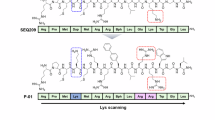
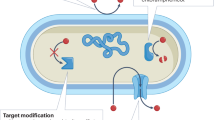
Abstract
The emergence of multidrug-resistant bacteria presents a critical threat to global health. These multidrug-resistant bacteria are often protected by complex cell envelopes that many antibiotics cannot penetrate, creating an important barrier to treatment. In response, targeting bacterial envelopes has long been recognized as an effective strategy, offering potential to bypass the challenges of drug entry and efflux resistance mechanisms. Moreover, many unique bacterial envelope sites remain clinically untapped, and new compounds directed at them have the potential to diversify the space of antimicrobial mechanisms, lowering the risk for cross-resistance. Compounds that target non-proteinaceous envelope components, such as lipopolysaccharide or prenylated peptidoglycan-precursors, are particularly attractive owing to their reduced susceptibility to antimicrobial resistance development. In this Review, we explore both recently discovered compounds and established envelope-targeting antibiotics, including compounds that target Gram-positive bacteria, more complex Gram-negative bacteria and mycobacterial pathogens, shedding light on this still clinically underexplored and vital therapeutic approach.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Naghavi, M. et al. Global burden of bacterial antimicrobial resistance 1990–2021: a systematic analysis with forecasts to 2050. Lancet 404, 1199–1226 (2024).
Okeke, I. N. et al. The scope of the antimicrobial resistance challenge. Lancet 403, 2426–2438 (2024).
Theuretzbacher, U., Jumde, R. P., Hennessy, A., Cohn, J. & Piddock, L. J. V. Global health perspectives on antibacterial drug discovery and the preclinical pipeline. Nat. Rev. Microbiol. https://doi.org/10.1038/s41579-025-01167-w (2025).
Lewis, K. et al. Sophisticated natural products as antibiotics. Nature 632, 39–49 (2024).
World Health Organization. WHO bacterial priority pathogens list, 2024: bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance (WHO, 2024).
Dulberger, C. L., Rubin, E. J. & Boutte, C. C. The mycobacterial cell envelope — a moving target. Nat. Rev. Microbiol. 18, 47–59 (2019).
Richter, M. F. et al. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 545, 299–304 (2017).
Walesch, S. et al. Fighting antibiotic resistance — strategies and (pre)clinical developments to find new antibacterials. EMBO Rep. 24, e56033 (2022).
Breukink, E. & de Kruijff, B. Lipid II as a target for antibiotics. Nat. Rev. Drug Discov. 5, 321–323 (2006).
Lazzaro, B. P., Zasloff, M. & Rolff, J. Antimicrobial peptides: application informed by evolution. Science 368, eaau5480 (2020).
Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 415, 389–395 (2002).
Torres, M. D. T., Melo, M. C. R., Crescenzi, O., Notomista, E. & de la Fuente-Nunez, C. Mining for encrypted peptide antibiotics in the human proteome. Nat. Biomed. Eng. 6, 67–75 (2021).
Turner, N. A. et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat. Rev. Microbiol. 17, 203–218 (2019).
Taylor, S. D. Synthesis, mechanism of action, and SAR studies on the cyclic lipopeptide antibiotic daptomycin. Can. J. Chem. 102, 414–424 (2024).
Hartley, M. D. & Imperiali, B. At the membrane frontier: a prospectus on the remarkable evolutionary conservation of polyprenols and polyprenyl-phosphates. Arch. Biochem. Biophysics 517, 83–97 (2012).
Sheldrick, G. M., Jones, P. G., Kennard, O., Williams, D. H. & Smith, G. A. Structure of vancomycin and its complex with acetyl-D-alanyl-D-alanine. Nature 271, 223–225 (1978).
Centers for Disease Control and Prevention. Reduced susceptibility of Staphylococcus aureus to vancomycin--Japan, 1996. MMWR Morb. Mortal. Wkly Rep. 46, 624–626 (1997).
van Groesen, E., Innocenti, P. & Martin, N. I. Recent advances in the development of semisynthetic glycopeptide antibiotics: 2014–2022. ACS Infect. Dis. 8, 1381–1407 (2022).
Brčić, J., Tong, A., Wender, P. A. & Cegelski, L. Conjugation of vancomycin with a single arginine improves efficacy against mycobacteria by more effective peptidoglycan targeting. J. Medicinal Chem. 66, 10226–10237 (2023).
