Abstract
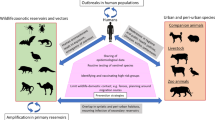
The risk of viral pathogen transmission between humans and animals (spillover events) and subsequent spread has been increasing due to human impacts on the planet, which lead to changes in the interactions between humans, animals, ecosystems and their pathogens. Key factors (drivers) that increase the risk of disease emergence include climate change, urbanization, land-use changes and global travel, all of which can alter human–animal–environment interactions and increase the likelihood of zoonotic spillovers and vector-borne diseases. Incorporating data on these drivers (such as ecological shifts and patterns of animal movement) into disease surveillance systems can help identify hot spots for disease emergence, which could in theory enable earlier detection of outbreaks and, in turn, increase the effectiveness of intervention strategies. A One Health approach, emphasizing the interconnectedness of human, animal and environmental health, is advocated for addressing these complex challenges. Although conceptually clear and widely endorsed, implementation of One Health approaches towards primary prevention of spillovers is extremely challenging. Here, we summarize current knowledge on disease emergence and its drivers, and discuss how this knowledge could be used towards primary prevention and for the development of risk-targeted One Health early warning surveillance. We consider integrating innovative tools for diagnostics, surveillance and virus characterization, and propose an outlook towards more integrated prevention, early warning and control of emerging infections at the human–animal interface.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
14 October 2025
In the version of the article initially published, the Acknowledgements section was inadvertently omitted and has now been added to the HTML and PDF versions of the article.
References
Adepoju, P. Mpox declared a public health emergency. Lancet 404, e1–e2 (2024).
Lawrence, O. G., Ashish, K. J. & Alexandra, F. The Mpox global health emergency — a time for solidarity and equity. N. Engl. J. Med. 391, 1265–1267 (2024).
Taylor, L. PAHO: Americas report record dengue and Oropouche cases. BMJ 387, q2808 (2024).
Caserta, L. C. et al. Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle. Nature 634, 669–676 (2024). This study reports the unprecedented spillover and cow-to-cow transmission of HPAI H5N1 in US dairy cattle, highlighting a critical shift in the host range of the virus.
Banyard, A. C. et al. Detection and spread of high pathogenicity avian influenza virus H5N1 in the Antarctic region. Nat. Commun. 15, 7433 (2024).
Authority, E. F. S. et al. Avian influenza overview December 2024–March 2025. EFSA J. 23, e9352 (2025).
Krammer, F. & Schultz-Cherry, S. We need to keep an eye on avian influenza. Nat. Rev. Immunol. 23, 267–268 (2023).
Sah, R. et al. Concerns on H5N1 avian influenza given the outbreak in U.S. dairy cattle. Lancet Reg. Health Am. 35, 100785 (2024).
Kuiken, T., Fouchier, R. A. M. & Koopmans, M. P. G. Being ready for the next influenza pandemic? Lancet Infect. Dis. 23, 398–399 (2023).
Adisasmito, W. B. et al. One Health: a new definition for a sustainable and healthy future. PLoS Pathog. 18, e1010537 (2022). In this paper the OHHLEP presents an updated definition of One Health, which is now widely adopted.
Machalaba, C. M. & Karesh, W. B. Emerging infectious disease risk: shared drivers with environmental change. Rev. Sci. Tech. 36, 435–444 (2017).
Engering, A., Hogerwerf, L. & Slingenbergh, J. Pathogen–host–environment interplay and disease emergence. Emerg. Microbes Infect. 2, 1–7 (2013).
Jones, K. E. et al. Global trends in emerging infectious diseases. Nature 451, 990–993 (2008).
European Commission: Group of Chief Scientific Advisors and Directorate-General for Research and Innovation. One Health Governance in the European Union (Publications Office of the European Union, 2024).
Mora, C. et al. Over half of known human pathogenic diseases can be aggravated by climate change. Nat. Clim. Change 12, 869–875 (2022). This study presents a systematic review of peer reviewed publications that study the effects of climate change on infectious diseases that impact humans.
IPCC Climate Change 2022: Impacts, Adaptation, and Vulnerability (eds Pörtner, H.-O. et al.) (Cambridge Univ. Press, 2022).
Carlson, C. J. et al. Climate change increases cross-species viral transmission risk. Nature 607, 555–562 (2022). This review highlights that climate change impacts interactions and spillover events not only at the human–animal interface but also between animal species.
Greenville, A. C., Wardle, G. M. & Dickman, C. R. Extreme climatic events drive mammal irruptions: regression analysis of 100-year trends in desert rainfall and temperature. Ecol. Evol. 2, 2645–2658 (2012).
Rushing, C. S., Royle, J. A., Ziolkowski, D. J. & Pardieck, K. L. Migratory behavior and winter geography drive differential range shifts of eastern birds in response to recent climate change. Proc. Natl Acad. Sci. USA 117, 12897–12903 (2020).
Perry, A. L., Low, P. J., Ellis, J. R. & Reynolds, J. D. Climate change and distribution shifts in marine fishes. Science 308, 1912–1915 (2005).
Musmanni, G. D. The delta blues: why climate change adaptation is crucial in the world’s deltas. Global Centre of Adaptation https://gca.org/the-delta-blues-why-climate-change-adaptation-is-crucial-in-the-worlds-deltas/ (2022).
European Environment Agency. Urban Adaptation in Europe: What Works? (EEA, 2024).
Geneletti, D. & Zardo, L. Ecosystem-based adaptation in cities: an analysis of European urban climate adaptation plans. Land Use Policy 50, 38–47 (2016).
