J. J. C. et al. Recommendations for the introduction of metagenomic next-generation sequencing in clinical virology, part II: bioinformatic analysis and reporting. J. Clin. Virol. 138, 104812 (2021).
Sczyrba, A. et al. Critical assessment of metagenome interpretation — a benchmark of metagenomics software. Nat. Methods 14, 1063–1071 (2017).
Meyer, F. et al. Critical assessment of metagenome interpretation: the second round of challenges. Nat. Methods 19, 429–440 (2022).
Morfopoulou, S. & Plagnol, V. Bayesian mixture analysis for metagenomic community profiling. Bioinformatics 31, 2930–2938 (2015).
de Vries, J. J. C. et al. Benchmark of thirteen bioinformatic pipelines for metagenomic virus diagnostics using datasets from clinical samples. J. Clin. Virol. 141, 104908 (2021). Together with Lopez-Labrador et al. (2024), this benchmarking study compares laboratory and data analysis methods used by clinical virology laboratories across Europe.
Rajeev, S. et al. Investigation of acute encephalitis syndrome with implementation of metagenomic next generation sequencing in Nepal. BMC Infect. Dis. 24, 734 (2024).
O’Leary, N. A. et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 44, D733–D745 (2016).
Huson, D. H., Auch, A. F., Qi, J. & Schuster, S. C. MEGAN analysis of metagenomic data. Genome Res. 17, 377–386 (2007).
Wood, D. E. & Salzberg, S. L. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 15, R46 (2014).
Bharucha, T. et al. STROBE-metagenomics: a STROBE extension statement to guide the reporting of metagenomics studies. Lancet Infect. Dis. 20, e251–e260 (2020).
López-Labrador, F. X. et al. Recommendations for the introduction of metagenomic high-throughput sequencing in clinical virology, part I: wet lab procedure. J. Clin. Virol. 134, 104691 (2021).
Salter, S. J. et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 12, 87 (2014).
Chrisman, B. et al. The human “contaminome”: bacterial, viral, and computational contamination in whole genome sequences from 1000 families. Sci. Rep. 12, 9863 (2022).
Eisenhofer, R. et al. Contamination in low microbial biomass microbiome studies: issues and recommendations. Trends Microbiol. 27, 105–117 (2019).
Tan, J. K. et al. Laboratory validation of a clinical metagenomic next-generation sequencing assay for respiratory virus detection and discovery. Nat. Commun. 15, 9016 (2024).
Karius Test® receives FDA breakthrough device designation to aid in the diagnosis of infectious disease. Karius https://kariusdx.com/resources/press-releases/karius-test-r-receives-fda-breakthrough-device-designation-to-aid-in-the-diagnosis-of-infectious-disease (2024).
Pathogenomix granted FDA breakthrough device designation. BioSpace https://www.biospace.com/pathogenomix-granted-fda-breakthrough-device-designation (2022).
Klaften, M. et al. P20 Clinical metagenomics from cell-free DNA: overview and lessons learned from 4 years as a clinical metagenomics provider. JAC Antimicrob. Resist. 6, dlad143.024 (2024).
First CE-IVD marked NGS-based metagenomics test for blood pathogens. Rapid Microbiology https://www.rapidmicrobiology.com/news/first-ce-ivd-marked-ngs-based-metagenomics-test-for-blood-pathogens (2016).
Chen, X. et al. Comparison of traditional methods and high-throughput genetic sequencing in the detection of pathogens in pulmonary infectious diseases. Ann. Transl. Med. 9, 702 (2021).
Vijayvargiya, P. et al. Metagenomic shotgun sequencing of blood to identify bacteria and viruses in leukemic febrile neutropenia. PLoS ONE 17, e0269405 (2022).
Laboratory developed tests. FDA https://www.fda.gov/medical-devices/in-vitro-diagnostics/laboratory-developed-tests (2025).