Padilla, M. S. T. L. & Nowick, J. S. Vancomycin–teixobactin conjugates. J. Am. Chem. Soc. https://doi.org/10.1021/jacs.4c17175 (2025).
Rahn, H. P. et al. Biguanide-vancomycin conjugates are effective broad-spectrum antibiotics against actively growing and biofilm-associated Gram-positive and Gram-negative ESKAPE pathogens and Mycobacteria. J. Am. Chem. Soc. 146, 22541–22552 (2024).
Malabarba, A. & Goldstein, B. P. Origin, structure, and activity in vitro and in vivo of dalbavancin. J. Antimicrob. Chemother. 55, ii15–ii20 (2005).
Cooper, R. D. G. et al. Reductive alkylation of glycopeptide antibiotics: synthesis and antibacterial activity. J. Antibiot. 49, 575–581 (1996).
van Groesen, E. et al. Semisynthetic guanidino lipoglycopeptides with potent in vitro and in vivo antibacterial activity. Sci. Transl. Med. 16, eabo4736 (2024).
Ling, L. L. et al. A new antibiotic kills pathogens without detectable resistance. Nature 517, 455–459 (2015).
Shukla, R. et al. Teixobactin kills bacteria by a two-pronged attack on the cell envelope. Nature 608, 390–396 (2022).
Shukla, R. et al. Mode of action of teixobactins in cellular membranes. Nat. Commun. 11, 2848 (2020).
Yang, H., Chen, K. H. & Nowick, J. S. Elucidation of the teixobactin pharmacophore. ACS Chem. Biol. 11, 1823–1826 (2016).
Shukla, R. et al. An antibiotic from an uncultured bacterium binds to an immutable target. Cell 186, 4059–4073.e27 (2023).
Wirtz, D. A. et al. Biosynthesis and mechanism of action of the cell wall targeting antibiotic hypeptin. Angew. Chem. Int. Ed. 60, 13579–13586 (2021).
Jekhmane, S. et al. Host defence peptide plectasin targets bacterial cell wall precursor lipid II by a calcium-sensitive supramolecular mechanism. Nat. Microbiol. 9, 1778–1791 (2024).
Loll, P. J. et al. Vancomycin forms ligand-mediated supramolecular complexes. J. Mol. Biol. 385, 200–211 (2009).
Lawrence, W. S. et al. Teixobactin: a resistance-evading antibiotic for treating anthrax. ACS Infect. Dis. https://doi.org/10.1021/acsinfecdis.4c00835 (2025).
Jones, C. R., Lai, G. H., Padilla, M. S. T. L. & Nowick, J. S. Investigation of isobactin analogues of teixobactin. ACS Med. Chem. Lett. 15, 1136–1142 (2024).
Ludwig, K. C. et al. The dual mode of antibacterial action of the synthetic small molecule DCAP involves lipid II binding. J. Am. Chem. Soc. 146, 24855–24862 (2024).
Reithuber, E. et al. THCz: small molecules with antimicrobial activity that block cell wall lipid intermediates. Proc. Natl Acad. Sci. USA 118, e2108244118 (2021).
Johnson, B. A., Anker, H. & Meleney, F. L. Bacitracin: a new antibiotic produced by a member of the B. subtilis group. Science 102, 376–377 (1945).
Economou, N. J., Cocklin, S. & Loll, P. J. High-resolution crystal structure reveals molecular details of target recognition by bacitracin. Proc. Natl Acad. Sci. USA 110, 14207–14212 (2013).
Buijs, N. P. et al. A classic antibiotic reimagined: rationally designed bacitracin variants exhibit potent activity against vancomycin-resistant pathogens. Proc. Natl Acad. Sci. USA 121, e2315310121 (2024).
Genkins, G. Bacitracin nephropathy. J. Am. Med. Assoc. 155, 894–897 (1954).
Culp, E. J. et al. Evolution-guided discovery of antibiotics that inhibit peptidoglycan remodelling. Nature 578, 582–587 (2020).
Steenbergen, J. N., Alder, J., Thorne, G. M. & Tally, F. P. Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections. J. Antimicrob. Chemother. 55, 283–288 (2005).
Grein, F. et al. Ca2+-Daptomycin targets cell wall biosynthesis by forming a tripartite complex with undecaprenyl-coupled intermediates and membrane lipids. Nat. Commun. 11, 1455 (2020).
