Abstract
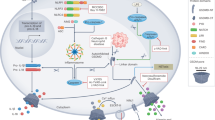
Pyroptosis, a novel mode of inflammatory cell death, is executed by membrane pore-forming gasdermin (GSDM) family members in response to extracellular or intracellular injury cues and is characterized by a ballooning cell morphology, plasma membrane rupture and the release of inflammatory mediators such as interleukin-1β (IL-1β), IL-18 and high mobility group protein B1 (HMGB1). It is a key effector mechanism for host immune defence and surveillance against invading pathogens and aberrant cancerous cells, and contributes to the onset and pathogenesis of inflammatory and autoimmune diseases. Manipulating the pore-forming activity of GSDMs and pyroptosis could lead to novel therapeutic strategies. In this Review, we discuss the current knowledge regarding how GSDM-mediated pyroptosis is initiated, executed and regulated, its roles in physiological and pathological processes, and the crosstalk between different modes of programmed cell death. We also highlight the development of drugs that target pyroptotic pathways for disease treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Friedlander, A. M. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J. Biol. Chem. 261, 7123–7126 (1986).
Zychlinsky, A., Prevost, M. C. & Sansonetti, P. J. Shigella flexneri induces apoptosis in infected macrophages. Nature 358, 167–169 (1992).
Monack, D. M., Raupach, B., Hromockyj, A. E. & Falkow, S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl Acad. Sci. USA 93, 9833–9838 (1996).
Lindgren, S. W., Stojiljkovic, I. & Heffron, F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc. Natl Acad. Sci. USA 93, 4197–4201 (1996).
Chen, Y., Smith, M. R., Thirumalai, K. & Zychlinsky, A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 15, 3853–3860 (1996).
Hilbi, H. et al. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 273, 32895–32900 (1998).
Hersh, D. et al. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl Acad. Sci. USA 96, 2396–2401 (1999).
Brennan, M. A. & Cookson, B. T. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 38, 31–40 (2000).
Cookson, B. T. & Brennan, M. A. Pro-inflammatory programmed cell death. Trends Microbiol. 9, 113–114 (2001). This article is the first to present the term inflammatory caspase-mediated cell death pyroptosis.
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 (2002).
Latz, E., Xiao, T. S. & Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411 (2013).
Broz, P. & Dixit, V. M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16, 407–420 (2016).
Barnett, K. C., Li, S., Liang, K. & Ting, J. P. A 360° view of the inflammasome: mechanisms of activation, cell death, and diseases. Cell 186, 2288–2312 (2023).
Hara, H. et al. The NLRP6 inflammasome recognizes lipoteichoic acid and regulates Gram-positive pathogen infection. Cell 175, 1651–1664.e14 (2018).
Zhao, Y. et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477, 596–600 (2011).
Kofoed, E. M. & Vance, R. E. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477, 592–595 (2011).
Rathinam, V. A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010).
Kanneganti, T. D. et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440, 233–236 (2006).
Sutterwala, F. S. et al. Critical role for NALP3/CIAS1/cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24, 317–327 (2006).
Deng, M. et al. The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in sepsis. Immunity 49, 740–753.e7 (2018).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. & Miao, E. A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Wandel, M. P. et al. Guanylate-binding proteins convert cytosolic bacteria into caspase-4 signaling platforms. Nat. Immunol. 21, 880–891 (2020).
Dickinson, M. S. et al. LPS-aggregating proteins GBP1 and GBP2 are each sufficient to enhance caspase-4 activation both in cellulo and in vitro. Proc. Natl Acad. Sci. USA 120, e2216028120 (2023).
Santos, J. C. et al. Human GBP1 binds LPS to initiate assembly of a caspase-4 activating platform on cytosolic bacteria. Nat. Commun. 11, 3276 (2020).
Santos, J. C. et al. LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation. EMBO J. 37, e98089 (2018).
Pilla, D. M. et al. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc. Natl Acad. Sci. USA 111, 6046–6051 (2014).
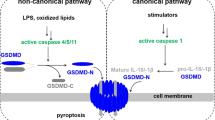
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015). Together with Shi et al. (2015), this article reports that GSDMD is cleaved and activated by inflammatory caspases, which are sufficient and required to trigger pyroptosis.
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016).
Ding, J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016).
Aglietti, R. A. et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc. Natl Acad. Sci. USA 113, 7858–7863 (2016).
Sborgi, L. et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 35, 1766–1778 (2016).