Vogeser, M., Brüggemann, M., Lennerz, J., Stenzinger, A. & Gassner, U. M. Laboratory-developed tests in the new European Union 2017/746 Regulation: opportunities and risks. Clin. Chem. 68, 40–42 (2022).
Gihawi, A. et al. Major data analysis errors invalidate cancer microbiome findings. mBio 14, e0160723 (2023).
Johnson, T., Jamrozik, E., Ramachandran, P. & Johnson, S. Clinical metagenomics: ethical issues. J. Med. Microbiol. 74, 001967 (2025).
Charalampous, T. et al. Routine metagenomics service for ICU patients with respiratory infection. Am. J. Respir. Crit. Care Med. 209, 164–174 (2024).
Zhu, Y. et al. Diagnostic performance and clinical impact of metagenomic next-generation sequencing for pediatric infectious diseases. J. Clin. Microbiol. 61, e0011523 (2023).
Lin, K. et al. Clinical application and drug-use-guidance value of metagenomic next-generation sequencing in central nervous system infection. Am. J. Transl. Res. 15, 47–62 (2023).
Piantadosi, A. et al. Enhanced virus detection and metagenomic sequencing in patients with meningitis and encephalitis. mBio 12, e0114321 (2021).
Junier, T. et al. Viral metagenomics in the clinical realm: lessons learned from a Swiss-wide ring trial. Genes 10, 655 (2019).
Morfopoulou, S. et al. Human coronavirus OC43 associated with fatal encephalitis. N. Engl. J. Med. 375, 497–498 (2016).
Winter, S. et al. Fatal encephalitis caused by Newcastle disease virus in a child. Acta Neuropathol. 142, 605–608 (2021).
Maimaris, J. et al. Safety and diagnostic utility of brain biopsy and metagenomics in decision-making for patients with inborn errors of immunity (IEI) and unexplained neurological manifestations. J. Clin. Immunol. 45, 86 (2025).
Erickson, T. A. et al. Infectious and autoimmune causes of encephalitis in children. Pediatrics 145, e20192543 (2020).
Coletti Moja, M., Riva, G. & Catalfamo, E. Dual drug-induced aseptic meningoencephalitis: more than a suggestion. SAGE Open Med. Case Rep. 9, 2050313X211021179 (2021).
Pallerla, S. R. et al. Diagnosis of pathogens causing bacterial meningitis using Nanopore sequencing in a resource-limited setting. Ann. Clin. Microbiol. Antimicrob. 21, 39 (2022).
Jain, S. et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N. Engl. J. Med. 373, 415–427 (2015).
Onwuchekwa, C. et al. Underascertainment of respiratory syncytial virus infection in adults due to diagnostic testing limitations: a systematic literature review and meta-analysis. J. Infect. Dis. 228, 173–184 (2023).
Gaston, D. C. et al. Evaluation of metagenomic and targeted next-generation sequencing workflows for detection of respiratory pathogens from bronchoalveolar lavage fluid specimens. J. Clin. Microbiol. 60, e0052622 (2022).
Zhou, H. et al. Clinical impact of metagenomic next-generation sequencing of bronchoalveolar lavage in the diagnosis and management of pneumonia: a multicenter prospective observational study. J. Mol. Diagn. 23, 1259–1268 (2021).
Mao, Y. et al. Detection of Coccidioides posadasii in a patient with meningitis using metagenomic next-generation sequencing: a case report. BMC Infect. Dis. 21, 968 (2021).
Osterman, A. et al. Travel-associated neurological disease terminated in a postmortem diagnosed atypical HSV-1 encephalitis after high-dose steroid therapy — a case report. BMC Infect. Dis. 20, 150 (2020).
Williams, E. et al. Case report: confirmation by metagenomic sequencing of visceral leishmaniasis in an immunosuppressed returned traveler. Am. J. Trop. Med. Hyg. 103, 1930–1933 (2020).