Müller, A. et al. Daptomycin inhibits cell envelope synthesis by interfering with fluid membrane microdomains. Proc. Natl Acad. Sci. USA 113, E7077–E7086 (2016).
Kotsogianni, I., Wood, T. M., Alexander, F. M., Cochrane, S. A. & Martin, N. I. Binding studies reveal phospholipid specificity and its role in the calcium-dependent mechanism of action of daptomycin. ACS Infect. Dis. 7, 2612–2619 (2021).
Moreira, R. & Taylor, S. D. The chiral target of daptomycin is the 2R,2′S stereoisomer of phosphatidylglycerol. Angew. Chem. Int. Ed. 61, e202114858 (2021).
Machhua, P. et al. Daptomycin forms a stable complex with phosphatidylglycerol for selective uptake to bacterial membrane. eLife 13, RP93267 (2025).
Rimal, B. et al. The effects of daptomycin on cell wall biosynthesis in Enterococcal faecalis. Sci. Rep. 13, 12227 (2023).
Zhang, T., Muraih, J. K., MacCormick, B., Silverman, J. & Palmer, M. Daptomycin forms cation- and size-selective pores in model membranes. Bioch. Biophys. Acta Biomembranes 1838, 2425–2430 (2014).
Beriashvili, D. et al. Mechanistic studies on the effect of membrane lipid acyl chain composition on daptomycin pore formation. Chem. Phys. Lipids 216, 73–79 (2018).
Howe, A. & Sofou, S. Daptomycin-induced lipid phases on model lipid bilayers: effect of lipid type and of lipid leaflet order on membrane permeability. J. Phys. Chem. B 125, 5775–5785 (2021).
Tran, T. T., Munita, J. M. & Arias, C. A. Mechanisms of drug resistance: daptomycin resistance. Ann. N. Y. Acad. Sci. 1354, 32–53 (2015).
Nguyen, A. H., Hood, K. S., Mileykovskaya, E., Miller, W. R. & Tran, T. T. Bacterial cell membranes and their role in daptomycin resistance: a review. Front. Mol. Biosci. 9, 1035574 (2022).
Makitrynskyy, R. et al. Olikomycin A–A novel calcium-dependent lipopeptide with antibiotic activity against multidrug-resistant bacteria. Chemistry 31, e202403985 (2024).
Goodyear, J., Diamandas, M., Moreira, R. & Taylor, S. D. The calcium-dependent antibiotics: structure–activity relationships and determination of their lipid target. ACS Infect. Dis. 11, 226–237 (2024).
Bertani, B. & Ruiz, N. Function and biogenesis of lipopolysaccharides. EcoSal Plus https://doi.org/10.1128/ecosalplus.ESP-0001-2018 (2018).
Simpson, B. W. & Trent, M. S. Pushing the envelope: LPS modifications and their consequences. Nat. Rev. Microbiol. 17, 403–416 (2019).
Clifton, L. A. et al. Effect of divalent cation removal on the structure of Gram-negative bacterial outer membrane models. Langmuir 31, 404–412 (2014).
MacNair, C. R., Brown, E. D. & Dunman, P. Outer membrane disruption overcomes intrinsic, acquired, and spontaneous antibiotic resistance. mBio 11, e01615-20 (2020).
MacNair, C. R., Rutherford, S. T. & Tan, M.-W. Alternative therapeutic strategies to treat antibiotic-resistant pathogens. Nat. Rev. Microbiol. 22, 262–275 (2023).
MacNair, C. R. et al. Preclinical development of pentamidine analogs identifies a potent and nontoxic antibiotic adjuvant. ACS Infect. Dis. 8, 768–777 (2022).
Zavascki, A. P., Goldani, L. Z., Li, J. & Nation, R. L. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J. Antimicrob. Chemother. 60, 1206–1215 (2007).
Nang, S. C. et al. Rescuing the last-line polymyxins: achievements and challenges. Pharmacol. Rev. 73, 679–728 (2021).
Trimble, M. J., Mlynárčik, P., Kolář, M. & Hancock, R. E. W. Polymyxin: alternative mechanisms of action and resistance. Cold Spring Harb. Perspect. Med. 6, a025288 (2016).
Velkov, T., Thompson, P. E., Nation, R. L. & Li, J. Structure−activity relationships of polymyxin antibiotics. J. Med. Chem. 53, 1898–1916 (2009).