Lindsay, S. W., Wilson, A., Golding, N., Scott, T. W. & Takken, W. Improving the built environment in urban areas to control Aedes aegypti-borne diseases. Bull. World Health Organ. 95, 607–608 (2017).
Rocklöv, J. et al. Decision-support tools to build climate resilience against emerging infectious diseases in Europe and beyond. Lancet Reg. Health Eur. 32, 100701 (2023).
de Cock, M. P., Esser, H. J., van der Poel, W. H. M., Sprong, H. & Maas, M. Higher rat abundance in greener urban areas. Urban Ecosystems 27, 1389–1401 (2024).
Traweger, D., Travnitzky, R., Moser, C., Walzer, C. & Bernatzky, G. Habitat preferences and distribution of the brown rat (Rattus norvegicus Berk.) in the city of Salzburg (Austria): implications for an urban rat management. J. Pest. Sci. 79, 113–125 (2006).
de Cock, M. P. et al. Increased rat-borne zoonotic disease hazard in greener urban areas. Sci. Total. Environ. 896, 165069 (2023).
Kibret, S., McCartney, M., Lautze, J., Nhamo, L. & Yan, G. The impact of large and small dams on malaria transmission in four basins in Africa. Sci. Rep. 11, 13355 (2021).
de Souza, W. M. & Weaver, S. C. Effects of climate change and human activities on vector-borne diseases. Nat. Rev. Microbiol. 22, 476–491 (2024).
Mordecai, E. A., Ryan, S. J., Caldwell, J. M., Shah, M. M. & LaBeaud, A. D. Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planet. Health 4, e416–e423 (2020). This study shows how climate change may have different effects across regions, vectors and pathogens.
FAOSTAT. Land Use Statistics and Indicators 2000–2021. Global, Regional and Country Trends Analytical Brief 71 (FAO, 2023).
Ellis, E. C., Klein Goldewijk, K., Siebert, S., Lightman, D. & Ramankutty, N. Anthropogenic transformation of the biomes, 1700 to 2000. Glob. Ecol. Biogeogr. 19, 589–606 (2010).
Livestock, Environment and Development Initiative. Livestock’s Long Shadow: Environmental Issues and Options (LEAD & FAO, 2006).
Greenspoon, L. et al. The global biomass of wild mammals. Proc. Natl Acad. Sci. USA 120, e2204892120 (2023).
Wegner, G. I. et al. Averting wildlife-borne infectious disease epidemics requires a focus on socio-ecological drivers and a redesign of the global food system. eClinicalMedicine 47, 101386 (2022).
Sikkema, R. S. et al. Risks of SARS-CoV-2 transmission between free-ranging animals and captive mink in the Netherlands. Transbound. Emerg. Dis. 69, 3339–3349 (2022).
Isabella, M. et al. Emergence of a highly pathogenic avian influenza virus from a low-pathogenic progenitor. J. Virol. 88, 4375–4388 (2014).
Agüero, M. et al. Highly pathogenic avian influenza A (H5N1) virus infection in farmed minks, Spain, October 2022. Eurosurveillance 28, 2300001 (2023).
Domańska-Blicharz, K. et al. Cryptic SARS-CoV-2 lineage identified on two mink farms as a possible result of long-term undetected circulation in an unknown animal reservoir, Poland, November 2022 to January 2023. Eurosurveillance 28, 2300188 (2023).
Pendrill, F. et al. Disentangling the numbers behind agriculture-driven tropical deforestation. Science 377, eabm9267 (2022).
Faust, C. L. et al. Pathogen spillover during land conversion. Ecol. Lett. 21, 471–483 (2018).
Walsh, M. G., Mor, S. M., Maity, H. & Hossain, S. Forest loss shapes the landscape suitability of Kyasanur Forest disease in the biodiversity hotspots of the Western Ghats, India. Int. J. Epidemiol. 48, 1804–1814 (2019).
Green, J., Schmidt-Burbach, J. & Elwin, A. Taking stock of wildlife farming: a global perspective. Glob. Ecol. Conserv. 43, e02452 (2023).
Biao, K. et al. Molecular evolution analysis and geographic investigation of severe acute respiratory syndrome coronavirus-like virus in palm civets at an animal market and on farms. J. Virol. 79, 11892–11900 (2005).
Nelson, M. I. et al. Global migration of influenza A viruses in swine. Nat. Commun. 6, 1–11 (2015).
Brown, V. R. et al. Risks of introduction and economic consequences associated with African swine fever, classical swine fever and foot-and-mouth disease: a review of the literature. Transbound. Emerg. Dis. 68, 1910–1965 (2021).
Moyen, N. et al. Avian influenza transmission risk along live poultry trading networks in Bangladesh. Sci. Rep. 11, 19962 (2021).
Lankau, E. W. et al. Prevention and control of rabies in an age of global travel: a review of travel- and trade-associated rabies events—US, 1986–2012. Zoonoses Public. Health 61, 305–316 (2014).
Worobey, M. et al. The Huanan market was the early epicenter of SARS-CoV-2 emergence. Science 377, 951–959 (2022).
Aguirre, A. A., Catherina, R., Frye, H. & Shelley, L. Illicit wildlife trade, wet markets, and COVID-19: preventing future pandemics. World Med. Health Policy 12, 256–265 (2020).
Zhou, P. et al. Avian influenza A (H7N9) virus and mixed live poultry–animal markets in Guangdong province: a perfect storm in the making? Emerg. Microbes Infect. 4, 1–3 (2015).
Milbank, C. & Vira, B. Wildmeat consumption and zoonotic spillover: contextualising disease emergence and policy responses. Lancet Planet. Health 6, e439–e448 (2022).