Woodworth, M. H. et al. Sentinel case of Candida auris in the Western United States following prolonged occult colonization in a returned traveler from India. Microb. Drug Resist. 25, 677–680 (2019).
Gao, Y., Qu, M., Song, C., Yin, L. & Zhang, M. Cerebral vasculitis caused by Talaromyces marneffei and Aspergillus niger in a HIV-positive patient: a case report and literature review. J. Neurovirol. 28, 274–280 (2022).
Camprubí-Ferrer, D. et al. Assessing viral metagenomics for the diagnosis of acute undifferentiated fever in returned travellers: a multicenter cohort study. J. Travel Med. 31, taae029 (2024).
Jerome, H. et al. Metagenomic next-generation sequencing aids the diagnosis of viral infections in febrile returning travellers. J. Infect. 79, 383–388 (2019).
Reyes, A. et al. Viral metagenomic sequencing in a cohort of international travellers returning with febrile illness. J. Clin. Virol. 143, 104940 (2021).
Van Poelvoorde, L. A. E. et al. Whole-genome-based phylogenomic analysis of the Belgian 2016–2017 influenza A(H3N2) outbreak season allows improved surveillance. Microb. Genom. 7, 000643 (2021).
Amman, F. et al. Viral variant-resolved wastewater surveillance of SARS-CoV-2 at national scale. Nat. Biotechnol. 40, 1814–1822 (2022).
Singh, P., Sharma, K., Bhargava, A. & Negi, S. S. Genomic characterization of influenza A (H1N1)pdm09 and SARS-CoV-2 from influenza like illness (ILI) and severe acute respiratory illness (SARI) cases reported between July–December, 2022. Sci. Rep. 14, 10660 (2024).
Zhang, S. et al. Evolutionary trajectory and characteristics of Mpox virus in 2023 based on a large-scale genomic surveillance in Shenzhen, China. Nat. Commun. 15, 7452 (2024).
Fyles, F. et al. Surveillance towards preventing paediatric incidence of respiratory syncytial virus attributable respiratory tract infection in primary and secondary/tertiary healthcare settings in Merseyside, Cheshire and Bristol, UK. BMJ Open Respir. Res. 10, e001457 (2023).
Mahdi, H. A. et al. Syndromic surveillance of respiratory-tract infections and hand hygiene practice among pilgrims attended Hajj in 2021: a cohort study. BMC Infect. Dis. 22, 578 (2022).
Kazazian, L. et al. A toolkit for planning and implementing acute febrile illness (AFI) surveillance. PLoS Glob. Public Health 4, e0003115 (2024).
Tomashek, K. M. et al. Clinical and epidemiologic characteristics of dengue and other etiologic agents among patients with acute febrile illness, Puerto Rico, 2012–2015. PLoS Negl. Trop. Dis. 11, e0005859 (2017).
Kayiwa, J. T. et al. Confirmation of Zika virus infection through hospital-based sentinel surveillance of acute febrile illness in Uganda, 2014–2017. J. Gen. Virol. 99, 1248–1252 (2018).
Shih, D. C. et al. Incorporating COVID-19 into acute febrile illness surveillance systems, Belize, Kenya, Ethiopia, Peru, and Liberia, 2020–2021. Emerg. Infect. Dis. 28, S34–S41 (2022).
Liu, J. et al. Development of a TaqMan array card for acute-febrile-illness outbreak investigation and surveillance of emerging pathogens, including Ebola virus. J. Clin. Microbiol. 54, 49–58 (2020).
Rhee, C. et al. Global knowledge gaps in acute febrile illness etiologic investigations: a scoping review. PLoS Negl. Trop. Dis. 13, e0007792 (2019).
Bohl, J. A. et al. Discovering disease-causing pathogens in resource-scarce Southeast Asia using a global metagenomic pathogen monitoring system. Proc. Natl Acad. Sci. USA 119, e2115285119 (2022).