Savenko, M., Vácha, R., Ramseyer, C. & Rivel, T. Role of divalent ions in membrane models of polymyxin-sensitive and resistant Gram-negative bacteria. J. Chem. Inf. Modeling 65, 1476–1491 (2025).
Buchholz, K. R. et al. Potent activity of polymyxin B is associated with long-lived super-stoichiometric accumulation mediated by weak-affinity binding to lipid A. Nat. Commun. 15, 4733 (2024).
Manioglu, S. et al. Antibiotic polymyxin arranges lipopolysaccharide into crystalline structures to solidify the bacterial membrane. Nat. Commun. 13, 6195 (2022).
Benn, G., Silhavy, T. J., Kleanthous, C. & Hoogenboom, B. W. Antibiotics and hexagonal order in the bacterial outer membrane. Nat. Commun. 14, 4772 (2023).
Manioglu, S. et al. Reply to: antibiotics and hexagonal order in the bacterial outer membrane. Nat. Commun. 14, 4773 (2023).
Borrelli, C. et al. Polymyxin B lethality requires energy-dependent outer membrane disruption. Nat. Microbiol. https://doi.org/10.1038/s41564-025-02133-1 (2025).
Sabnis, A. et al. Colistin kills bacteria by targeting lipopolysaccharide in the cytoplasmic membrane. eLife 10, e65836 (2021).
Gadar, K. et al. Disrupting iron homeostasis can potentiate colistin activity and overcome colistin resistance mechanisms in Gram-negative bacteria. Commun. Biol. 6, 937 (2023).
Sampson, T. R. et al. Rapid killing of Acinetobacter baumannii by polymyxins is mediated by a hydroxyl radical death pathway. Antimicrobial Agents Chemother. 56, 5642–5649 (2012).
Ledger, E. V. K., Sabnis, A. & Edwards, A. M. Polymyxin and lipopeptide antibiotics: membrane-targeting drugs of last resort. Microbiology 168, 001136 (2022).
Wang, R. et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun. 9, 1179 (2018).
MacNair, C. R. et al. Overcoming mcr-1 mediated colistin resistance with colistin in combination with other antibiotics. Nat. Commun. 9, 458 (2018).
Humphrey, M. et al. Colistin resistance in Escherichia coli confers protection of the cytoplasmic but not outer membrane from the polymyxin antibiotic. Microbiology 167, 001104 (2021).
Carfrae, L. A. et al. Inhibiting fatty acid synthesis overcomes colistin resistance. Nat. Microbiol. 8, 1026–1038 (2023).
Wang, Z. et al. A naturally inspired antibiotic to target multidrug-resistant pathogens. Nature 601, 606–611 (2022).
Roberts, K. D. et al. A synthetic lipopeptide targeting top-priority multidrug-resistant Gram-negative pathogens. Nat. Commun. 13, 1625 (2022).
Slingerland, C. J. et al. Semisynthetic polymyxins with potent antibacterial activity and reduced kidney cell toxicity. RSC Med. Chem. 14, 2417–2425 (2023).
Yang, H.-X. et al. Design, synthesis, and bioactivity investigation of cyclic lipopeptide antibiotics containing eight to nine amino acids. J. Med. Chem. 66, 2524–2541 (2023).
Slingerland, C. J. & Martin, N. I. Recent advances in the development of polymyxin antibiotics: 2010-2023. ACS Infect. Dis. 10, 1056–1079 (2024).
Li, J. et al. Total and semisyntheses of polymyxin analogues with 2-thr or 10-thr modifications to decipher the structure-activity relationship and improve the antibacterial activity. J. Med. Chem. 64, 5746–5765 (2021).
Patil, N. A. et al. Critical role of position 10 residue in the polymyxin antimicrobial activity. J. Med. Chem. 66, 2865–2876 (2023).
Slingerland, C. J. et al. Synthesis and evaluation of polymyxins bearing reductively labile disulfide-linked lipids. J. Med. Chem. 65, 15878–15892 (2022).
Brown, P. et al. Design of next generation polymyxins with lower toxicity: the discovery of SPR206. ACS Infect. Dis. 5, 1645–1656 (2019).
Outeda-Garcia, M. et al. The novel polymyxin analogue SPR206 exhibits higher activity than colistin against both colistin-susceptible and colistin-resistant strains of Acinetobacter baumannii. Antimicrob. Agents Chemother. 69, e0194024 (2025).
