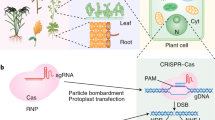
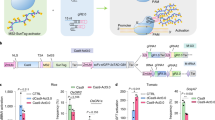
Abstract
Growing world population and deteriorating climate conditions necessitate the development of new crops with high yields and resilience. CRISPR–Cas-mediated genome engineering presents unparalleled opportunities to engineer crop varieties cheaper, easier and faster than ever. In this Review, we discuss how the CRISPR–Cas toolbox has rapidly expanded from Cas9 and Cas12 to include different Cas orthologues and engineered variants. We present various CRISPR–Cas-based methods, including base editing and prime editing, which are used for precise genome, epigenome and transcriptome engineering, and methods used to deliver the genome editors into plants, such as bacterial-mediated and viral-mediated transformation. We then discuss how promoter editing and chromosome engineering are used in crop breeding for trait engineering and fixation, and important applications of CRISPR–Cas in crop improvement, such as de novo domestication and enhancing tolerance to abiotic stresses. We conclude with discussing future prospects of plant genome engineering.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Searchinger, T., Waite, R., Hanson, C. & Ranganathan, J. Creating a Sustainable Food Future: A Menu of Solutions to Feed Nearly 10 Billion People By 2050. (World Resources Institute, 2019).
van Dijk, M., Morley, T., Rau, M. L. & Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010-2050. Nat. Food 2, 494–501 (2021).
World Resources Institute. Climate Watch Historical GHG Emissions. https://www.climatewatchdata.org/ghg-emissions (accessed 1 August 2024).
Ritchie, H. & Roser, M. Water Use and Stress. How Much Water Do We Use? How Did it Change Over Time? https://ourworldindata.org/water-use-stress (accessed 20 January 2025).
Zhao, C. et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl Acad. Sci. USA 114, 9326–9331 (2017).
Food and Agriculture Organization. The future of food and agriculture — trends and challenges. (2017).
Cassman, K. G. & Grassini, P. A global perspective on sustainable intensification research. Nat. Sustain. 3, 262–268 (2020).
Gao, C. Genome engineering for crop improvement and future agriculture. Cell 184, 1621–1635 (2021).
Grassini, P., Eskridge, K. M. & Cassman, K. G. Distinguishing between yield advances and yield plateaus in historical crop production trends. Nat. Commun. 4, 2918 (2013).
Ray, D. K., Ramankutty, N., Mueller, N. D., West, P. C. & Foley, J. A. Recent patterns of crop yield growth and stagnation. Nat. Commun. 3, 1293 (2012).
Malzahn, A., Lowder, L. & Qi, Y. Plant genome editing with TALEN and CRISPR. Cell Biosci. 7, 21 (2017).
Molla, K. A., Sretenovic, S., Bansal, K. C. & Qi, Y. Precise plant genome editing using base editors and prime editors. Nat. Plants 7, 1166–1187 (2021).
Zhang, Y., Malzahn, A. A., Sretenovic, S. & Qi, Y. The emerging and uncultivated potential of CRISPR technology in plant science. Nat. Plants 5, 778–794 (2019).
Wang, J. Y. & Doudna, J. A. CRISPR technology: a decade of genome editing is only the beginning. Science 379, eadd8643 (2023).
Food and Agriculture Organization. Gene editing and agrifood systems. (FAO, 2022).
Food and Agriculture Organization. Gene editing and food safety — technical considerations and potential relevance to the work of Codex Alimentarius. (FAO, 2023).
Tuncel, A. et al. Genome-edited foods. Nat. Rev. Bioeng. 1, 799–816 (2023).
Makarova, K. S. et al. Evolutionary classification of CRISPR–Cas systems: a burst of class 2 and derived variants. Nat. Rev. Microbiol. 18, 67–83 (2020).
Shan, Q. et al. Targeted genome modification of crop plants using a CRISPR–Cas system. Nat. Biotechnol. 31, 686–688 (2013).
Nekrasov, V., Staskawicz, B., Weigel, D., Jones, J. D. & Kamoun, S. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31, 691–693 (2013).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Nishimasu, H. et al. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 156, 935–949 (2014).
Zhu, H., Li, C. & Gao, C. Applications of CRISPR–Cas in agriculture and plant biotechnology. Nat. Rev. Mol. Cell Biol. 21, 661–677 (2020).
Agudelo, D. et al. Versatile and robust genome editing with Streptococcus thermophilus CRISPR1–Cas9. Genome Res. 30, 107–117 (2020).
Ran, F. A. et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015).
Chatterjee, P., Jakimo, N. & Jacobson, J. M. Minimal PAM specificity of a highly similar SpCas9 ortholog. Sci. Adv. 4, eaau0766 (2018).
Chatterjee, P. et al. An engineered ScCas9 with broad PAM range and high specificity and activity. Nat. Biotechnol. 38, 1154–1158 (2020).
Zhong, Z. et al. Efficient plant genome engineering using a probiotic sourced CRISPR–Cas9 system. Nat. Commun. 14, 6102 (2023).
Cui, Z. et al. FrCas9 is a CRISPR/Cas9 system with high editing efficiency and fidelity. Nat. Commun. 13, 1425 (2022).
Hirano, H. et al. Structure and engineering of Francisella novicida Cas9. Cell 164, 950–961 (2016).
Hou, Z. et al. Efficient genome engineering in human pluripotent stem cells using Cas9 from Neisseria meningitidis. Proc. Natl Acad. Sci. USA 110, 15644–15649 (2013).
Edraki, A. et al. A compact, high-accuracy Cas9 with a dinucleotide PAM for In vivo genome editing. Mol. Cell 73, 714–726 e714 (2019).
Kleinstiver, B. P. et al. Engineered CRISPR–Cas9 nucleases with altered PAM specificities. Nature 523, 481–485 (2015).
Miller, S. M. et al. Continuous evolution of SpCas9 variants compatible with non-G PAMs. Nat. Biotechnol. 38, 471–481 (2020).
Chatterjee, P. et al. A Cas9 with PAM recognition for adenine dinucleotides. Nat. Commun. 11, 2474 (2020).
Yamamoto, A., Ishida, T., Yoshimura, M., Kimura, Y. & Sawa, S. Developing heritable mutations in Arabidopsis thaliana using a modified CRISPR/Cas9 toolkit comprising PAM-altered Cas9 variants and gRNAs. Plant. Cell Physiol. 60, 2255–2262 (2019).
Sretenovic, S. et al. Expanding plant genome-editing scope by an engineered iSpyMacCas9 system that targets A-rich PAM sequences. Plant. Commun. 2, 100101 (2021).
Hu, X. et al. Expanding the range of CRISPR/Cas9 genome editing in rice. Mol. Plant. 9, 943–945 (2016).
Hu, X., Meng, X., Liu, Q., Li, J. & Wang, K. Increasing the efficiency of CRISPR–Cas9–VQR precise genome editing in rice. Plant. Biotechnol. J. 16, 292–297 (2018).