Oguzie, J. U. et al. Metagenomic surveillance uncovers diverse and novel viral taxa in febrile patients from Nigeria. Nat. Commun. 14, 4693 (2023).
Levine, Z. C. et al. Investigating the etiologies of non-malarial febrile illness in Senegal using metagenomic sequencing. Nat. Commun. 15, 747 (2024).
Guidelines for environmental surveillance of poliovirus circulation. World Health Organization IRIS https://iris.who.int/handle/10665/67854 (2003).
Mercier, E. et al. Municipal and neighbourhood level wastewater surveillance and subtyping of an influenza virus outbreak. Sci. Rep. 12, 15777 (2022).
Wolfe, M. K. et al. Wastewater-based detection of two influenza outbreaks. Environ. Sci. Technol. Lett. 9, 687–692 (2022).
Boehm, A. B. et al. Wastewater concentrations of human influenza, metapneumovirus, parainfluenza, respiratory syncytial virus, rhinovirus, and seasonal coronavirus nucleic-acids during the COVID-19 pandemic: a surveillance study. Lancet Microbe 4, e340–e348 (2023).
Oghuan, J. et al. Wastewater analysis of Mpox virus in a city with low prevalence of Mpox disease: an environmental surveillance study. Lancet Reg. Health Am. 28, 100639 (2023).
Brunner, F. S. et al. Utility of wastewater genomic surveillance compared to clinical surveillance to track the spread of the SARS-CoV-2 Omicron variant across England. Water Res. 247, 120804 (2023).
Martínez-Puchol, S. et al. Characterisation of the sewage virome: comparison of NGS tools and occurrence of significant pathogens. Sci. Total Environ. 713, 136604 (2020).
Child, H. T. et al. Comparison of metagenomic and targeted methods for sequencing human pathogenic viruses from wastewater. mBio 14, e01468-23 (2023). This study provides a detailed comparison of different untargeted and targeted approaches used for wastewater surveillance.
Tisza, M. et al. Wastewater sequencing reveals community and variant dynamics of the collective human virome. Nat. Commun. 14, 6878 (2023).
Sirleaf, E. J. & Clark, H. Report of the independent panel for pandemic preparedness and response: making COVID-19 the last pandemic. Lancet 398, 101–103 (2021).
Ko, K. K. K., Chng, K. R. & Nagarajan, N. Metagenomics-enabled microbial surveillance. Nat. Microbiol. 7, 486–496 (2022). This review presents a comprehensive overview of the use of metagenomics for pathogen surveillance.
Cherry, J. D. & Krogstad, P. SARS: the first pandemic of the 21st century. Pediatr. Res. 56, 1–5 (2004).
Saxena, S. K., Mishra, N., Saxena, R. & Saxena, S. Swine flu: influenza A/H1N1 2009: the unseen and unsaid. Future Microbiol. 4, 945–947 (2009).
Zaki, A. M., Boheemen, S., van Bestebroer, T. M., Osterhaus, A. D. M. E. & Fouchier, R. A. M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 367, 1814–1820 (2012).
Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020).
Grange, Z. L. et al. Ranking the risk of animal-to-human spillover for newly discovered viruses. Proc. Natl Acad. Sci. USA 118, e2002324118 (2021).
Gebreyes, W. A. et al. The global one health paradigm: challenges and opportunities for tackling infectious diseases at the human, animal, and environment interface in low-resource settings. PLoS Negl. Trop. Dis. 8, e3257 (2014).
Taylor, L. H., Latham, S. M. & Woolhouse, M. E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356, 983–989 (2001).
Van Brussel, K. & Holmes, E. C. Zoonotic disease and virome diversity in bats. Curr. Opin. Virol. 52, 192–202 (2022).
Liu, W. J. et al. Surveillance of SARS-CoV-2 at the Huanan seafood market. Nature 631, 402–408 (2024).