Chen, X. et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 26, 1007–1020 (2016). Together with Liu et al. (2016), Ding et al. (2016), Aglietti et al. (2016) and Sborgi et al. (2016), this article demonstrates that GSDMD-NT binds with high affinity to acidic phospholipids, oligomerizes and forms pores on plasma or organelle membranes to cause pyroptosis.
Angosto-Bazarra, D. et al. Evolutionary analyses of the gasdermin family suggest conserved roles in infection response despite loss of pore-forming functionality. BMC Biol. 20, 9 (2022).
Shi, J., Gao, W. & Shao, F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 42, 245–254 (2017).
Liu, X., Xia, S., Zhang, Z., Wu, H. & Lieberman, J. Channelling inflammation: gasdermins in physiology and disease. Nat. Rev. Drug Discov. 20, 384–405 (2021).
Liu, Y. et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci. Immunol. 5, eaax7969 (2020).
Wei, Y. et al. GSDME-mediated pyroptosis promotes the progression and associated inflammation of atherosclerosis. Nat. Commun. 14, 929 (2023).
Cao, Z. et al. Pyroptosis in diabetes and diabetic nephropathy. Clin. Chim. Acta 531, 188–196 (2022).
You, R. et al. Pyroptosis and its role in autoimmune disease: a potential therapeutic target. Front. Immunol. 13, 841732 (2022).
Kanneganti, A. et al. GSDMD is critical for autoinflammatory pathology in a mouse model of familial Mediterranean fever. J. Exp. Med. 215, 1519–1529 (2018).
Xiao, J. et al. Gasdermin D mediates the pathogenesis of neonatal-onset multisystem inflammatory disease in mice. PLoS Biol. 16, e3000047 (2018).
Liu, Z. et al. Structures of the Gasdermin D C-terminal domains reveal mechanisms of autoinhibition. Structure 26, 778–784.e3 (2018).
Liu, Z. et al. Crystal structures of the full-length murine and human gasdermin D reveal mechanisms of autoinhibition, lipid binding, and oligomerization. Immunity 51, 43–49.e4 (2019).
Lunny, D. P. et al. Mutations in gasdermin 3 cause aberrant differentiation of the hair follicle and sebaceous gland. J. Invest. Dermatol. 124, 615–621 (2005).
Porter, R. M. et al. Defolliculated (dfl): a dominant mouse mutation leading to poor sebaceous gland differentiation and total elimination of pelage follicles. J. Invest. Dermatol. 119, 32–37 (2002).
Li, J. et al. Gsdma3 is required for hair follicle differentiation in mice. Biochem. Biophys. Res. Commun. 403, 18–23 (2010).
Runkel, F. et al. The dominant alopecia phenotypes Bareskin, Rex-denuded, and Reduced Coat 2 are caused by mutations in gasdermin 3. Genomics 84, 824–835 (2004).
Tanaka, S. et al. A new Gsdma3 mutation affecting anagen phase of first hair cycle. Biochem. Biophys. Res. Commun. 359, 902–907 (2007).
Sato, H. et al. A new mutation Rim3 resembling Re(den) is mapped close to retinoic acid receptor ɑ (Rara) gene on mouse chromosome 11. Mamm. Genome 9, 20–25 (1998).
Lin, P. H., Lin, H. Y., Kuo, C. C. & Yang, L. T. N-terminal functional domain of gasdermin A3 regulates mitochondrial homeostasis via mitochondrial targeting. J. Biomed. Sci. 22, 44 (2015).
Zhou, Y. et al. Gsdma3 mutation causes bulge stem cell depletion and alopecia mediated by skin inflammation. Am. J. Pathol. 180, 763–774 (2012).
Kumar, S. et al. Gsdma3I359N is a novel ENU-induced mutant mouse line for studying the function of Gasdermin A3 in the hair follicle and epidermis. J. Dermatol. Sci. 67, 190–192 (2012).
Deng, W. et al. Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis. Nature 602, 496–502 (2022).
LaRock, D. L. et al. Group A Streptococcus induces GSDMA-dependent pyroptosis in keratinocytes. Nature 605, 527–531 (2022). Together with Deng et al. (2022), this article reports that SpeB cleaves and activates GSDMA to initiate pyroptosis in keratinocytes.
Katoh, M. & Katoh, M. Evolutionary recombination hotspot around GSDML–GSDM locus is closely linked to the oncogenomic recombination hotspot around the PPP1R1B–ERBB2–GRB7 amplicon. Int. J. Oncol. 24, 757–763 (2004).
Zhong, X. et al. Structural mechanisms for regulation of GSDMB pore-forming activity. Nature 616, 598–605 (2023).
Wang, C. et al. Structural basis for GSDMB pore formation and its targeting by IpaH7.8. Nature 616, 590–597 (2023).