Spero Therapeutics. Spero Therapeutics Announces Fourth Quarter and Full Year 2024 Operating Results and Provides a Business Update. https://www.globenewswire.com/news-release/2025/03/27/3050978/0/en/Spero-Therapeutics-Announces-Fourth-Quarter-and-Full-Year-2024-Operating-Results-and-Provides-a-Business-Update.html (2025).
Jiang, X. et al. Structure-interaction relationship of polymyxins with lung surfactant. J. Med. Chem. 66, 16109–16119 (2023).
Li, S. et al. Pharmacokinetics and safety of EVER206, a novel polymyxin antimicrobial, in healthy Chinese subjects. Antimicrob. Agents Chemother. 68, e0156323 (2024).
Lepak, A. J., Wang, W. & Andes, D. R. Pharmacodynamic evaluation of MRX-8, a novel polymyxin, in the neutropenic mouse thigh and lung infection models against Gram-negative pathogens. Antimicrob. Agents Chemother. 64, e01517-20 (2020).
Qu, X. et al. Pharmacokinetics and nephrotoxicity of polymyxin MRX-8 in rats: a novel agent against resistant Gram-negative bacteria. Antibiotics 13, 354 (2024).
MicuRX. Pipeline. https://www.micurxchina.com/en/pipeline (2025).
Corbett, D. et al. Potentiation of antibiotic activity by a novel cationic peptide: potency and spectrum of activity of SPR741. Antimicrob. Agents Chemother. 61, e00200-17 (2017).
French, S. et al. Potentiation of antibiotics against Gram-negative bacteria by polymyxin B analogue SPR741 from unique perturbation of the outer membrane. ACS Infect. Dis. 6, 1405–1412 (2020).
Zurawski, D. V. et al. SPR741, an antibiotic adjuvant, potentiates the in vitro and in vivo activity of rifampin against clinically relevant extensively drug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 61, e01239-17 (2017).
Song, M. et al. A broad-spectrum antibiotic adjuvant reverses multidrug-resistant Gram-negative pathogens. Nat. Microbiol. 5, 1040–1050 (2020).
Sabnis, A. & Edwards, A. M. Lipopolysaccharide as an antibiotic target. Bioch. Biophys. Acta Mol. Cell Res. 1870, 119507 (2023).
Törk, L., Moffatt, C. B., Bernhardt, T. G., Garner, E. C. & Kahne, D. Single-molecule dynamics show a transient lipopolysaccharide transport bridge. Nature 623, 814–819 (2023).
Zhang, T. et al. Native mass spectrometry and structural studies reveal modulation of MsbA–nucleotide interactions by lipids. Nat. Commun. 15, 5946 (2024).
Wu, H. et al. Research progress of LpxC inhibitor on Gram-negative bacteria. Eur. J. Medicinal Chem. 289, 117440 (2025).
Huseby, D. L. et al. Antibiotic class with potent in vivo activity targeting lipopolysaccharide synthesis in Gram-negative bacteria. Proc. Natl Acad. Sci. USA 121, e2317274121 (2024).
Thélot, F. A. et al. Distinct allosteric mechanisms of first-generation MsbA inhibitors. Science 374, 580–585 (2021).
Verma, V. A. et al. Discovery of inhibitors of the lipopolysaccharide transporter MsbA: from a screening hit to potent wild-type Gram-negative activity. J. Medicinal Chem. 65, 4085–4120 (2022).
Wang, H. et al. Cerastecins inhibit membrane lipooligosaccharide transport in drug-resistant Acinetobacter baumannii. Nat. Microbiol. 9, 1244–1255 (2024).
Zhang, Q. et al. Re-sensitization of mcr carrying multidrug resistant bacteria to colistin by silver. Proc. Natl Acad. Sci. USA 119, e2119417119 (2022).
Srinivas, N. et al. Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa. Science 327, 1010–1013 (2010).
Li, D. & Schneider-Futschik, E. K. Current and emerging inhaled antibiotics for chronic pulmonary Pseudomonas aeruginosa and Staphylococcus aureus infections in cystic fibrosis. Antibiotics 12, 484 (2023).
Pahil, K. S. et al. A new antibiotic traps lipopolysaccharide in its intermembrane transporter. Nature 625, 572–577 (2024).
Zampaloni, C. et al. A novel antibiotic class targeting the lipopolysaccharide transporter. Nature 625, 566–571 (2024).
Vetterli, S. U. et al. Thanatin targets the intermembrane protein complex required for lipopolysaccharide transport in Escherichia coli. Sci. Adv. 4, eaau2634 (2018).