Hu, J. H. et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature 556, 57–63 (2018).
Nishimasu, H. et al. Engineered CRISPR–Cas9 nuclease with expanded targeting space. Science 361, 1259–1262 (2018).
Walton, R. T., Christie, K. A., Whittaker, M. N. & Kleinstiver, B. P. Unconstrained genome targeting with near-PAMless engineered CRISPR–Cas9 variants. Science 368, 290–296 (2020).
Ren, B. et al. Cas9-NG greatly expands the targeting scope of the genome-editing toolkit by recognizing NG and other atypical PAMs in rice. Mol. Plant. 12, 1015–1026 (2019).
Ren, J. et al. Expanding the scope of genome editing with SpG and SpRY variants in rice. Sci. China Life Sci 64, 1784–1787 (2021).
Endo, M. et al. Genome editing in plants by engineered CRISPR–Cas9 recognizing NG PAM. Nat. Plants 5, 14–17 (2019).
Hua, K., Tao, X., Han, P., Wang, R. & Zhu, J. K. Genome engineering in rice using Cas9 Variants that recognize NG PAM sequences. Mol. Plant. 12, 1003–1014 (2019).
Zhong, Z. et al. Improving plant genome editing with high-fidelity xCas9 and non-canonical PAM-targeting Cas9-NG. Mol. Plant. 12, 1027–1036 (2019).
Xu, Z. et al. SpRY greatly expands the genome editing scope in rice with highly flexible PAM recognition. Genome Biol. 22, 6 (2021).
Wang, J. et al. xCas9 expands the scope of genome editing with reduced efficiency in rice. Plant. Biotechnol. J. 17, 709–711 (2019).
Ge, Z. et al. Engineered xCas9 and SpCas9-NG variants broaden PAM recognition sites to generate mutations in Arabidopsis plants. Plant. Biotechnol. J. 17, 1865–1867 (2019).
Niu, Q. et al. Expanding the scope of CRISPR/Cas9-mediated genome editing in plants using an xCas9 and Cas9-NG hybrid. J. Integr. Plant. Biol. 62, 398–402 (2020).
Zhang, C. et al. Genome engineering in plant using an efficient CRISPR-xCas9 toolset with an expanded PAM compatibility. Front. Genome Ed. 2, 618385 (2020).
Ren, Q. et al. PAM-less plant genome editing using a CRISPR-SpRY toolbox. Nat. Plants 7, 25–33 (2021).
Yan, W. X. et al. Functionally diverse type V CRISPR–Cas systems. Science 363, 88–91 (2019).
Zetsche, B. et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR–Cas system. Cell 163, 759–771 (2015).
Yin, X. et al. CRISPR–Cas9 and CRISPR–Cpf1 mediated targeting of a stomatal developmental gene EPFL9 in rice. Plant. Cell Rep. 36, 745–757 (2017).
Tang, X. et al. A CRISPR–Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat. Plants 3, 17018 (2017).
Kim, H. et al. CRISPR/Cpf1-mediated DNA-free plant genome editing. Nat. Commun. 8, 14406 (2017).
Endo, A., Masafumi, M., Kaya, H. & Toki, S. Efficient targeted mutagenesis of rice and tobacco genomes using Cpf1 from Francisella novicida. Sci. Rep. 6, 38169 (2016).
Xu, R. et al. Generation of targeted mutant rice using a CRISPR–Cpf1 system. Plant. Biotechnol. J. 15, 713–717 (2017).
Wang, M., Mao, Y., Lu, Y., Tao, X. & Zhu, J. K. Multiplex gene editing in rice using the CRISPR–Cpf1 system. Mol. Plant. 10, 1011–1013 (2017).
Begemann, M. B. et al. Precise insertion and guided editing of higher plant genomes using Cpf1 CRISPR nucleases. Sci. Rep. 7, 11606 (2017).
Hu, X., Wang, C., Liu, Q., Fu, Y. & Wang, K. Targeted mutagenesis in rice using CRISPR–Cpf1 system. J. Genet. Genomics 44, 71–73 (2017).
Malzahn, A. A. et al. Application of CRISPR–Cas12a temperature sensitivity for improved genome editing in rice, maize, and Arabidopsis. BMC Biol. 17, 9 (2019).
Bernabe-Orts, J. M. et al. Assessment of Cas12a-mediated gene editing efficiency in plants. Plant. Biotechnol. J. 17, 1971–1984 (2019).
Lee, K. et al. Activities and specificities of CRISPR/Cas9 and Cas12a nucleases for targeted mutagenesis in maize. Plant. Biotechnol. J. 17, 362–372 (2019).
Zhang, Y. et al. Expanding the scope of plant genome engineering with Cas12a orthologs and highly multiplexable editing systems. Nat. Commun. 12, 1944 (2021).
Li, G., Zhang, Y., Dailey, M. & Qi, Y. Hs1Cas12a and Ev1Cas12a confer efficient genome editing in plants. Front. Genome Ed. 5, 1251903 (2023).
Gao, L. et al. Engineered Cpf1 variants with altered PAM specificities. Nat. Biotechnol. 35, 789–792 (2017).
Zhong, Z. et al. Plant genome editing using FnCpf1 and LbCpf1 nucleases at redefined and altered PAM sites. Mol. Plant. 11, 999–1002 (2018).
Zhang, L. et al. Boosting genome editing efficiency in human cells and plants with novel LbCas12a variants. Genome Biol. 24, 102 (2023).
Schindele, P. & Puchta, H. Engineering CRISPR/LbCas12a for highly efficient, temperature-tolerant plant gene editing. Plant. Biotechnol. J. 18, 1118–1120 (2020).
Xin, C. et al. Enhanced editing efficiency in Arabidopsis with a LbCas12a variant harboring D156R and E795L mutations. aBIOTECH 5, 117–126 (2024).
Strecker, J. et al. Engineering of CRISPR–Cas12b for human genome editing. Nat. Commun. 10, 212 (2019).
Ming, M. et al. CRISPR–Cas12b enables efficient plant genome engineering. Nat. Plants 6, 202–208 (2020).
Wu, F. et al. Targeted mutagenesis in Arabidopsis thaliana using CRISPR–Cas12b/C2c1. J. Integr. Plant. Biol. 62, 1653–1658 (2020).
Duan, Z. et al. An engineered Cas12i nuclease that is an efficient genome editing tool in animals and plants. Innovation 5, 100564 (2024).
Han, X., Chen, Y., Liu, R., Zhu, J.-K. & Duan, Z. Engineering hfCas12Max for improved gene editing efficiency. Innov. Life 2, 100067 (2024).
Pausch, P. et al. CRISPR–CasΦ from huge phages is a hypercompact genome editor. Science 369, 333–337 (2020).
Pausch, P. et al. DNA interference states of the hypercompact CRISPR–CasΦ effector. Nat. Struct. Mol. Biol. 28, 652–661 (2021).