Vibin, J., Chamings, A., Klaassen, M., Bhatta, T. R. & Alexandersen, S. Metagenomic characterisation of avian parvoviruses and picornaviruses from Australian wild ducks. Sci. Rep. 10, 12800 (2020).
Delaune, D. et al. A novel SARS-CoV-2 related coronavirus in bats from Cambodia. Nat. Commun. 12, 6563 (2021).
Cibulski, S. P. et al. Detection of multiple viruses in oropharyngeal samples from Brazilian free-tailed bats (Tadarida brasiliensis) using viral metagenomics. Arch. Virol. 166, 207–212 (2021).
Liao, F. et al. Metagenomics of gut microbiome for migratory seagulls in Kunming City revealed the potential public risk to human health. BMC Genom. 24, 269 (2023).
Grard, G. et al. A novel rhabdovirus associated with acute hemorrhagic fever in Central Africa. PLoS Pathog. 8, e1002924 (2012).
Hou, X. et al. Using artificial intelligence to document the hidden RNA virosphere. Cell 187, 6929–6942.e16 (2024).
Sweeney, T. E., Shidham, A., Wong, H. R. & Khatri, P. A comprehensive time-course-based multicohort analysis of sepsis and sterile inflammation reveals a robust diagnostic gene set. Sci. Transl. Med. 7, 287ra71 (2015).
Woods, C. W. et al. A host transcriptional signature for presymptomatic detection of infection in humans exposed to influenza H1N1 or H3N2. PLoS ONE 8, e52198 (2013).
Tsalik, E. L. et al. Host gene expression classifiers diagnose acute respiratory illness etiology. Sci. Transl. Med. 8, 322ra11 (2016).
Habgood-Coote, D. et al. Diagnosis of childhood febrile illness using a multi-class blood RNA molecular signature. Med 4, 635–654.e5 (2023).
Ramilo, O. et al. Gene expression patterns in blood leukocytes discriminate patients with acute infections. Blood 109, 2066–2077 (2006).
Mejias, A. et al. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med. 10, e1001549 (2013).
Gu, W. et al. Rapid pathogen detection by metagenomic next-generation sequencing of infected body fluids. Nat. Med. 27, 115–124 (2021).
Ramachandran, P. S. et al. Integrating central nervous system metagenomics and host response for diagnosis of tuberculosis meningitis and its mimics. Nat. Commun. 13, 1675 (2022).
Loy, C. J. et al. Plasma cell-free RNA signatures of inflammatory syndromes in children. Proc. Natl Acad. Sci. USA 121, e2403897121 (2024).
Wang, Y. et al. Plasma cell-free RNA characteristics in COVID-19 patients. Genome Res. 32, 228–241 (2022).
Chang, A. et al. Circulating cell-free RNA in blood as a host response biomarker for detection of tuberculosis. Nat. Commun. 15, 4949 (2024).
Tzani-Tzanopoulou, P. et al. Interactions of bacteriophages and bacteria at the airway mucosa: new insights into the pathophysiology of asthma. Front. Allergy 1, 617240 (2021).
Rolain, J.-M. et al. Genomic analysis of an emerging multiresistant Staphylococcus aureus strain rapidly spreading in cystic fibrosis patients revealed the presence of an antibiotic inducible bacteriophage. Biol. Direct 4, 1 (2009).
Pride, D. T. et al. Evidence of a robust resident bacteriophage population revealed through analysis of the human salivary virome. ISME J. 6, 915–926 (2012).
Li, R., Li, J. & Zhou, X. Lung microbiome: new insights into the pathogenesis of respiratory diseases. Signal Transduct. Target. Ther. 9, 19 (2024).
Willner, D. et al. Metagenomic analysis of respiratory tract DNA viral communities in cystic fibrosis and non-cystic fibrosis individuals. PLoS ONE 4, e7370 (2009).
Lakbar, I., Singer, M. & Leone, M. 2030: will we still need our microbiologist? Intensiv. Care Med. 49, 1232–1234 (2023).