Kong, Q. et al. Alternative splicing of GSDMB modulates killer lymphocyte-triggered pyroptosis. Sci. Immunol. 8, eadg3196 (2023).
Zhou, Z. et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 368, eaaz7548 (2020).
Voskoboinik, I., Whisstock, J. C. & Trapani, J. A. Perforin and granzymes: function, dysfunction and human pathology. Nat. Rev. Immunol. 15, 388–400 (2015).
Hou, J. et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 22, 1264–1275 (2020).
Zhang, J.-y et al. The metabolite α-KG induces GSDMC-dependent pyroptosis through death receptor 6-activated caspase-8. Cell Res. 31, 980–997 (2021).
Yang, L. et al. Intraepithelial mast cells drive gasdermin C-mediated type 2 immunity. Immunity 57, 1056–1070.e5 (2024).
Orning, P. et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 362, 1064–1069 (2018).
Sarhan, J. et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl Acad. Sci. USA 115, E10888–E10897 (2018).
Zhang, H. et al. TMEM173 drives lethal coagulation in sepsis. Cell Host Microbe 27, 556–570.e6 (2020).
Schwarzer, R., Jiao, H., Wachsmuth, L., Tresch, A. & Pasparakis, M. FADD and caspase-8 regulate gut homeostasis and inflammation by controlling MLKL- and GSDMD-mediated death of intestinal epithelial cells. Immunity 52, 978–993.e6 (2020).
Hughes, S. A. et al. Caspase-8-driven apoptotic and pyroptotic crosstalk causes cell death and IL-1β release in X-linked inhibitor of apoptosis (XIAP) deficiency. EMBO J. 42, e110468 (2023).
Wu, S. et al. Targeting STING elicits GSDMD-dependent pyroptosis and boosts anti-tumor immunity in renal cell carcinoma. Oncogene 43, 1534–1548 (2024).
Kambara, H. et al. Gasdermin D exerts anti-inflammatory effects by promoting neutrophil death. Cell Rep. 22, 2924–2936 (2018).
Burgener, S. S. et al. Cathepsin G inhibition by Serpinb1 and Serpinb6 prevents programmed necrosis in neutrophils and monocytes and reduces GSDMD-driven inflammation. Cell Rep. 27, 3646–3656.e5 (2019).
Karmakar, M. et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat. Commun. 11, 2212 (2020).
Du, G. et al. ROS-dependent S-palmitoylation activates cleaved and intact gasdermin D. Nature 630, 437–446 (2024).
Chen, W. et al. Allergen protease-activated stress granule assembly and gasdermin D fragmentation control interleukin-33 secretion. Nat. Immunol. 23, 1021–1030 (2022).
Van Laer, L. et al. Nonsyndromic hearing impairment is associated with a mutation in DFNA5. Nat. Genet. 20, 194–197 (1998).
Park, H. J. et al. Evidence for a founder mutation causing DFNA5 hearing loss in East Asians. J. Hum. Genet. 55, 59–62 (2010).
Cheng, J. et al. A novel DFNA5 mutation, IVS8 + 4 A>G, in the splice donor site of intron 8 causes late-onset non-syndromic hearing loss in a Chinese family. Clin. Genet. 72, 471–477 (2007).
Bischoff, A. M. et al. A novel mutation identified in the DFNA5 gene in a Dutch family: a clinical and genetic evaluation. Audiol. Neurootol. 9, 34–46 (2004).
Yu, C. et al. A 3-nucleotide deletion in the polypyrimidine tract of intron 7 of the DFNA5 gene causes nonsyndromic hearing impairment in a Chinese family. Genomics 82, 575–579 (2003).
Op de Beeck, K. et al. The DFNA5 gene, responsible for hearing loss and involved in cancer, encodes a novel apoptosis-inducing protein. Eur. J. Hum. Genet. 19, 965–973 (2011).
Van Rossom, S. et al. The splicing mutant of the human tumor suppressor protein DFNA5 induces programmed cell death when expressed in the yeast Saccharomyces cerevisiae. Front. Oncol. 2, 77 (2012).
Nadol, J. B. Jr, Handzel, O. & Amr, S. Histopathology of the human inner ear in a patient with sensorineural hearing loss caused by a variant in DFNA5. Otol. Neurotol. 36, 1616–1621 (2015).
Zhou, B. et al. Full-length GSDME mediates pyroptosis independent from cleavage. Nat. Cell Biol. 26, 1545–1557 (2024).
Wang, Y. et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 (2017).