Duan, Z. et al. Molecular basis for DNA cleavage by the hypercompact Cas12j-SF05. Cell Discov. 9, 117 (2023).
Bigelyte, G. et al. Miniature type V-F CRISPR–Cas nucleases enable targeted DNA modification in cells. Nat. Commun. 12, 6191 (2021).
Kim, D. Y. et al. Efficient CRISPR editing with a hypercompact Cas12f1 and engineered guide RNAs delivered by adeno-associated virus. Nat. Biotechnol. 40, 94–102 (2022).
Hino, T. et al. An AsCas12f-based compact genome-editing tool derived by deep mutational scanning and structural analysis. Cell 186, 4920–4935 e4923 (2023).
Xu, X. et al. Engineered miniature CRISPR–Cas system for mammalian genome regulation and editing. Mol. Cell 81, 4333–4345 e4334 (2021).
Xiang, G. et al. Evolutionary mining and functional characterization of TnpB nucleases identify efficient miniature genome editors. Nat. Biotechnol. 42, 745–757 (2024).
Karvelis, T. et al. Transposon-associated TnpB is a programmable RNA-guided DNA endonuclease. Nature 599, 692–696 (2021).
Li, Q. et al. Genome editing in plants using the TnpB transposase system. aBIOTECH 5, 225–230 (2024).
Karmakar, S. et al. A miniature alternative to Cas9 and Cas12: transposon-associated TnpB mediates targeted genome editing in plants. Plant Biotechnol. J. 22, 2950–2953 (2024).
Zhang, R. et al. IsDge10 is a hypercompact TnpB nuclease that confers efficient genome editing in rice. Plant Commun. 5, 101068 (2024).
Abudayyeh, O. O. et al. RNA targeting with CRISPR–Cas13. Nature 550, 280–284 (2017).
Konermann, S. et al. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell 173, 665–676 e614 (2018).
Mahas, A., Aman, R. & Mahfouz, M. CRISPR–Cas13d mediates robust RNA virus interference in plants. Genome Biol. 20, 263 (2019).
Aman, R. et al. RNA virus interference via CRISPR/Cas13a system in plants. Genome Biol. 19, 1 (2018).
Sharma, V. K. et al. CRISPR guides induce gene silencing in plants in the absence of Cas. Genome Biol. 23, 6 (2022).
Brouns, S. J. et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321, 960–964 (2008).
Osakabe, K. et al. Genome editing in plants using CRISPR type I-D nuclease. Commun. Biol. 3, 648 (2020).
Li, Y. et al. Targeted large fragment deletion in plants using paired crRNAs with type I CRISPR system. Plant. Biotechnol. J. 21, 2196–2208 (2023).
Li, S., Zhang, Y., Xia, L. & Qi, Y. CRISPR–Cas12a enables efficient biallelic gene targeting in rice. Plant. Biotechnol. J. 18, 1351–1353 (2020).
Wolter, F. & Puchta, H. In planta gene targeting can be enhanced by the use of CRISPR/Cas12a. Plant. J. 100, 1083–1094 (2019).
Atkins, P. A. & Voytas, D. F. Overcoming bottlenecks in plant gene editing. Curr. Opin. Plant. Biol. 54, 79–84 (2020).
Cermak, T., Baltes, N. J., Cegan, R., Zhang, Y. & Voytas, D. F. High-frequency, precise modification of the tomato genome. Genome Biol. 16, 232 (2015).
Gil-Humanes, J. et al. High-efficiency gene targeting in hexaploid wheat using DNA replicons and CRISPR/Cas9. Plant. J. 89, 1251–1262 (2017).
Barone, P. et al. Efficient gene targeting in maize using inducible CRISPR–Cas9 and marker-free donor template. Mol. Plant. 13, 1219–1227 (2020).
Wolter, F., Klemm, J. & Puchta, H. Efficient in planta gene targeting in Arabidopsis using egg cell-specific expression of the Cas9 nuclease of Staphylococcus aureus. Plant. J. 94, 735–746 (2018).
Miki, D., Zhang, W., Zeng, W., Feng, Z. & Zhu, J. K. CRISPR/Cas9-mediated gene targeting in Arabidopsis using sequential transformation. Nat. Commun. 9, 1967 (2018).
Sun, Y. et al. Engineering herbicide-resistant rice plants through CRISPR/Cas9-mediated homologous recombination of acetolactate synthase. Mol. Plant. 9, 628–631 (2016).
Qi, Y. et al. Increasing frequencies of site-specific mutagenesis and gene targeting in Arabidopsis by manipulating DNA repair pathways. Genome Res. 23, 547–554 (2013).
Schreiber, T. et al. Efficient scar-free knock-ins of several kilobases in plants by engineered CRISPR–Cas endonucleases. Mol. Plant. 17, 824–837 (2024).
Vu, T. V. et al. Highly efficient homology-directed repair using CRISPR/Cpf1-geminiviral replicon in tomato. Plant. Biotechnol. J. 18, 2133–2143 (2020).
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016).
Gaudelli, N. M. et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017).
Li, B., Sun, C., Li, J. & Gao, C. Targeted genome-modification tools and their advanced applications in crop breeding. Nat. Rev. Genet. 25, 603–622 (2024).
Huang, J. et al. Discovery of deaminase functions by structure-based protein clustering. Cell 186, 3182–3195 e3114 (2023).
Sretenovic, S. et al. Exploring C-to-G base editing in rice, tomato, and poplar. Front. Genome Ed. 3, 756766 (2021).
Li, Y. et al. Engineering a plant A-to-K base editor with improved performance by fusion with a transactivation module. Plant. Commun. 4, 100667 (2023).
Liu, L. et al. Developing guanine base editors for G-to-T editing in rice. J. Integr. Plant. Biol. 66, 1557–1560 (2024).
Li, C. et al. Targeted, random mutagenesis of plant genes with dual cytosine and adenine base editors. Nat. Biotechnol. 38, 875–882 (2020).
Li, C. et al. SWISS: multiplexed orthogonal genome editing in plants with a Cas9 nickase and engineered CRISPR RNA scaffolds. Genome Biol. 21, 141 (2020).
Zhang, A. et al. Directed evolution rice genes with randomly multiplexed sgRNAs assembly of base editors. Plant. Biotechnol. J. 21, 2597–2610 (2023).
Neugebauer, M. E. et al. Evolution of an adenine base editor into a small, efficient cytosine base editor with low off-target activity. Nat. Biotechnol. 41, 673–685 (2023).
Fan, T. et al. High performance TadA-8e derived cytosine and dual base editors with undetectable off-target effects in plants. Nat. Commun. 15, 5103 (2024).
Gaillochet, C. et al. Systematic optimization of Cas12a base editors in wheat and maize using the ITER platform. Genome Biol. 24, 6 (2023).
Cheng, Y. et al. CRISPR–Cas12a base editors confer efficient multiplexed genome editing in rice. Plant. Commun. 4, 100601 (2023).