Xia, W., Hughes, J., Robertson, D. & Jiang, X. How one pandemic led to another: was African swine fever virus (ASFV) the disruption contributing to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emergence? Preprint at Preprints.org https://doi.org/10.20944/preprints202102.0590.v2 (2022).
Lytras, S., Xia, W., Hughes, J., Jiang, X. & Robertson, D. L. The animal origin of SARS-CoV-2. Science 373, 968–970 (2021).
Kowarik, I. Novel urban ecosystems, biodiversity, and conservation. Environ. Pollut. 159, 1974–1983 (2011).
Johnson, M. T. J. & Munshi-South, J. Evolution of life in urban environments. Science 358, eaam8327 (2017). This review describes and analyses the effects of urbanization on the evolution of microorganisms, plants and animals, and how this affects interactions with humans.
Bradley, C. A. & Altizer, S. Urbanization and the ecology of wildlife diseases. Trends Ecol. Evolution 22, 95–102 (2007).
Egan, S., Barbosa, A. D., Feng, Y., Xiao, L. & Ryan, U. Critters and contamination: zoonotic protozoans in urban rodents and water quality. Water Res. 251, 121165 (2024).
Akhtardanesh, B. et al. Survey of common infectious diseases in urban foxes (Vulpes spp.) in southeastern Iran. J. Wildl. Dis. 60, 77–85 (2024).
Naderi, S. et al. Zooanthroponotic transmission of SARS-CoV-2 and host-specific viral mutations revealed by genome-wide phylogenetic analysis. eLife 12, e83685 (2023).
Gibb, R. et al. Zoonotic host diversity increases in human-dominated ecosystems. Nature 584, 398–402 (2020).
United Nations Department of Economic and Social Affairs. World Urbanization Prospects: The 2018 Revision (United Nations, 2018).
Seto, K. C., Sánchez-Rodríguez, R. & Fragkias, M. The new geography of contemporary urbanization and the environment. Annu. Rev. Environ. Resour. 35, 167–194 (2010).
Schneider, A., Friedl, M. A. & Potere, D. A new map of global urban extent from MODIS satellite data. Environ. Res. Lett. 4, 044003 (2009).
United Nations Human Settlements Programme. World Cities Report 2022: Envisaging the Future of Cities (UN Habitat, 2022).
Coltart, C. E. M., Lindsey, B., Ghinai, I., Johnson, A. M. & Heymann, D. L. The Ebola outbreak, 2013–2016: old lessons for new epidemics. Philos. Trans. R. Soc. B: Biol. Sci. 372, 20160297 (2017).
Dellicour, S. et al. Phylodynamic assessment of intervention strategies for the West African Ebola virus outbreak. Nat. Commun. 9, 2222 (2018).
Dudas, G. et al. Virus genomes reveal factors that spread and sustained the Ebola epidemic. Nature 544, 309–315 (2017).
World Health Organization & United Nations Human Settlements Programme. Hidden Cities: Unmasking and Overcoming Health Inequities in Urban Settings (WHO & UN Habitat, 2010).
Levin, A. T. et al. Assessing the burden of COVID-19 in developing countries: systematic review, meta-analysis and public policy implications. BMJ Glob. Health 7, e008477 (2022).
Wachtler, B. et al. Socioeconomic inequalities and COVID-19—a review of the current international literature. J. Health Monit. 5, 3–17 (2020).
Parolin, Z. & Lee, E. K. The role of poverty and racial discrimination in exacerbating the health consequences of COVID-19. Lancet Reg. Health Am. 7, 100178 (2022).
Van Damme, P. et al. Hepatitis A virus infection. Nat. Rev. Dis. Primers 9, 51 (2023).
Boussaa, S., Pesson, B. & Boumezzough, A. Phlebotomine sandflies (Diptera: Psychodidae) of Marrakech city, Morocco. Ann. Tropical Med. Parasitol. 101, 715–724 (2007).
Kabaria, C. W., Gilbert, M., Noor, A. M., Snow, R. W. & Linard, C. The impact of urbanization and population density on childhood Plasmodium falciparum parasite prevalence rates in Africa. Malar. J. 16, 1–10 (2017).
Giles, J. R. et al. The duration of travel impacts the spatial dynamics of infectious diseases. Proc. Natl Acad. Sci. USA 117, 22572–22579 (2020).
Liu, K. et al. Population movement, city closure in Wuhan, and geographical expansion of the COVID-19 infection in China in January 2020. Clin. Infect. Dis. 71, 2045–2051 (2020).
Volz, E. et al. Evaluating the effects of SARS-CoV-2 spike mutation D614G on transmissibility and pathogenicity. Cell 184, 64–75.e11 (2021).
Castelli, F. & Sulis, G. Migration and infectious diseases. Clin. Microbiol. Infect. 23, 283–289 (2017).
World Health Organization. SARS outbreak contained worldwide. WHO https://www.who.int/news/item/05-07-2003-sars-outbreak-contained-worldwide (2003).
Bell, D. M. & World Health Organization Working Group on International and Community Transmission of SARS. Public health interventions and SARS spread, 2003. Emerg. Infect. Dis. 10, 1900–1906 (2004).
Stegeman, A. et al. Avian influenza A virus (H7N7) epidemic in the Netherlands in 2003: course of the epidemic and effectiveness of control measures. J. Infect. Dis. 190, 2088–2095 (2004).
World Health Organization. Strengthening Health Emergency Prevention, Preparedness, Response and Resilience (HEPR & WHO, 2023).