Gauthier, N. P. G. et al. Validation of an automated, end-to-end metagenomic sequencing assay for agnostic detection of respiratory viruses. J. Infect. Dis. 230, e1245–e1253 (2024).
Hoffmann, A., Timm, A., Johnson, C., Rupp, S. & Grumaz, C. Automation of customizable library preparation for next-generation sequencing into an open microfluidic platform. Sci. Rep. 14, 17150 (2024).
World Health Organization. The World Health Report 2007: A Safer Future: Global Public Health Security in the 21st Century (WHO, 2007).
Charon, J., Buchmann, J. P., Sadiq, S. & Holmes, E. C. RdRp-scan: a bioinformatic resource to identify and annotate divergent RNA viruses in metagenomic sequence data. Virus Evol. 8, veac082 (2022).
Olendraite, I., Brown, K. & Firth, A. E. Identification of RNA virus-derived RdRp sequences in publicly available transcriptomic data sets. Mol. Biol. Evol. 40, msad060 (2023).
Zhao, J. et al. Farmed fur animals harbour viruses with zoonotic spillover potential. Nature 634, 228–233 (2024).
Kawasaki, J., Suzuki, T. & Hamada, M. Hidden challenges in evaluating spillover risk of zoonotic viruses using machine learning models. Commun. Med. 5, 187 (2025).
Mollentze, N., Babayan, S. A. & Streicker, D. G. Identifying and prioritizing potential human-infecting viruses from their genome sequences. PLoS Biol. 19, e3001390 (2021).
Panca, M. et al. Evaluating the cost implications of integrating SARS-CoV-2 genome sequencing for infection prevention and control investigation of nosocomial transmission within hospitals. J. Hosp. Infect. 139, 23–32 (2023).
Stirrup, O. et al. Rapid feedback on hospital onset SARS-CoV-2 infections combining epidemiological and sequencing data. eLife 10, e65828 (2021).
Fu, Z. et al. Pathogen quantitative efficacy of different spike-in internal controls and clinical application in central nervous system infection with metagenomic sequencing. Microbiol. Spectr. 11, e01139-23 (2023).
Camacho, C. et al. BLAST+: architecture and applications. BMC Bioinform. 10, 421 (2009).
Cadenas-Castrejón, E., Verleyen, J., Boukadida, C., Díaz-González, L. & Taboada, B. Evaluation of tools for taxonomic classification of viruses. Brief. Funct. Genom. 22, 31–41 (2023).
Fan, J., Huang, S. & Chorlton, S. D. BugSeq: a highly accurate cloud platform for long-read metagenomic analyses. BMC Bioinform. 22, 160 (2021).
Portik, D. M., Brown, C. T. & Pierce-Ward, N. T. Evaluation of taxonomic classification and profiling methods for long-read shotgun metagenomic sequencing datasets. BMC Bioinform. 23, 541 (2022).
Kim, D., Song, L., Breitwieser, F. P. & Salzberg, S. L. Centrifuge: rapid and sensitive classification of metagenomic sequences. Genome Res. 26, 1721–1729 (2016).
Carbo, E. C. et al. Performance of five metagenomic classifiers for virus pathogen detection using respiratory samples from a clinical cohort. Pathogens 11, 340 (2022).
Ounit, R., Wanamaker, S., Close, T. J. & Lonardi, S. CLARK: fast and accurate classification of metagenomic and genomic sequences using discriminative k-mers. BMC Genom. 16, 236 (2015).
Kalantar, K. L. et al. IDseq — an open source cloud-based pipeline and analysis service for metagenomic pathogen detection and monitoring. GigaScience 9, giaa111 (2020).
Alawi, M. et al. DAMIAN: an open source bioinformatics tool for fast, systematic and cohort based analysis of microorganisms in diagnostic samples. Sci. Rep. 9, 16841 (2019).