Jin, S. et al. Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice. Science 364, 292–295 (2019).
Grunewald, J. et al. CRISPR DNA base editors with reduced RNA off-target and self-editing activities. Nat. Biotechnol. 37, 1041–1048 (2019).
Grunewald, J. et al. Transcriptome-wide off-target RNA editing induced by CRISPR-guided DNA base editors. Nature 569, 433–437 (2019).
Anzalone, A. V., Koblan, L. W. & Liu, D. R. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38, 824–844 (2020).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019).
Lin, Q. et al. Prime genome editing in rice and wheat. Nat. Biotechnol. 38, 582–585 (2020).
Jiang, Y. et al. Optimized prime editing efficiently generates glyphosate-resistant rice plants carrying homozygous TAP-IVS mutation in EPSPS. Mol. Plant. 15, 1646–1649 (2022).
Zong, Y. et al. An engineered prime editor with enhanced editing efficiency in plants. Nat. Biotechnol. 40, 1394–1402 (2022).
Lin, Q. et al. High-efficiency prime editing with optimized, paired pegRNAs in plants. Nat. Biotechnol. 39, 923–927 (2021).
Liu, X. et al. Conditional knockdown of OsMLH1 to improve plant prime editing systems without disturbing fertility in rice. Genome Biol. 25, 131 (2024).
Cao, Z. et al. PE6c greatly enhances prime editing in transgenic rice plants. J. Integr. Plant Biol. 66, 1864–1870 (2024).
Xu, R. et al. Engineering PE6 prime editors to efficiently insert tags in rice. Plant Biotechnol. J. 22, 3383–3385 (2024).
Ni, P. et al. Efficient and versatile multiplex prime editing in hexaploid wheat. Genome Biol. 24, 156 (2023).
Vu, T. V. et al. Optimized dicot prime editing enables heritable desired edits in tomato and Arabidopsis. Nat. Plants 10, 1502–1513 (2024).
Anzalone, A. V. et al. Programmable deletion, replacement, integration and inversion of large DNA sequences with twin prime editing. Nat. Biotechnol. 40, 731–740 (2022).
Sun, C. et al. Precise integration of large DNA sequences in plant genomes using PrimeRoot editors. Nat. Biotechnol. 42, 316–327 (2024).
Liu, P. et al. Transposase-assisted target-site integration for efficient plant genome engineering. Nature 631, 593–600 (2024).
Gallego-Bartolome, J. et al. Co-targeting RNA polymerases IV and V promotes efficient de novo DNA methylation in Arabidopsis. Cell 176, 1068–1082 e1019 (2019).
Papikian, A., Liu, W., Gallego-Bartolome, J. & Jacobsen, S. E. Site-specific manipulation of Arabidopsis loci using CRISPR–Cas9 SunTag systems. Nat. Commun. 10, 729 (2019).
Ghoshal, B., Picard, C. L., Vong, B., Feng, S. & Jacobsen, S. E. CRISPR-based targeting of DNA methylation in Arabidopsis thaliana by a bacterial CG-specific DNA methyltransferase. Proc. Natl Acad. Sci. USA 118, e2125016118 (2021).
Oberkofler, V. & Baurle, I. Inducible epigenome editing probes for the role of histone H3K4 methylation in Arabidopsis heat stress memory. Plant. Physiol. 189, 703–714 (2022).
Nunez, J. K. et al. Genome-wide programmable transcriptional memory by CRISPR-based epigenome editing. Cell 184, 2503–2519 e2517 (2021).
Liu, S. et al. Hypercompact CRISPR–Cas12j2 (CasΦ) enables genome editing, gene activation, and epigenome editing in plants. Plant. Commun. 3, 100453 (2022).
Tang, J., Chen, S. & Jia, G. Detection, regulation, and functions of RNA N6-methyladenosine modification in plants. Plant. Commun. 4, 100546 (2023).
Liu, X. M., Zhou, J., Mao, Y., Ji, Q. & Qian, S. B. Programmable RNA N6-methyladenosine editing by CRISPR–Cas9 conjugates. Nat. Chem. Biol. 15, 865–871 (2019).
Wilson, C., Chen, P. J., Miao, Z. & Liu, D. R. Programmable m6A modification of cellular RNAs with a Cas13-directed methyltransferase. Nat. Biotechnol. 38, 1431–1440 (2020).
Li, J. et al. Targeted mRNA demethylation using an engineered dCas13b–ALKBH5 fusion protein. Nucleic Acids Res. 48, 5684–5694 (2020).
Shi, C. et al. Programmable RNA N6-methyladenosine editing with CRISPR/dCas13a in plants. Plant. Biotechnol. J. 22, 1867–1880 (2024).
Amabile, A. et al. Inheritable silencing of endogenous genes by hit-and-run targeted epigenetic editing. Cell 167, 219–232 e214 (2016).
Cappelluti, M. A. et al. Durable and efficient gene silencing in vivo by hit-and-run epigenome editing. Nature 627, 416–423 (2024).
Piatek, A. et al. RNA-guided transcriptional regulation in planta via synthetic dCas9-based transcription factors. Plant. Biotechnol. J. 13, 578–589 (2015).
Lowder, L. G. et al. A CRISPR/Cas9 toolbox for multiplexed plant genome editing and transcriptional regulation. Plant. Physiol. 169, 971–985 (2015).
Lowder, L. G. et al. Robust transcriptional activation in plants using multiplexed CRISPR-Act2.0 and mTALE-Act systems. Mol. Plant. 11, 245–256 (2018).
Li, Z. et al. A potent Cas9-derived gene activator for plant and mammalian cells. Nat. Plants 3, 930–936 (2017).
Selma, S. et al. Strong gene activation in plants with genome-wide specificity using a new orthogonal CRISPR/Cas9-based programmable transcriptional activator. Plant. Biotechnol. J. 17, 1703–1705 (2019).
Casas-Mollano, J. A., Zinselmeier, M. H., Sychla, A. & Smanski, M. J. Efficient gene activation in plants by the MoonTag programmable transcriptional activator. Nucleic Acids Res. 51, 7083–7093 (2023).
Pan, C. et al. CRISPR-Act3.0 for highly efficient multiplexed gene activation in plants. Nat. Plants 7, 942–953 (2021).
Pan, C. et al. Boosting plant genome editing with a versatile CRISPR-Combo system. Nat. Plants 8, 513–525 (2022).
Pan, C. & Qi, Y. CRISPR-Combo-mediated orthogonal genome editing and transcriptional activation for plant breeding. Nat. Protoc. 18, 1760–1794 (2023).
Chen, Z., Debernardi, J. M., Dubcovsky, J. & Gallavotti, A. Recent advances in crop transformation technologies. Nat. Plants 8, 1343–1351 (2022).
Chen, L. et al. A method for the production and expedient screening of CRISPR/Cas9-mediated non-transgenic mutant plants. Hortic. Res. 5, 13 (2018).