Singer, B. J. et al. Development of prediction models to identify hotspots of schistosomiasis in endemic regions to guide mass drug administration. Proc. Natl Acad. Sci. USA 121, e2315463120 (2024).
Kasbergen, L. M. R. et al. Multi-antigen serology and a diagnostic algorithm for the detection of arbovirus infections as novel tools for arbovirus preparedness in southeast Europe (MERMAIDS-ARBO): a prospective observational study. Lancet Infect. Dis. 25, 678–689 (2025).
Sigfrid, L. et al. Prevalence, clinical management, and outcomes of adults hospitalised with endemic arbovirus illness in southeast Europe (MERMAIDS-ARBO): a prospective observational study. Lancet Infect. Dis. 25, 690–700 (2025).
Schmidt, T. L. et al. Incursion pathways of the Asian tiger mosquito (Aedes albopictus) into Australia contrast sharply with those of the yellow fever mosquito (Aedes aegypti). Pest. Manag. Sci. 76, 4202–4209 (2020).
Oliveira, S., Rocha, J., Sousa, C. A. & Capinha, C. Wide and increasing suitability for Aedes albopictus in Europe is congruent across distribution models. Sci. Rep. 11, 9916 (2021).
Nakase, T., Giovanetti, M., Obolski, U. & Lourenço, J. Global transmission suitability maps for dengue virus transmitted by Aedes aegypti from 1981 to 2019. Sci Data 10, 275 (2023).
Dagostin, F. et al. Ecological and environmental factors affecting the risk of tick-borne encephalitis in Europe, 2017 to 2021. Euro. Surveill. 28, 2300121 (2023).
Farooq, Z. et al. European projections of West Nile virus transmission under climate change scenarios. One Health 16, 100509 (2023).
Romero-Alvarez, D., Escobar, L. E., Auguste, A. J., Del Valle, S. Y. & Manore, C. A. Transmission risk of Oropouche fever across the Americas. Infect. Dis. Poverty 12, 47 (2023).
Kjær, L. J. et al. Potential drivers of human tick-borne encephalitis in the Örebro region of Sweden, 2010–2021. Sci. Rep. 13, 7685 (2023).
Chemison, A., Ramstein, G., Jones, A., Morse, A. & Caminade, C. Ability of a dynamical climate sensitive disease model to reproduce historical Rift valley fever outbreaks over Africa. Sci. Rep. 14, 3904 (2024).
Nabi, G. et al. Bats and birds as viral reservoirs: a physiological and ecological perspective. Sci. Total. Env. 754, 142372 (2021).
Guth, S. et al. Bats host the most virulent—but not the most dangerous—zoonotic viruses. Proc. Natl Acad. Sci. USA 119, e2113628119 (2022).
Mollentze, N. & Streicker, D. G. Viral zoonotic risk is homogenous among taxonomic orders of mammalian and avian reservoir hosts. Proc. Natl Acad. Sci. USA 117, 9423–9430 (2020). This study proposes a host-neutral explanation for differences in the number of zoonotic pathogens among animal groups.
Ribeiro, R. et al. Incorporating environmental heterogeneity and observation effort to predict host distribution and viral spillover from a bat reservoir. Proc. Biol. Sci. 290, 20231739 (2023).
Beyer, R. M., Manica, A. & Mora, C. Shifts in global bat diversity suggest a possible role of climate change in the emergence of SARS-CoV-1 and SARS-CoV-2. Sci. Total. Env. 767, 145413 (2021).
Forero-Muñoz, N. R. et al. The coevolutionary mosaic of bat betacoronavirus emergence risk. Virus Evol. 10, vead079 (2024). This study shows that predicted virus hot spots based on the combined presence and richness of bat species may not be correct when they are compared with coronavirus co-evolution patterns.
Warmuth, V. M., Metzler, D. & Zamora-Gutierrez, V. Human disturbance increases coronavirus prevalence in bats. Sci. Adv. 9, eadd0688 (2023).
Rulli, M. C., D’Odorico, P., Galli, N. & Hayman, D. T. S. Land-use change and the livestock revolution increase the risk of zoonotic coronavirus transmission from rhinolophid bats. Nat. Food 2, 409–416 (2021).
Nyakarahuka, L. et al. Ecological niche modeling for filoviruses: a risk map for Ebola and marburg virus disease outbreaks in Uganda. PLoS Curr. https://doi.org/10.1371/currents.outbreaks.07992a87522e1f229c7cb023270a2af1 (2017).
Muylaert, R. L. et al. Using drivers and transmission pathways to identify SARS-like coronavirus spillover risk hotspots. Nat. Commun. 14, 6854 (2023).
Sánchez, C. A. et al. A strategy to assess spillover risk of bat SARS-related coronaviruses in Southeast Asia. Nat. Commun. 13, 4380 (2022).
Horigan, V. et al. Assessing the quality of data for drivers of disease emergence. Sci. Tech. Rev. 42, 90–102 (2023).
Si, Y., Xin, Q., Prins, H. H. T., de Boer, W. F. & Gong, P. Improving the quantification of waterfowl migration with remote sensing and bird tracking. Sci. Bull. 60, 1984–1993 (2015).
Johnson, E. et al. Applications and advances in acoustic monitoring for infectious disease epidemiology. Trends Parasitol. 39, 386–399 (2023). This study highlights the promise of integrating ecological methodologies in infectious disease research.
González-Pérez, M. I. et al. Field evaluation of an automated mosquito surveillance system which classifies Aedes and Culex mosquitoes by genus and sex. Parasites Vectors 17, 97 (2024).