Buchfink, B., Xie, C. & Huson, D. H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12, 59–60 (2015).
Lin, H.-H. & Liao, Y.-C. drVM: a new tool for efficient genome assembly of known eukaryotic viruses from metagenomes. GigaScience 6, gix003 (2017).
Tithi, S. S., Aylward, F. O., Jensen, R. V. & Zhang, L. FastViromeExplorer: a pipeline for virus and phage identification and abundance profiling in metagenomics data. PeerJ 6, e4227 (2018).
Fernandes, J. F. et al. Unbiased metagenomic next-generation sequencing of blood from hospitalized febrile children in Gabon. Emerg. Microbes Infect. 9, 1242–1244 (2020).
Vilsker, M. et al. Genome Detective: an automated system for virus identification from high-throughput sequencing data. Bioinformatics 35, 871–873 (2019).
Schmitz, D. et al. Accessible viral metagenomics for public health and clinical domains with Jovian. Sci. Rep. 14, 26018 (2024).
Menzel, P., Ng, K. L. & Krogh, A. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat. Commun. 7, 11257 (2016).
Wood, D. E., Lu, J. & Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 20, 257 (2019).
Lu, J., Breitwieser, F. P., Thielen, P. & Salzberg, S. L. Bracken: estimating species abundance in metagenomics data. PeerJ Comput. Sci. 3, e104 (2017).
Breitwieser, F. P., Baker, D. N. & Salzberg, S. L. KrakenUniq: confident and fast metagenomics classification using unique k-mer counts. Genome Biol. 19, 198 (2018).
Minot, S. S., Krumm, N. & Greenfield, N. B. One Codex: a sensitive and accurate data platform for genomic microbial identification. Preprint at bioRxiv https://doi.org/10.1101/027607 (2015).
Scheuch, M., Höper, D. & Beer, M. RIEMS: a software pipeline for sensitive and comprehensive taxonomic classification of reads from metagenomics datasets. BMC Bioinform. 16, 69 (2015).
Dadi, T. H., Renard, B. Y., Wieler, L. H., Semmler, T. & Reinert, K. SLIMM: species level identification of microorganisms from metagenomes. PeerJ 5, e3138 (2017).
Benoit, P. et al. Seven-year performance of a clinical metagenomic next-generation sequencing test for diagnosis of central nervous system infections. Nat. Med. 30, 3522–3533 (2024).
Lin, J. et al. Vipie: web pipeline for parallel characterization of viral populations from multiple NGS samples. BMC Genom. 18, 378 (2017).
Claro, I. M. et al. Rapid viral metagenomics using SMART-9N amplification and nanopore sequencing. Wellcome Open Res. 6, 241 (2021).
Tsitsiklis, A. et al. Lower respiratory tract infections in children requiring mechanical ventilation: a multicentre prospective surveillance study incorporating airway metagenomics. Lancet Microbe 3, e284–e293 (2022).
Minor, N. R. et al. Metagenomic sequencing detects human respiratory and enteric viruses in air samples collected from congregate settings. Sci. Rep. 13, 21398 (2023).
Severe acute hepatitis of unknown aetiology in children — multi-country. World Health Organization https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON400 (2022).
Gutierrez Sanchez, L. H. et al. A case series of children with acute hepatitis and human adenovirus infection. N. Engl. J. Med. 387, 620–630 (2022).
Kelgeri, C. et al. Clinical spectrum of children with acute hepatitis of unknown cause. N. Engl. J. Med. 387, 611–619 (2022).
Hodcroft, E. B. et al. Spread of a SARS-CoV-2 variant through Europe in the summer of 2020. Nature 595, 707–712 (2021).
Caserta, L. C. et al. Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle. Nature 634, 669–676 (2024).
Uhart, M. M. et al. Epidemiological data of an influenza A/H5N1 outbreak in elephant seals in Argentina indicates mammal-to-mammal transmission. Nat. Commun. 15, 9516 (2024).