Banfalvi, Z., Csakvari, E., Villanyi, V. & Kondrak, M. Generation of transgene-free PDS mutants in potato by Agrobacterium-mediated transformation. BMC Biotechnol. 20, 25 (2020).
Huang, X. et al. Transgene-free genome editing of vegetatively propagated and perennial plant species in the T0 generation via a co-editing strategy. Nat. Plants 9, 1591–1597 (2023).
Nishizawa-Yokoi, A. & Toki, S. A piggyBac-mediated transgenesis system for the temporary expression of CRISPR/Cas9 in rice. Plant. Biotechnol. J. 19, 1386–1395 (2021).
Svitashev, S., Schwartz, C., Lenderts, B., Young, J. K. & Mark Cigan, A. Genome editing in maize directed by CRISPR–Cas9 ribonucleoprotein complexes. Nat. Commun. 7, 13274 (2016).
Liang, Z. et al. Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat. Commun. 8, 14261 (2017).
Liu, J. et al. Genome-scale sequence disruption following biolistic transformation in rice and maize. Plant. Cell 31, 368–383 (2019).
Hamada, H. et al. Biolistic-delivery-based transient CRISPR/Cas9 expression enables in planta genome editing in wheat. Sci. Rep. 8, 14422 (2018).
Imai, R. et al. In planta particle bombardment (iPB): a new method for plant transformation and genome editing. Plant. Biotechnol. 37, 171–176 (2020).
Li, J. F. et al. Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat. Biotechnol. 31, 688–691 (2013).
Laforest, L. C. & Nadakuduti, S. S. Advances in delivery mechanisms of CRISPR gene-editing reagents in plants. Front. Genome Ed. 4, 830178 (2022).
Woo, J. W. et al. DNA-free genome editing in plants with preassembled CRISPR–Cas9 ribonucleoproteins. Nat. Biotechnol. 33, 1162–1164 (2015).
Andersson, M. et al. Genome editing in potato via CRISPR–Cas9 ribonucleoprotein delivery. Physiol. Plant. 164, 378–384 (2018).
Liu, Y. et al. Establishment of a DNA-free genome editing and protoplast regeneration method in cultivated tomato (Solanum lycopersicum). Plant. Cell Rep. 41, 1843–1852 (2022).
Fossi, M., Amundson, K., Kuppu, S., Britt, A. & Comai, L. Regeneration of Solanum tuberosum plants from protoplasts induces widespread genome instability. Plant. Physiol. 180, 78–86 (2019).
Ali, Z. et al. Efficient virus-mediated genome editing in plants using the CRISPR/Cas9 system. Mol. Plant. 8, 1288–1291 (2015).
Ellison, E. E. et al. Multiplexed heritable gene editing using RNA viruses and mobile single guide RNAs. Nat. Plants 6, 620–624 (2020).
Nagalakshmi, U., Meier, N., Liu, J. Y., Voytas, D. F. & Dinesh-Kumar, S. P. High-efficiency multiplex biallelic heritable editing in Arabidopsis using an RNA virus. Plant. Physiol. 189, 1241–1245 (2022).
Liu, D. et al. Heritable base-editing in Arabidopsis using RNA viral vectors. Plant. Physiol. 189, 1920–1924 (2022).
Li, T. et al. Highly efficient heritable genome editing in wheat using an RNA virus and bypassing tissue culture. Mol. Plant. 14, 1787–1798 (2021).
Liu, D. et al. Heritable, multinucleotide deletions in plants using viral delivery of a repair exonuclease and guide RNAs. Plant. Physiol. 194, 2229–2239 (2024).
Liu, Q., Zhao, C., Sun, K., Deng, Y. & Li, Z. Engineered biocontainable RNA virus vectors for non-transgenic genome editing across crop species and genotypes. Mol. Plant. 16, 616–631 (2023).
Ma, X., Zhang, X., Liu, H. & Li, Z. Highly efficient DNA-free plant genome editing using virally delivered CRISPR–Cas9. Nat. Plants 6, 773–779 (2020).
Yang, L., Machin, F., Wang, S., Saplaoura, E. & Kragler, F. Heritable transgene-free genome editing in plants by grafting of wild-type shoots to transgenic donor rootstocks. Nat. Biotechnol. 41, 958–967 (2023).
Cao, X. et al. Cut-dip-budding delivery system enables genetic modifications in plants without tissue culture. Innovation 4, 100345 (2023).
Rodriguez-Leal, D., Lemmon, Z. H., Man, J., Bartlett, M. E. & Lippman, Z. B. Engineering quantitative trait variation for crop improvement by genome editing. Cell 171, 470–480.e478 (2017).
Liu, L. et al. Enhancing grain-yield-related traits by CRISPR–Cas9 promoter editing of maize CLE genes. Nat. Plants 7, 287–294 (2021).
Zhou, S. et al. Fine-tuning rice heading date through multiplex editing of the regulatory regions of key genes by CRISPR–Cas9. Plant. Biotechnol. J. 22, 751–758 (2024).
Zhou, J. et al. An efficient CRISPR–Cas12a promoter editing system for crop improvement. Nat. Plants 9, 588–604 (2023).
Patel-Tupper, D. et al. Multiplexed CRISPR–Cas9 mutagenesis of rice PSBS1 noncoding sequences for transgene-free overexpression. Sci. Adv. 10, eadm7452 (2024).
Yao, Q. et al. Efficient and multiplex gene upregulation in plants through CRISPR/Cas-mediated knock-in of enhancers. Mol. Plant. 17, 1472–1483 (2024).
Tanaka, M. et al. Boric acid intercepts 80S ribosome migration from AUG-stop by stabilizing eRF1. Nat. Chem. Biol. 20, 605–614 (2024).
Xu, G. et al. uORF-mediated translation allows engineered plant disease resistance without fitness costs. Nature 545, 491–494 (2017).
Zhang, H. et al. Genome editing of upstream open reading frames enables translational control in plants. Nat. Biotechnol. 36, 894–898 (2018).
Xing, S. et al. Fine-tuning sugar content in strawberry. Genome Biol. 21, 230 (2020).
Xue, C. et al. Tuning plant phenotypes by precise, graded downregulation of gene expression. Nat. Biotechnol. 41, 1758–1764 (2023).
Shen, R. et al. In-locus gene silencing in plants using genome editing. New Phytol 243, 2501–2511 (2024).
Li, Y. R. & Liu, M. J. Prevalence of alternative AUG and non-AUG translation initiators and their regulatory effects across plants. Genome Res. 30, 1418–1433 (2020).
Wang, H. et al. Genome editing of 3′ UTR-embedded inhibitory region enables generation of gene knock-up alleles in plants. Plant. Commun. 5, 100745 (2024).
Pan, C., Li, G., Bandyopadhyay, A. & Qi, Y. Guide RNA library-based CRISPR screens in plants: opportunities and challenges. Curr. Opin. Biotechnol. 79, 102883 (2023).