Ruppert, K. M., Kline, R. J. & Rahman, M. S. Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: a systematic review in methods, monitoring, and applications of global eDNA. Glob. Ecol. Conserv. 17, e00547 (2019).
Park, H.-A., Jung, H., On, J., Park, S. K. & Kang, H. Digital epidemiology: use of digital data collected for non-epidemiological purposes in epidemiological studies. Healthc. Inform. Res. 24, 253–262 (2018).
Dugas, A. F. et al. Influenza forecasting with Google Flu trends. PLoS ONE 8, e56176 (2013).
Rocklöv, J. et al. Using big data to monitor the introduction and spread of Chikungunya, Europe, 2017. Emerg. Infect. Dis. 25, 1041 (2019).
Wu, J. et al. Mobile health technology combats COVID-19 in China. J. Infect. 82, 159–198 (2021).
Brownstein, J. S., Rader, B., Astley, C. M. & Tian, H. Advances in artificial intelligence for infectious-disease surveillance. N. Engl. J. Med. 388, 1597–1607 (2023).
Maganga, G. D. et al. Genetic diversity and ecology of coronaviruses hosted by cave-dwelling bats in Gabon. Sci. Rep. 10, 1–13 (2020).
Bai, R. et al. Exploring utility of genomic epidemiology to trace origins of highly pathogenic influenza A/H7N9 in Guangdong. Virus Evol. 6, veaa097 (2020).
Atama, N. C. et al. Evaluation of the use of alternative sample types for mosquito-borne flavivirus surveillance: using Usutu virus as a model. One Health 15, 100456 (2022).
Hotta, K. et al. Antibody survey on avian influenza viruses using egg yolks of ducks in Hanoi between 2010 and 2012. Vet. Microbiol. 166, 179–183 (2013).
de Rooij, M. M. T. et al. Occupational and environmental exposure to SARS-CoV-2 in and around infected mink farms. Occup. Env. Med. 78, 893–899 (2021).
Hendriksen, R. S. et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat. Commun. 10, 1124 (2019).
Nieuwenhuijse, D. F. et al. Setting a baseline for global urban virome surveillance in sewage. Sci. Rep. 10, 13748 (2020). This study shows the potential of environmental samples that may benefit pandemic preparedness.
Lu, J. et al. Capturing noroviruses circulating in the population: sewage surveillance in Guangdong, China (2013–2018). Water Res. 196, 116990 (2021).
Tisza, M. et al. Wastewater sequencing reveals community and variant dynamics of the collective human virome. Nat. Commun. 14, 6878 (2023).
Kutter, J. S. et al. Small quantities of respiratory syncytial virus RNA only in large droplets around infants hospitalized with acute respiratory infections. Antimicrob. Resist. Infect. Control. 10, 100 (2021).
Grayson, S. A., Griffiths, P. S., Perez, M. K. & Piedimonte, G. Detection of airborne respiratory syncytial virus in a pediatric acute care clinic. Pediatr. Pulmonol. 52, 684–688 (2017).
Gaide, N. et al. Viral tropism and detection of clade 2.3.4.4b H5N8 highly pathogenic avian influenza viruses in feathers of ducks and geese. Sci. Rep. 11, 5928 (2021).
Cheung, P. P. et al. Identifying the species-origin of faecal droppings used for avian influenza virus surveillance in wild-birds. J. Clin. Virol. 46, 90–93 (2009).
Smith, S. E. et al. Emerging technologies in the study of the virome. Curr. Opin. Virol. 54, 101231 (2022).
Kwok, K. T. T., Nieuwenhuijse, D. F., Phan, M. V. T. & Koopmans, M. P. G. Virus metagenomics in farm animals: a systematic review. Viruses 12, 107 (2020).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021). This work presents a key artificial intelligence tool that has transformed timely prediction of pathogen properties.
Madani, A. et al. Large language models generate functional protein sequences across diverse families. Nat. Biotechnol. 41, 1099–1106 (2023).
Plowright, R. K. et al. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 15, 502–510 (2017).
Warren, C. J. & Sawyer, S. L. Identifying animal viruses in humans. Science 379, 982–983 (2023).
One Health High-Level Expert Panel. Prevention of zoonotic spillover: from relying on response to reducing the risk at source. PLoS Pathog. 19, e1011504 (2023). In this work the OHHLEP advocates for primary prevention.
Fritz, S. et al. Citizen science and the United Nations Sustainable Development Goals. Nat. Sustain. 2, 922–930 (2019).
Pocock, M. J. O., Tweddle, J. C., Savage, J., Robinson, L. D. & Roy, H. E. The diversity and evolution of ecological and environmental citizen science. PLoS ONE 12, e0172579 (2017).
Palmer, J. R. B. et al. Citizen science provides a reliable and scalable tool to track disease-carrying mosquitoes. Nat. Commun. 8, 916 (2017).
Cohnstaedt, L. W., Ladner, J., Campbell, L. R., Busch, N. & Barrera, R. Determining mosquito distribution from egg data: the role of the citizen scientist. Am. Biol. Teach. 78, 317–322 (2016).
Murindahabi, M. M. et al. Citizen science for monitoring the spatial and temporal dynamics of malaria vectors in relation to environmental risk factors in Ruhuha, Rwanda. Malar. J. 20, 1–18 (2021).
Kampen, H. et al. Approaches to passive mosquito surveillance in the EU. Parasites Vectors 8, 1–13 (2015).
Larsen, L. Why citizen scientists are gathering DNA from hundreds of lakes-on the same day. Nature https://www.nature.com/articles/d41586-024-00520-y (2024).