Banyard, A. C. et al. Detection and spread of high pathogenicity avian influenza virus H5N1 in the Antarctic region. Nat. Commun. 15, 7433 (2024).
Wu, F. et al. A new coronavirus associated with human respiratory disease in China. Nature 579, 265–269 (2020).
Brito, A. F. et al. Global disparities in SARS-CoV-2 genomic surveillance. Nat. Commun. 13, 7003 (2022).
Tegally, H. et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 592, 438–443 (2021).
Faria, N. R. et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science 372, 815–821 (2021).
Smyth, D. S. et al. Tracking cryptic SARS-CoV-2 lineages detected in NYC wastewater. Nat. Commun. 13, 635 (2022).
Karthikeyan, S. et al. Wastewater sequencing reveals early cryptic SARS-CoV-2 variant transmission. Nature 609, 101–108 (2022).
Morvan, M. et al. An analysis of 45 large-scale wastewater sites in England to estimate SARS-CoV-2 community prevalence. Nat. Commun. 13, 4313 (2022).
Otieno, J. R. et al. Global genomic surveillance of monkeypox virus. Nat. Med. 31, 342–350 (2025).
Nicholls, S. M. et al. CLIMB-COVID: continuous integration supporting decentralised sequencing for SARS-CoV-2 genomic surveillance. Genome Biol. 22, 196 (2021).
The COVID-19 Genomics UK (COG-UK) Consortium. An integrated national scale SARS-CoV-2 genomic surveillance network. Lancet Microbe 1, e99–e100 (2020).
Tegally, H. et al. The evolving SARS-CoV-2 epidemic in Africa: insights from rapidly expanding genomic surveillance. Science 378, eabq5358 (2022).
Sahadeo, N. S. D. et al. Implementation of genomic surveillance of SARS-CoV-2 in the Caribbean: lessons learned for sustainability in resource-limited settings. PLoS Glob. Public Health 3, e0001455 (2023).
UK to create world-first ‘early warning system’ for pandemics. GOV.UK https://www.gov.uk/government/news/uk-to-create-world-first-early-warning-system-for-pandemics (2024).
Acknowledgements
The authors acknowledge the clinical metagenomics team at Great Ormond Street Hospital and colleagues at University College London Genomics. O.E.T.M., S.B. and S.M. acknowledge the support of the National Institute for Health Research Blood and Transplant Research Unit in Genomics to Enhance Microbiology Screening (NIHR BTRU-GEMS). All research at Great Ormond Street Hospital NHS Foundation Trust and UCL Great Ormond Street Institute of Child Health is made possible by the NIHR Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
J.B. receives research support from Illumina PLC, Oxford Nanopore Technologies, and Twist Bioscience. O.E.T.M., S.B. and J.B. are authors on a patent application concerning the use of target capture sequencing with ONT. S.M. declares no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Charles Chiu; Jelle Matthijnssens, who submitted a co-report with Mustafa Karatas; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ISO/IEC 17025:2017: https://www.virology.uzh.ch/de/services.html
NCBI RefSeq: https://www.ncbi.nlm.nih.gov/refseq/
Six sequences of the novel coronavirus: https://virological.org/t/novel-2019-coronavirus-genome/319
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Torres Montaguth, O.E., Buddle, S., Morfopoulou, S. et al. Clinical metagenomics for diagnosis and surveillance of viral pathogens. Nat Rev Microbiol 24, 61–75 (2026). https://doi.org/10.1038/s41579-025-01223-5
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41579-025-01223-5
This article is cited by
-
Tick-borne viruses: discovery, clinical aspects, influencing factors and control
Nature Reviews Microbiology (2026)
-
Metagenomics and its impact on environmental and therapeutic microbiology
Archives of Microbiology (2026)
-
Maternal health status is associated with paired maternal and cord blood virome and mother-to-infant transmission
npj Biofilms and Microbiomes (2025)