Gaillochet, C., Develtere, W. & Jacobs, T. B. CRISPR screens in plants: approaches, guidelines, and future prospects. Plant. Cell 33, 794–813 (2021).
Lu, Y. et al. Genome-wide targeted mutagenesis in rice using the CRISPR/Cas9 system. Mol. Plant. 10, 1242–1245 (2017).
Meng, X. et al. Construction of a genome-wide mutant library in rice using CRISPR/Cas9. Mol. Plant. 10, 1238–1241 (2017).
Xu, R., Liu, X., Li, J., Qin, R. & Wei, P. Identification of herbicide resistance OsACC1 mutations via in planta prime-editing-library screening in rice. Nat. Plants 7, 888–892 (2021).
Birchler, J. A., Yao, H. & Chudalayandi, S. Unraveling the genetic basis of hybrid vigor. Proc. Natl Acad. Sci. USA 103, 12957–12958 (2006).
Wang, C. et al. Clonal seeds from hybrid rice by simultaneous genome engineering of meiosis and fertilization genes. Nat. Biotechnol. 37, 283–286 (2019).
Khanday, I., Skinner, D., Yang, B., Mercier, R. & Sundaresan, V. A male-expressed rice embryogenic trigger redirected for asexual propagation through seeds. Nature 565, 91–95 (2019).
Marimuthu, M. P. et al. Synthetic clonal reproduction through seeds. Science 331, 876 (2011).
Liu, C. et al. Synthetic apomixis enables stable transgenerational transmission of heterotic phenotypes in hybrid rice. Plant. Commun. 4, 100470 (2023).
Wang, Y. et al. Harnessing clonal gametes in hybrid crops to engineer polyploid genomes. Nat. Genet. 56, 1075–1079 (2024).
Westermann, J., Srikant, T., Gonzalo, A., Tan, H. S. & Bomblies, K. Defective pollen tube tip growth induces neo-polyploid infertility. Science 383, eadh0755 (2024).
Kelliher, T. et al. One-step genome editing of elite crop germplasm during haploid induction. Nat. Biotechnol. 37, 287–292 (2019).
Impens, L. et al. Combining multiplex gene editing and doubled haploid technology in maize. N. Phytol. 239, 1521–1532 (2023).
Ronspies, M. et al. Massive crossover suppression by CRISPR–Cas-mediated plant chromosome engineering. Nat. Plants 8, 1153–1159 (2022).
Schmidt, C. et al. Changing local recombination patterns in Arabidopsis by CRISPR/Cas mediated chromosome engineering. Nat. Commun. 11, 4418 (2020).
Beying, N., Schmidt, C., Pacher, M., Houben, A. & Puchta, H. CRISPR–Cas9-mediated induction of heritable chromosomal translocations in Arabidopsis. Nat. Plants 6, 638–645 (2020).
Lu, Y. et al. A donor-DNA-free CRISPR/Cas-based approach to gene knock-up in rice. Nat. Plants 7, 1445–1452 (2021).
Schwartz, C. et al. CRISPR–Cas9-mediated 75.5-Mb inversion in maize. Nat. Plants 6, 1427–1431 (2020).
Hu, H. et al. Unravelling inversions: technological advances, challenges, and potential impact on crop breeding. Plant. Biotechnol. J. 22, 544–554 (2024).
Liu, J. et al. GW5 acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice. Nat. Plants 3, 17043 (2017).
Zhang, Y. et al. Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat. Commun. 7, 12617 (2016).
Wang, W. et al. Gene editing of the wheat homologs of TONNEAU1-recruiting motif encoding gene affects grain shape and weight in wheat. Plant. J. 100, 251–264 (2019).
Xu, R. et al. Rapid improvement of grain weight via highly efficient CRISPR/Cas9-mediated multiplex genome editing in rice. J. Genet. Genomics 43, 529–532 (2016).
Zhou, J. et al. Multiplex QTL editing of grain-related genes improves yield in elite rice varieties. Plant. Cell Rep. 38, 475–485 (2019).
Kwon, C. T. et al. Rapid customization of Solanaceae fruit crops for urban agriculture. Nat. Biotechnol. 38, 182–188 (2020).
Song, X. et al. IPA1 functions as a downstream transcription factor repressed by D53 in strigolactone signaling in rice. Cell Res. 27, 1128–1141 (2017).
Song, X. et al. Targeting a gene regulatory element enhances rice grain yield by decoupling panicle number and size. Nat. Biotechnol. 40, 1403–1411 (2022).
Wang, S. et al. Architecture design of cucurbit crops for enhanced productivity by a natural allele. Nat. Plants 8, 1394–1407 (2022).
Meyer, R. S. & Purugganan, M. D. Evolution of crop species: genetics of domestication and diversification. Nat. Rev. Genet. 14, 840–852 (2013).
Gasparini, K., Moreira, J. D. R., Peres, L. E. P. & Zsogon, A. De novo domestication of wild species to create crops with increased resilience and nutritional value. Curr. Opin. Plant. Biol. 60, 102006 (2021).
Li, T. et al. Domestication of wild tomato is accelerated by genome editing. Nat. Biotechnol. 36, 1160–1163 (2018).
Zsogon, A. et al. De novo domestication of wild tomato using genome editing. Nat. Biotechnol. 36, 1211–1216 (2018).
Lemmon, Z. H. et al. Rapid improvement of domestication traits in an orphan crop by genome editing. Nat. Plants 4, 766–770 (2018).
Yu, H. et al. A route to de novo domestication of wild allotetraploid rice. Cell 184, 1156–1170 e1114 (2021).
Enciso-Rodriguez, F. et al. Overcoming self-incompatibility in diploid potato using CRISPR–Cas9. Front. Plant. Sci. 10, 376 (2019).
Ye, M. et al. Generation of self-compatible diploid potato by knockout of S-RNase. Nat. Plants 4, 651–654 (2018).
Tuncel, A. & Qi, Y. CRISPR/Cas mediated genome editing in potato: past achievements and future directions. Plant. Sci. 325, 111474 (2022).
Zaidi, S. S., Mahas, A., Vanderschuren, H. & Mahfouz, M. M. Engineering crops of the future: CRISPR approaches to develop climate-resilient and disease-resistant plants. Genome Biol. 21, 289 (2020).
Gupta, A., Rico-Medina, A. & Cano-Delgado, A. I. The physiology of plant responses to drought. Science 368, 266–269 (2020).
Reuters. Kenya Lifts Ban on Genetically Modified Crops in Response to Drought. https://www.reuters.com/world/africa/kenya-lifts-ban-genetically-modified-crops-response-drought-2022-10-04/ (2022).
Shelake, R. M. et al. Engineering drought and salinity tolerance traits in crops through CRISPR-mediated genome editing: targets, tools, challenges, and perspectives. Plant. Commun. 3, 100417 (2022).
Liao, S. Y. et al. CRISPR/Cas9-induced mutagenesis of semi-rolled leaf1,2 confers curled leaf phenotype and drought tolerance by influencing protein expression patterns and ROS scavenging in rice (Oryza sativa L.). Agronomy-Basel 9, 728 (2019).