Fiorentino, F. et al. Dynamics of an LPS translocon induced by substrate and an antimicrobial peptide. Nat. Chem. Biol. 17, 187–195 (2020).
Schuster, M. et al. Peptidomimetic antibiotics disrupt the lipopolysaccharide transport bridge of drug-resistant Enterobacteriaceae. Sci. Adv. 9, eadg3683 (2023).
Gu, Y. et al. Structural basis of outer membrane protein insertion by the BAM complex. Nature 531, 64–69 (2016).
Bakelar, J., Buchanan, S. K. & Noinaj, N. The structure of the β-barrel assembly machinery complex. Science 351, 180–186 (2016).
dos Santos, T. M. A. et al. Native β-barrel substrates pass through two shared intermediates during folding on the BAM complex. Proc. Natl Acad. Sci. USA 121, e2409672121 (2024).
Imai, Y. et al. A new antibiotic selectively kills Gram-negative pathogens. Nature 576, 459–464 (2019).
Kaur, H. et al. The antibiotic darobactin mimics a β-strand to inhibit outer membrane insertase. Nature 593, 125–129 (2021).
Groß, S. et al. Improved broad-spectrum antibiotics against Gram-negative pathogens via darobactin biosynthetic pathway engineering. Chem. Sci. 12, 11882–11893 (2021).
Miller, R. D. et al. Computational identification of a systemic antibiotic for Gram-negative bacteria. Nat. Microbiol. 7, 1661–1672 (2022).
Sun, D. et al. The discovery and structural basis of two distinct state-dependent inhibitors of BamA. Nat. Commun. 15, 8718 (2024).
Luther, A. et al. Chimeric peptidomimetic antibiotics against Gram-negative bacteria. Nature 576, 452–458 (2019).
Javed, A. et al. Chimeric peptidomimetic antibiotic efficiently neutralizes lipopolysaccharides (LPS) and bacteria-induced activation of RAW macrophages. ACS Infect. Dis. 9, 518–526 (2023).
Muñoz, K. A. et al. A Gram-negative-selective antibiotic that spares the gut microbiome. Nature 630, 429–436 (2024).
Smith, P. A. et al. Optimized arylomycins are a new class of Gram-negative antibiotics. Nature 561, 189–194 (2018).
World Health Organization. Global Tuberculosis Report 2019 https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1 (2019).
Dartois, V. A. & Rubin, E. J. Anti-tuberculosis treatment strategies and drug development: challenges and priorities. Nat. Rev. Microbiol. 20, 685–701 (2022).
Wu, M.-L., Aziz, D. B., Dartois, V. & Dick, T. NTM drug discovery: status, gaps and the way forward. Drug Discov. Today 23, 1502–1519 (2018).
Conradie, F. et al. Bedaquiline–pretomanid–linezolid regimens for drug-resistant tuberculosis. N. Engl. J. Med. 387, 810–823 (2022).
Forget, E. J. & Menzies, D. Adverse reactions to first-line antituberculosis drugs. Expert Opin. Drug Saf. 5, 231–249 (2006).
Daley, C. L. et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur. Respiratory J. 56, 2000535 (2020).
Russell, D. G., Cardona, P.-J., Kim, M.-J., Allain, S. & Altare, F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat. Immunol. 10, 943–948 (2009).
Jarlier, V. & Nikaido, H. Permeability barrier to hydrophilic solutes in Mycobacterium chelonei. J. Bacteriol. 172, 1418–1423 (1990).
Nessar, R., Cambau, E., Reyrat, J. M., Murray, A. & Gicquel, B. Mycobacterium abscessus: a new antibiotic nightmare. J. Antimicrob. Chemother. 67, 810–818 (2012).
Jarlier, V. & Nikaido, H. Mycobacterial cell wall: structure and role in natural resistance to antibiotics. FEMS Microbiol. Lett. 123, 11–18 (1994).
Nguyen, T. Q. et al. Exploring antibiotic resistance mechanisms in Mycobacterium abscessus for enhanced therapeutic approaches. Front. Microbiol. 15, 1331508 (2024).
Alderwick, L. J., Harrison, J., Lloyd, G. S. & Birch, H. L. The mycobacterial cell wall— peptidoglycan and arabinogalactan. Cold Spring Harb. Perspect. Med. 5, a021113 (2015).
Sparks, I. L. et al. Lipoarabinomannan mediates localized cell wall integrity during division in mycobacteria. Nat. Commun. 15, 2191 (2024).