Poen, M. J. et al. Local amplification of highly pathogenic avian influenza H5N8 viruses in wild birds in the Netherlands, 2016 to 2017. Eurosurveillance 23, 17-00449 (2018).
Münger, E. et al. One Health approach uncovers emergence and dynamics of Usutu and West Nile viruses in the Netherlands. Nat. Commun. 16, 7883 (2025). This extensive study describes how collaboration between different expertise, as well as involvement of citizen science, can lead to important insights in zoonotic virus ecology.
World Health Organizaton. mHealth: New Horizons for Health Through Mobile Technologies (WHO, 2011).
Kaarj, K., Akarapipad, P. & Yoon, J.-Y. Simpler, faster, and sensitive Zika virus assay using smartphone detection of loop-mediated isothermal amplification on paper microfluidic chips. Sci. Rep. 8, 1–11 (2018).
Geneviève, L. D. et al. Participatory disease surveillance systems: ethical framework. J. Med. Internet Res. 21, e12273 (2019).
Koppeschaar, C. E. et al. Influenzanet: citizens among 10 countries collaborating to monitor influenza in Europe. JMIR Public. Health Surveill. 3, e7429 (2017).
Elliot, A. J. et al. Self-sampling for community respiratory illness: a new tool for national virological surveillance. Eurosurveillance 20, 21058 (2015).
Su, X. et al. A novel internet sampling for HIV surveillance: feasibility of self-sampling and preparation of DBS for delivery detection of HIV total nucleic acid and complementarity to sentinel surveillance. BMC Infect. Dis. 23, 509 (2023).
Karesh, W. B. et al. Ecology of zoonoses: natural and unnatural histories. Lancet 380, 1936–1945 (2012). This key study proposes a framework of disease emergence.
Corman, V. M. et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro. Surveill. 25, 2000045 (2020).
Wille, M., Geoghegan, J. L. & Holmes, E. C. How accurately can we assess zoonotic risk? PLoS Biol. 19, e3001135 (2021).
Wasik, B. R. et al. Onward transmission of viruses: how do viruses emerge to cause epidemics after spillover? Philos. Trans. R. Soc. B 374, 20190017 (2019).
Zhang, X.-A. et al. A zoonotic Henipavirus in febrile patients in China. N. Engl. J. Med. 387, 470–472 (2022). This study is an example of how a human risk population can serve as the sentinel for novel zoonotic viruses.
Porta, M. S., Greenland, S., Hernán, M., dos Santos Silva, I. & Last, J. M. A Dictionary of Epidemiology (Oxford Univ. Press, 2014).
Hui, D. S. et al. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect. Dis. 18, e217–e227 (2018).
Wang, H., de Paulo, K. J. I. d. A., Gültzow, T., Zimmermann, H. M. L. & Jonas, K. J. Brief report: determinants of potential sexual activity reduction in the face of the mpox epidemic. Int. J. Behav. Med. 32, 308–324 (2024).
Wilkinson, A., Parker, M., Martineau, F. & Leach, M. Engaging ‘communities’: anthropological insights from the West African Ebola epidemic. Philos. Trans. R. Soc. B: Biol. Sci. 372, 20160305 (2017).
Tan, C. C. S. et al. Transmission of SARS-CoV-2 from humans to animals and potential host adaptation. Nat. Commun. 13, 2988 (2022).
Koopmans, M. SARS-CoV-2 and the human–animal interface: outbreaks on mink farms. Lancet Infect. Dis. 21, 18–19 (2020).
Hallmaier-Wacker, L. K., Munster, V. J. & Knauf, S. Disease reservoirs: from conceptual frameworks to applicable criteria. Emerg. Microbes Infect. 6, 1–5 (2017).
Food and Agriculture Organization, World Organisation for Animal Health & World Health Orgaization. Joint statement on the prioritization of monitoring SARS-CoV-2 infection in wildlife and preventing the formation of animal reservoirs. WHO https://www.who.int/news/item/07-03-2022-joint-statement-on-the-prioritization-of-monitoring-sars-cov-2-infection-in-wildlife-and-preventing-the-formation-of-animal-reservoirs (2022).
Hoffmann, M. et al. SARS-CoV-2 mutations acquired in mink reduce antibody-mediated neutralization. Cell Rep. 35, 109017 (2021).
Hayman, D. T. S. et al. Developing One Health surveillance systems. One Health 17, 100617 (2023).
Bordier, M., Uea-Anuwong, T., Binot, A., Hendrikx, P. & Goutard, F. L. Characteristics of One Health surveillance systems: a systematic literature review. Preventive Vet. Med. 181, 104560 (2020).
Vredenberg, I. et al. Assessing the use of different surveillance components to detect highly pathogenic avian influenza outbreaks in poultry in the Netherlands in low-and high-risk years. Transbound. Emerg. Dis. 2025, 7441785 (2025).
Errecaborde, K. M. et al. Factors that enable effective One Health collaborations — a scoping review of the literature. PLoS ONE 14, e0224660 (2019).
Sikkema, R. & Koopmans, M. One Health training and research activities in Western Europe. Infect. Ecol. Epidemiol. 6, 33703 (2016).
World Health Organization. WHO Pandemic Agreement (WHO, 2025). This work presents the adoption of the WHO Pandemic Agreement, an important milestone towards coordinated, fair preparation and response to future pandemics.
Stephen, C. & Berezowski, J. Reflective practice is a prerequisite for One Health development. One Health Outlook 6, 13 (2024).