Liu, S. et al. Mapping regulatory variants controlling gene expression in drought response and tolerance in maize. Genome Biol. 21, 163 (2020).
Gabay, G. et al. Dosage differences in 12-OXOPHYTODIENOATE REDUCTASE genes modulate wheat root growth. Nat. Commun. 14, 539 (2023).
Shi, J. et al. Overexpression of ARGOS genes modifies plant sensitivity to ethylene, leading to improved drought tolerance in both Arabidopsis and Maize. Plant. Physiol. 169, 266–282 (2015).
Shi, J. et al. ARGOS8 variants generated by CRISPR–Cas9 improve maize grain yield under field drought stress conditions. Plant. Biotechnol. J. 15, 207–216 (2017).
Tian, T. et al. Genome assembly and genetic dissection of a prominent drought-resistant maize germplasm. Nat. Genet. 55, 496–506 (2023).
Mao, H. et al. Variation in cis-regulation of a NAC transcription factor contributes to drought tolerance in wheat. Mol. Plant. 15, 276–292 (2022).
Nascimento, F. D. S. et al. Gene editing for plant resistance to abiotic factors: a systematic review. Plants 12, 305 (2023).
Bowerman, A. F. et al. Potential abiotic stress targets for modern genetic manipulation. Plant. Cell 35, 139–161 (2023).
Zong, Y. et al. Efficient C-to-T base editing in plants using a fusion of nCas9 and human APOBEC3A. Nat. Biotechnol. 36, 950–953 (2018).
Butt, H. et al. Engineering herbicide resistance via prime editing in rice. Plant. Biotechnol. J. 18, 2370–2372 (2020).
Liu, X. et al. A CRISPR–Cas9-mediated domain-specific base-editing screen enables functional assessment of ACCase variants in rice. Plant. Biotechnol. J. 18, 1845–1847 (2020).
Ren, Q. et al. Improved plant cytosine base editors with high editing activity, purity, and specificity. Plant. Biotechnol. J. 19, 2052–2068 (2021).
Pacesa, M., Pelea, O. & Jinek, M. Past, present, and future of CRISPR genome editing technologies. Cell 187, 1076–1100 (2024).
Ferreira da Silva, J. et al. Click editing enables programmable genome writing using DNA polymerases and HUH endonucleases. Nat. Biotechnol. https://doi.org/10.1038/s41587-024-02324-x (2024).
Durrant, M. G. et al. Bridge RNAs direct programmable recombination of target and donor DNA. Nature 630, 984–993 (2024).
Yu, G. et al. Prediction of efficiencies for diverse prime editing systems in multiple cell types. Cell 186, 2256–2272 e2223 (2023).
Baek, M. et al. Accurate prediction of protein-nucleic acid complexes using RoseTTAFoldNA. Nat. Methods 21, 117–121 (2024).
Sulis, D. B. et al. Multiplex CRISPR editing of wood for sustainable fiber production. Science 381, 216–221 (2023).
Stokstad, E. European Parliament votes to ease regulation of gene-edited crops. Science https://doi.org/10.1126/science.zdjbra4 (2024).
Puchta, H. Regulation of gene-edited plants in Europe: from the valley of tears into the shining sun? aBIOTECH 5, 231–238 (2024).
Atimango, A. O., Wesana, J., Kalule, S. W., Verbeke, W. & De Steur, H. Genome editing in food and agriculture: from regulations to consumer perspectives. Curr. Opin. Biotechnol. 87, 103127 (2024).
Li, J. et al. Genome editing mediated by SpCas9 variants with broad non-canonical PAM compatibility in plants. Mol. Plant. 14, 352–360 (2021).
Chen, X. et al. Advancing PAM-less genome editing in soybean using CRISPR-SpRY. Hortic. Res. 11, uhae160 (2024).
Steinert, J., Schiml, S., Fauser, F. & Puchta, H. Highly efficient heritable plant genome engineering using Cas9 orthologues from Streptococcus thermophilus and Staphylococcus aureus. Plant. J. 84, 1295–1305 (2015).
Jia, H., Xu, J., Orbovic, V., Zhang, Y. & Wang, N. Editing citrus genome via SaCas9/sgRNA system. Front. Plant. Sci. 8, 2135 (2017).
Veillet, F., Kermarrec, M. P., Chauvin, L., Chauvin, J. E. & Nogue, F. CRISPR-induced indels and base editing using the Staphylococcus aureus Cas9 in potato. PLoS ONE 15, e0235942 (2020).
Kaya, H., Mikami, M., Endo, A., Endo, M. & Toki, S. Highly specific targeted mutagenesis in plants using Staphylococcus aureus Cas9. Sci. Rep. 6, 26871 (2016).
Wang, M. et al. Targeted base editing in rice with CRISPR/ScCas9 system. Plant. Biotechnol. J. 18, 1645–1647 (2020).
Xu, Y. et al. ScCas9 recognizes NNG protospacer adjacent motif in genome editing of rice. Sci. China Life Sci 63, 450–452 (2020).
Ma, G. et al. CRISPR/Sc++ -mediated genome editing in rice. J. Integr. Plant. Biol. 63, 1606–1610 (2021).
Liu, T. et al. The ScCas9++ variant expands the CRISPR toolbox for genome editing in plants. J. Integr. Plant. Biol. 63, 1611–1619 (2021).
He, Y. et al. Expanding plant genome editing scope and profiles with CRISPR-FrCas9 systems targeting palindromic TA sites. Plant Biotechnol. J. 22, 2488–2503 (2024).
Wang, H. et al. Developing a CRISPR/FrCas9 system for core promoter editing in rice. aBIOTECH 5, 189–195 (2024).
Zhang, T. et al. Establishing RNA virus resistance in plants by harnessing CRISPR immune system. Plant. Biotechnol. J. 16, 1415–1423 (2018).
Xu, R. et al. Genome editing with type II-C CRISPR–Cas9 systems from Neisseria meningitidis in rice. Plant. Biotechnol. J. 20, 350–359 (2022).
Jia, H., Orbovic, V. & Wang, N. CRISPR-LbCas12a-mediated modification of citrus. Plant. Biotechnol. J. 17, 1928–1937 (2019).
Li, B. et al. Robust CRISPR/Cpf1 (Cas12a)-mediated genome editing in allotetraploid cotton (Gossypium hirsutum). Plant. Biotechnol. J. 17, 1862–1864 (2019).
Li, S. et al. Expanding the scope of CRISPR/Cpf1-mediated genome editing in rice. Mol. Plant. 11, 995–998 (2018).
Schindele, P. et al. Enhancing gene editing and gene targeting efficiencies in Arabidopsis thaliana by using an intron-containing version of ttLbCas12a. Plant. Biotechnol. J. 21, 457–459 (2023).
Hui, F. et al. Robust CRISPR/Mb2Cas12a genome editing tools in cotton plants. Imeta 3, e209 (2024).