Dartois, V. & Dick, T. Therapeutic developments for tuberculosis and nontuberculous mycobacterial lung disease. Nat. Rev. Drug Discov. 23, 381–403 (2024).
Yadav, S. et al. DprE1 inhibitors: enduring aspirations for future antituberculosis drug discovery. ChemMedChem 18, e202300099 (2023).
Mikušová, K. et al. Decaprenylphosphoryl arabinofuranose, the donor of the D-arabinofuranosyl residues of mycobacterial arabinan, is formed via a two-step epimerization of decaprenylphosphoryl ribose. J. Bacteriol. 187, 8020–8025 (2005).
Chikhale, R. V., Barmade, M. A., Murumkar, P. R. & Yadav, M. R. Overview of the development of DprE1 inhibitors for combating the menace of tuberculosis. J. Med. Chem. 61, 8563–8593 (2018).
Makarov, V. et al. Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan synthesis. Science 324, 801–804 (2009).
Römpp, A. et al. The clinical-stage drug BTZ-043 accumulates in murine tuberculosis lesions and efficiently acts against Mycobacterium tuberculosis. Nat. Commun. 16, 826 (2025).
Makarov, V. & Mikušová, K. Development of macozinone for TB treatment: an update. Appl. Sci. 10, 2269 (2020).
Shirude, P. S. et al. Azaindoles: noncovalent DprE1 inhibitors from scaffold morphing efforts, kill Mycobacterium tuberculosis and are efficacious in vivo. J. Med. Chem. 56, 9701–9708 (2013).
Hariguchi, N. et al. OPC-167832, a novel carbostyril derivative with potent antituberculosis activity as a DprE1 inhibitor. Antimicrobial Agents Chemother. 64, e02020-19 (2020).
Kerantzas, C. A., Jacobs, W. R., Rubin, E. J. & Collier, R. J. Origins of combination therapy for tuberculosis: lessons for future antimicrobial development and application. mBio 8, e01586-16 (2017).
Zhang, L. et al. Structures of cell wall arabinosyltransferases with the anti-tuberculosis drug ethambutol. Science 368, 1211–1219 (2020).
Tan, Y. Z. et al. Cryo-EM structure of arabinosyltransferase EmbB from Mycobacterium smegmatis. Nat. Commun. 11, 3396 (2020).
Rozwarski, D. A., Grant, G. A., Barton, D. H. R., Jacobs, W. R. & Sacchettini, J. C. Modification of the NADH of the isoniazid target (InhA) from Mycobacterium tuberculosis. Science 279, 98–102 (1998).
Xu, Z., Meshcheryakov, V. A., Poce, G. & Chng, S.-S. MmpL3 is the flippase for mycolic acids in Mycobacteria. Proc. Natl Acad. Sci. USA 114, 7993–7998 (2017).
Waldor, M. K. et al. Structures of the mycobacterial membrane protein MmpL3 reveal its mechanism of lipid transport. PLoS Biol. 19, e3001370 (2021).
Adams, O. et al. Cryo-EM structure and resistance landscape of M. tuberculosis MmpL3: an emergent therapeutic target. Structure 29, 1182–1191.e4 (2021).
Degiacomi, G. et al. Essentiality of mmpL3 and impact of its silencing on Mycobacterium tuberculosis gene expression. Sci. Rep. 7, 43495 (2017).
North, E. J., Schwartz, C. P., Zgurskaya, H. I. & Jackson, M. Recent advances in mycobacterial membrane protein large 3 inhibitor drug design for mycobacterial infections. Expert Opin. Drug Discov. 18, 707–724 (2023).
Jia, L. et al. Pharmacodynamics and pharmacokinetics of SQ109, a new diamine-based antitubercular drug. Br. J. Pharmacol. 144, 80–87 (2009).
Stevens, C. M. et al. Proton transfer activity of the reconstituted Mycobacterium tuberculosis MmpL3 is modulated by substrate mimics and inhibitors. Proc. Natl Acad. Sci. USA 119, e2113963119 (2022).
Zhang, B. et al. Crystal structures of membrane transporter MmpL3, an anti-TB drug target. Cell 176, 636–648.e13 (2019).
Yang, X. et al. Structural basis for the inhibition of mycobacterial MmpL3 by NITD-349 and SPIRO. J. Mol. Biol. 432, 4426–4434 (2020).