Halpin, K., Graham, K. & Durr, P. A. Sero-monitoring of horses demonstrates the Equivac® HeV Hendra virus vaccine to be highly effective in inducing neutralising antibody titres. Vaccines 9, 731 (2021).
Manyweathers, J. et al. Risk mitigation of emerging zoonoses: Hendra virus and non-vaccinating horse owners. Transbound. Emerg. Dis. 64, 1898–1911 (2017).
International Monetary Fund. World Economic Outlook: Countering the Cost-of-living Crisis (IMF, 2022).
Fan, V. Y., Jamison, D. T. & Summers, L. H. Pandemic risk: how large are the expected losses? Bull. World Health Organ. 96, 129 (2018).
World Bank. Putting Pandemics Behind Us: Investing in One Health to Reduce Risks of Emerging Infectious Diseases (World Bank, 2022).
Dobson, A. P. et al. Ecology and economics for pandemic prevention. Science 369, 379–381 (2020). This study attempts to quantify the costs and benefits of pandemic preparedness.
Butt, E. W. et al. Amazon deforestation causes strong regional warming. Proc. Natl Acad. Sci. USA 120, e2309123120 (2023).
Montanarella, L., Scholes, R. & Brainich, A. The Assessment Report on Land Degradation and Restoration: Summary for Policymakers (IPBES, 2018).
Pike, J., Bogich, T., Elwood, S., Finnoff, D. C. & Daszak, P. Economic optimization of a global strategy to address the pandemic threat. Proc. Natl Acad. Sci. USA 111, 18519–18523 (2014).
Sirleaf, E. J. & Clark, H. Report of the Independent Panel for Pandemic Preparedness and Response: making COVID-19 the last pandemic. Lancet 398, 101–103 (2021).
Sikkema, R. S. et al. Detection of West Nile virus in a common whitethroat (Curruca communis) and Culex mosquitoes in the Netherlands, 2020. Eurosurveillance 25, 2001704 (2020).
de Saint Lary, C.dB. et al. Assessing West Nile virus (WNV) and Usutu virus (USUV) exposure in bird ringers in the Netherlands: a high-risk group for WNV and USUV infection? One Health 16, 100533 (2023).
Streng, K. et al. Sentinel chicken surveillance reveals previously undetected circulation of West Nile virus in the Netherlands. Emerg. Microbes Infect. 13, 2406278 (2024).
Streng, K. et al. Orthoflavivirus surveillance in the Netherlands: insights from a serosurvey in horses & dogs and a questionnaire among horse owners. Zoonoses Public. Health 71, 900–910 (2024).
Tao, X., Liu, S., Zhu, W. & Rayner, S. Rabies surveillance and control in China over the last twenty years. Biosaf. Health 3, 142–147 (2021).
Liu, H. et al. Rabies viruses in specific wild fur animals in northern China, 2017–2019. Transbound. Emerg. Dis. 67, 2307–2312 (2020).
Oude Munnink, B. B. et al. The next phase of SARS-CoV-2 surveillance: real-time molecular epidemiology. Nat. Med. 27, 1518–1524 (2021).
Holmes, E. C. et al. The origins of SARS-CoV-2: a critical review. Cell 184, 4848–4856 (2021).
Alm, E. et al. Geographical and temporal distribution of SARS-CoV-2 clades in the WHO European region, January to June 2020. Eurosurveillance 25, 2001410 (2020).
Voeten, H. et al. Unravelling the modes of transmission of SARS-CoV-2 during a nursing home outbreak: looking beyond the church super-spread event. Clin. Infect. Dis. 73, S163–S169 (2020).
Lu, L. et al. Adaptation, spread and transmission of SARS-CoV-2 in farmed minks and associated humans in the Netherlands. Nat. Commun. 12, 6802 (2021).
Mulder, M. et al. Reinfection of severe acute respiratory syndrome coronavirus 2 in an immunocompromised patient: a case report. Clin. Infect. Dis. 73, e2841–e2842 (2021).
Lu, L. et al. West Nile virus spread in Europe: phylogeographic pattern analysis and key drivers. PLoS Pathog. 20, e1011880 (2024). This work presents an extensive multi-institute phylogenetic analysis of WNV in Europe, identifying possible drivers for spread.
Attwood, S. W., Hill, S. C., Aanensen, D. M., Connor, T. R. & Pybus, O. G. Phylogenetic and phylodynamic approaches to understanding and combating the early SARS-CoV-2 pandemic. Nat. Rev. Genet. 23, 547–562 (2022).
Vaughan, T. G. et al. Estimating epidemic incidence and prevalence from genomic data. Mol. Biol. Evol. 36, 1804–1816 (2019).
Roosenhoff, R. et al. Influenza A/H3N2 virus infection in immunocompromised ferrets and emergence of antiviral resistance. PLoS ONE 13, e0200849 (2018).
Bai, R. et al. Antigenic variation of avian influenza A (H5N6) viruses, Guangdong province, China, 2014–2018. Emerg. Infect. Dis. 25, 1932 (2019).
Harvey, W. T. et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 19, 409–424 (2021).
Luca, B. et al. Highly pathogenic avian influenza H5N1 virus infections in wild red foxes (Vulpes vulpes) show neurotropism and adaptive virus mutations. Microbiol. Spectr. 11, e0286722 (2023).
Simmonds, P. & Aiewsakun, P. Virus classification—where do you draw the line? Arch. Virol. 163, 2037–2046 (2018).
Wang, J. et al. Individual bat virome analysis reveals co-infection and spillover among bats and virus zoonotic potential. Nat. Commun. 14, 4079 (2023).
Geoghegan, J. L. & Holmes, E. C. Predicting virus emergence amid evolutionary noise. Open. Biol. 7, 170189 (2017).