Wang, Q. et al. The application of a heat-inducible CRISPR/Cas12b (C2c1) genome editing system in tetraploid cotton (G. hirsutum) plants. Plant. Biotechnol. J. 18, 2436–2443 (2020).
Lv, P. et al. Genome editing in rice using CRISPR/Cas12i3. Plant. Biotechnol. J. 22, 379–385 (2024).
Li, Z. et al. Genome editing in plants using the compact editor CasΦ. Proc. Natl Acad. Sci. USA 120, e2216822120 (2023).
Sun, Y. et al. Engineer and split an efficient hypercompact CRISPR–CasΦ genome editor in plants. Plant. Commun. 5, 100881 (2024).
Ye, Z. et al. Efficient genome editing in rice with miniature Cas12f variants. aBIOTECH 5, 184–188 (2024).
Tang, X. et al. Genome editing in rice and tomato with a small Un1Cas12f1 nuclease. Plant. Genome 17, e20465 (2024).
Sukegawa, S., Nureki, O., Toki, S. & Saika, H. Genome editing in rice mediated by miniature size Cas nuclease SpCas12f. Front. Genome Ed. 5, 1138843 (2023).
Knott, G. J. et al. Broad-spectrum enzymatic inhibition of CRISPR–Cas12a. Nat. Struct. Mol. Biol. 26, 315–321 (2019).
Yang, H. & Patel, D. J. Structures, mechanisms and applications of RNA-centric CRISPR–Cas13. Nat. Chem. Biol. 20, 673–688 (2024).
Mieulet, D. et al. Turning rice meiosis into mitosis. Cell Res. 26, 1242–1254 (2016).
d’Erfurth, I. et al. Turning meiosis into mitosis. PLoS Biol. 7, e1000124 (2009).
Song, M. et al. Simultaneous production of high-frequency synthetic apomixis with high fertility and improved agronomic traits in hybrid rice. Mol. Plant. 17, 4–7 (2024).
Dan, J. et al. One-line hybrid rice with high-efficiency synthetic apomixis and near-normal fertility. Plant. Cell Rep. 43, 79 (2024).
Vernet, A. et al. High-frequency synthetic apomixis in hybrid rice. Nat. Commun. 13, 7963 (2022).
Huang, Y. et al. OsWUS-driven synthetic apomixis in hybrid rice. Plant. Commun. 6, 101136 (2024).
Lv, J. et al. Generation of paternal haploids in wheat by genome editing of the centromeric histone CENH3. Nat. Biotechnol. 38, 1397–1401 (2020).
Kelliher, T. et al. MATRILINEAL, a sperm-specific phospholipase, triggers maize haploid induction. Nature 542, 105–109 (2017).
Lowe, K. et al. Morphogenic regulators baby boom and wuschel improve monocot transformation. Plant. Cell 28, 1998–2015 (2016).
Debernardi, J. M. et al. A GRF-GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat. Biotechnol. 38, 1274–1279 (2020).
Wang, K. et al. The gene TaWOX5 overcomes genotype dependency in wheat genetic transformation. Nat. Plants 8, 110–117 (2022).
Maher, M. F. et al. Plant gene editing through de novo induction of meristems. Nat. Biotechnol. 38, 84–89 (2020).
Lian, Z. et al. Application of developmental regulators to improve in planta or in vitro transformation in plants. Plant. Biotechnol. J. 20, 1622–1635 (2022).
Author information
Authors and Affiliations
Contributions
A.T., C.P., J.S.C and Y.Q. researched data for the article, contributed substantially to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission. D.L. wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Zhenghe Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Activity window
-
Specific range of nucleotides within a target DNA sequence, where the base editor converts one nucleotide to another.
- Bystander mutations
-
Unintended genetic alterations that occur near the primary target site (often at adjacent bases within the activity window) owing to the activity of base editor.
- Cascade
-
An RNA-guided protein complex in type I CRISPR–Cas systems that recognizes target DNA sequences.
- CRISPR–Cas
-
Stands for clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein. A bacterial and archaeal adaptive immunity mechanism, in which CRISPR arrays (repeats interspersed with spacers) and Cas proteins identify and degrade viral nucleic acids.
- CRISPR RNA
-
In adaptive immunity in bacteria and archaea, a short RNA molecule that is complementary to a specific sequence of an invading nucleic acid and guides Cas proteins to cleave it.
- Crop yield potentials
-
Maximum possible agricultural outputs that can be achieved under optimal growing conditions. Represents the highest yield a crop can attain based on its genetic characteristics in the absence of limiting environmental factors.
- Filler DNA
-
Inadvertent integration of a segment(s) of the transduced plasmid DNA into the host genome at or near the site of the intended edit.
- Homology-directed repair
-
An error-free or high fidelity DNA double-strand break repair pathway, which uses a homologous template to guide repair, resulting in precise editing outcomes.
- Non-homologous end joining
-
An error-prone pathway of DNA double-strand break repair through direct joining of the DNA ends without requiring a homologous template. This process can introduce insertions or deletions of varying sizes.
- Primer-binding sequence
-
A short sequence included in the prime editing guide RNA that is complementary to the DNA strand targeted for editing; serves as the reverse transcriptase primer for initiation of DNA synthesis during the prime editing.
- Promoter bashing
-
Serial targeted deletions or mutagenesis of a promoter to optimize gene expression.
- Protospacer-adjacent motif
-
A short DNA sequence that is recognized by the Cas protein as a prerequisite for DNA targeting by a CRISPR–Cas system.
- Pyramiding mutations
-
Stacking or combining multiple beneficial genetic mutations or traits in a single organism, typically through selective breeding or genetic engineering.
- Replicons
-
DNA or RNA molecules capable of autonomous replication within a host cell, owing to harbouring sequences such as an origin of replication.
- Single guide RNA
-
(sgRNA). A fusion of CRISPR RNA and trans-activating CRISPR RNA. sgRNAs are used with certain Cas proteins such as Cas9 and Cas12b.
- Trans-activating CRISPR RNA
-
A short RNA encoded adjacent to the CRISPR locus, which can hybridize with the precursor CRISPR RNA and form a complex that guides the CRISPR-associated nuclease to the DNA target site.
- Transposase
-
An enzyme that catalyses the movement of transposable elements (or ‘jumping genes’) within the genome. It facilitates the excision of transposable elements from one location and their integration into another site in the DNA, contributing to genetic diversity.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tuncel, A., Pan, C., Clem, J.S. et al. CRISPR–Cas applications in agriculture and plant research. Nat Rev Mol Cell Biol 26, 419–441 (2025). https://doi.org/10.1038/s41580-025-00834-3
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41580-025-00834-3
This article is cited by
-
Application of the OsRPS5 promoter for CRISPR/Cas9-mediated genome editing in rice
Applied Biological Chemistry (2025)
-
Integrated biotechnological and AI innovations for crop improvement
Nature (2025)