Tenland, E. et al. A novel derivative of the fungal antimicrobial peptide plectasin is active against Mycobacterium tuberculosis. Tuberculosis 113, 231–238 (2018).
Martelli, G. et al. N-Thio-β-lactams targeting L,D-transpeptidase-2, with activity against drug-resistant strains of Mycobacterium tuberculosis. Cell Chem. Biol. 28, 1321–1332.e5 (2021).
de Munnik, M. et al. Biochemical and crystallographic studies of l,d-transpeptidase 2 from Mycobacterium tuberculosis with its natural monomer substrate. Commun. Biol. 7, 1173 (2024).
de Munnik, M. et al. High-throughput screen with the l,d-transpeptidase LdtMt2 of Mycobacterium tuberculosis reveals novel classes of covalently reacting inhibitors. Chem. Sci. 14, 7262–7278 (2023).
Ramón-García, S. et al. Sanfetrinem, an oral β-lactam antibiotic repurposed for the treatment of tuberculosis. Drug Resist. Update 80, 101213 (2025).
Süssmuth, R. D., Kulike-Koczula, M., Gao, P. & Kosol, S. Fighting antimicrobial resistance: innovative drugs in antibacterial research. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.202414325 (2025).
Santos-Júnior, C. D. et al. Discovery of antimicrobial peptides in the global microbiome with machine learning. Cell 187, 3761–3778.e16 (2024).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Liu, G. et al. Deep learning-guided discovery of an antibiotic targeting Acinetobacter baumannii. Nat. Chem. Biol. 19, 1342–1350 (2023).
Moffat, J. G., Vincent, F., Lee, J. A., Eder, J. & Prunotto, M. Opportunities and challenges in phenotypic drug discovery: an industry perspective. Nat. Rev. Drug Discov. 16, 531–543 (2017).
Klapper, M. et al. Natural products from reconstructed bacterial genomes of the Middle and Upper Paleolithic. Science 380, 619–624 (2023).
Wan, F., Torres, M. D. T., Peng, J. & de la Fuente-Nunez, C. Deep-learning-enabled antibiotic discovery through molecular de-extinction. Nat. Biomed. Eng. 8, 854–871 (2024).
Kühlbrandt, W. The resolution revolution. Science 343, 1443–1444 (2014).
Medeiros-Silva, J. et al. High-resolution NMR studies of antibiotics in cellular membranes. Nat. Commun. 9, 3963 (2018).
Parker, E. N. et al. Implementation of permeation rules leads to a FabI inhibitor with activity against Gram-negative pathogens. Nat. Microbiol. 5, 67–75 (2019).
Sarathy, J. P., Dartois, V. & Lee, E. J. D. The role of transport mechanisms in Mycobacterium tuberculosis drug resistance and tolerance. Pharmaceuticals 5, 1210–1235 (2012).
Hoffmann, C., Leis, A., Niederweis, M., Plitzko, J. M. & Engelhardt, H. Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc. Natl Acad. Sci. USA 105, 3963–3967 (2008).
Zuber, B. et al. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J. Bacteriol. 190, 5672–5680 (2008).
Chiaradia, L. et al. Dissecting the mycobacterial cell envelope and defining the composition of the native mycomembrane. Sci. Rep. 7, 12807 (2017).
Batt, S. M., Burke, C. E., Moorey, A. R. & Besra, G. S. Antibiotics and resistance: the two-sided coin of the mycobacterial cell wall. Cell Surf. 6, 100044 (2020).
Fleming, A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzæ. Br. J. Exp. Pathol. 10, 226–236 (1929).
Tipper, D. J. & Strominger, J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc. Natl Acad. Sci. USA 54, 1133–1141 (1965).
Bush, K. & Bradford, P. A. β-Lactams and β-lactamase inhibitors: an overview. Cold Spring Harb. Perspect. Med. 6, a025247 (2016).
Acknowledgements
Projects in the M.W. laboratory are supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 101045485 to M.W.). A.M.E. is supported by the Biotechnology and Biological Sciences Research Council (BB/Y003667/1) and the National Institute for Health and Care Research Imperial Biomedical Research Centre.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Paul J. Hergenrother, Michael Lobritz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ntallis, C., Martin, N.I., Edwards, A.M. et al. Bacterial cell envelope-targeting antibiotics. Nat Rev Microbiol 24, 183–196 (2026). https://doi.org/10.1038/s41579-025-01247-x
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41579-025-01247-x