Suttie, A. et al. Inventory of molecular markers affecting biological characteristics of avian influenza A viruses. Virus Genes. 55, 739–768 (2019).
Potocnakova, L., Bhide, M. & Pulzova, L. B. An introduction to B-cell epitope mapping and in silico epitope prediction. J. Immunol. Res. 2016, 6760830 (2016).
Joana, D. et al. Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proc. Natl Acad. Sci. USA 117, 22311–22322 (2020).
Borkenhagen, L. K., Allen, M. W. & Runstadler, J. A. Influenza virus genotype to phenotype predictions through machine learning: a systematic review: computational prediction of influenza phenotype. Emerg. Microbes Infect. 10, 1896–1907 (2021).
Zhou, L. et al. Retrospective detection and phylogenetic analysis of swine acute diarrhoea syndrome coronavirus in pigs in southern China. Transbound. Emerg. Dis. 66, 687–695 (2019).
Edwards, C. E. et al. Swine acute diarrhea syndrome coronavirus replication in primary human cells reveals potential susceptibility to infection. Proc. Natl Acad. Sci. USA 117, 26915–26925 (2020).
Li, H. et al. Human–animal interactions and bat coronavirus spillover potential among rural residents in southern China. Biosaf. Health 1, 84–90 (2019).
Schriml, L. M. et al. COVID-19 pandemic reveals the peril of ignoring metadata standards. Sci. Data 7, 188 (2020).
Chen, Z. et al. Global landscape of SARS-CoV-2 genomic surveillance and data sharing. Nat. Genet. 54, 499–507 (2022).
Murray, K. et al. A morbillivirus that caused fatal disease in horses and humans. Science 268, 94–97 (1995).
Plowright, R. K. et al. Ecological dynamics of emerging bat virus spillover. Proc. R. Soc. B: Biol. Sci. 282, 20142124 (2015).
Eby, P. et al. Pathogen spillover driven by rapid changes in bat ecology. Nature 613, 340–344 (2023). This work presents an extensive 25-year study of bat virus spillover in Australia, providing a knowledge base for the development of interventions for primary prevention of spillover.
Becker, D. J., Eby, P., Madden, W., Peel, A. J. & Plowright, R. K. Ecological conditions predict the intensity of Hendra virus excretion over space and time from bat reservoir hosts. Ecol. Lett. 26, 23–36 (2023).
World Health Organization. SARS-CoV-2 in Animals Used for Fur Farming: GLEWS+ Risk Assessment (FAO, WOAH & WHO, 2021).
Guan, Y. et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302, 276–278 (2003).
Oude Munnink, B. B. et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 371, 172–177 (2020). This work is the first report of large-scale spillover and spill-back of SARS-CoV-2 between humans and animals.
Lindh, E. et al. Highly pathogenic avian influenza A (H5N1) virus infection on multiple fur farms in the South and Central Ostrobothnia regions of Finland, July 2023. Eurosurveillance 28, 2300400 (2023).
Zhao, J. et al. Farmed fur animals harbour viruses with zoonotic spillover potential. Nature 634, 228–233 (2024). This study shows that farmed fur animals can be a reservoir for novel zoonotic viruses, in addition to known risks of SARS-CoV-2 and avian influenza virus infections and adaptation.
European Food Safety Authority et al. Drivers for a pandemic due to avian influenza and options for One Health mitigation measures. EFSA J. 22, e8735 (2024).
European Food Safety Authoity. SARS-CoV-2 in animals: susceptibility of animal species, risk for animal and public health, monitoring, prevention and control. EFSA J. 21, e07822 (2023).
Halstead, S. B. Three dengue vaccines—what now. N. Engl. J. Med. 390, 464–465 (2024).
Weber, W. C. et al. The approved live-attenuated Chikungunya virus vaccine (IXCHIQ®) elicits cross-neutralizing antibody breadth extending to multiple arthritogenic alphaviruses similar to the antibody breadth following natural infection. Vaccines 12, 893 (2024).
World Health Organization. Global Vector Control Response 2017–2030 (WHO & TDR, 2017).
Hickmann, M. Plant-covered residential towers in Chengdu attract mosquitos, repel tenants. The Architect's Newspaper https://www.archpaper.com/2020/09/plant-covered-residential-towers-chengdu-attract-mosquitos-repel-tenants/ (2020).
Walshe, D. P., Garner, P., Adeel, A. A., Pyke, G. H. & Burkot, T. R. Larvivorous fish for preventing malaria transmission. Cochrane Database Syst. Rev. 12, CD008090 (2017).
Willott, E. Restoring nature, without mosquitoes? Restor. Ecol. 12, 147–153 (2004).
Acknowledgements
R.S. and M.K. receive funding for One Health research through European Union’s Horizon 2020 research and innovation programme under grant agreement no. 874735 (VEO - Versatile emerging infectious disease observatory) and EU HERA/HADEA under grant agreement no. 101102733 (DURABLE).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Gregory Gray, who co-reviewed with Franciso Guerra; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
European countries banned fur farming: https://www.furfreealliance.com/fur-bans/
Reported human infections in the United States: https://www.cdc.gov/bird-flu/situation-summary/index.html
World Bank Pandemic Fund: https://www.thepandemicfund.org/
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sikkema, R.S., Koopmans, M. Viral emergence and pandemic preparedness in a One Health framework. Nat Rev Microbiol (2025). https://doi.org/10.1038/s41579-025-01243-1
Accepted:
Published:
DOI: https://doi.org/10.1038/s41579-025-01243-1