Rogers, C. et al. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 8, 14128 (2017). Together with Wang et al. (2017), this study reports that apoptotic caspase 3 mediates GSDME cleavage and activation, converting apoptosis into pyroptosis.
Zhang, Z. et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579, 415–420 (2020). Together with Zhou et al. (2020), this article reports that cytotoxic granzymes produced by killer lymphocytes cleave and activate GSDMs to induce tumour cell pyroptosis, which increases host antitumour immunity.
Billman, Z. P. et al. Caspase-1 activates gasdermin A in non-mammals. eLife 12, RP92362 (2024).
Jiang, S., Zhou, Z., Sun, Y., Zhang, T. & Sun, L. Coral gasdermin triggers pyroptosis. Sci. Immunol. 5, eabd2591 (2020).
Johnson, A. G. et al. Bacterial gasdermins reveal an ancient mechanism of cell death. Science 375, 221–225 (2022).
Johnson, A. G. et al. Structure and assembly of a bacterial gasdermin pore. Nature 628, 657–663 (2024).
Li, Y. et al. Cleavage-independent activation of ancient eukaryotic gasdermins and structural mechanisms. Science 384, adm9190 (2024). Together with Du et al. (2024) and Zhou et al. (2024), this article describes alternative activation of GSDM in a cleavage-independent manner.
Clave, C. et al. Fungal gasdermin-like proteins are controlled by proteolytic cleavage. Proc. Natl Acad. Sci. USA 119, e2109418119 (2022).
Miao, R. et al. Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis. Immunity 56, 2523–2541.e8 (2023).
Kondolf, H. C., D’Orlando, D. A., Dubyak, G. R. & Abbott, D. W. Protein engineering reveals that gasdermin A preferentially targets mitochondrial membranes over the plasma membrane during pyroptosis. J. Biol. Chem. 299, 102908 (2023).
van Meer, G., Voelker, D. R. & Feigenson, G. W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124 (2008).
Lorent, J. H. et al. Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat. Chem. Biol. 16, 644–652 (2020).
Mari, S. A. et al. Gasdermin-A3 pore formation propagates along variable pathways. Nat. Commun. 13, 2609 (2022).
Mulvihill, E. et al. Mechanism of membrane pore formation by human gasdermin-D. EMBO J. 37, e98321 (2018).
Liu, Y. et al. Visualization of perforin/gasdermin/complement-formed pores in real cell membranes using atomic force microscopy. Cell Mol. Immunol. 16, 611–620 (2019).
Ruan, J., Xia, S., Liu, X., Lieberman, J. & Wu, H. Cryo-EM structure of the gasdermin A3 membrane pore. Nature 557, 62–67 (2018).
Xia, S. et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 593, 607–611 (2021). Together with Zhong, X. et al. (2023), Wang, C. et al. (2023), Johnson et al. (2024), Li et al. (2024) and Ruan et al. (2018), this article describes the GSDM membrane pore structure resolved via cryo electron microscopy.
Akino, K. et al. Identification of DFNA5 as a target of epigenetic inactivation in gastric cancer. Cancer Sci. 98, 88–95 (2007).
Kim, M. S. et al. Aberrant promoter methylation and tumor suppressive activity of the DFNA5 gene in colorectal carcinoma. Oncogene 27, 3624–3634 (2008).
Saeki, N. et al. GASDERMIN, suppressed frequently in gastric cancer, is a target of LMO1 in TGF-β-dependent apoptotic signalling. Oncogene 26, 6488–6498 (2007).
Kothari, P. H. et al. Role of local CpG DNA methylation in mediating the 17q21 asthma susceptibility gasdermin B (GSDMB)/ORMDL sphingolipid biosynthesis regulator 3 (ORMDL3) expression quantitative trait locus. J. Allergy Clin. Immunol. 141, 2282–2286.e6 (2018).
Ning, H. et al. Enhancer decommissioning by MLL4 ablation elicits dsRNA–interferon signaling and GSDMD-mediated pyroptosis to potentiate anti-tumor immunity. Nat. Commun. 13, 6578 (2022).
Oltra, S. S. et al. Distinct GSDMB protein isoforms and protease cleavage processes differentially control pyroptotic cell death and mitochondrial damage in cancer cells. Cell Death Differ. 30, 1366–1381 (2023).
Croes, L. et al. Large-scale analysis of DFNA5 methylation reveals its potential as biomarker for breast cancer. Clin. Epigenetics 10, 51 (2018).
Hansen, J. M. et al. Pathogenic ubiquitination of GSDMB inhibits NK cell bactericidal functions. Cell 184, 3178–3191.e18 (2021).
Taabazuing, C. Y., Okondo, M. C. & Bachovchin, D. A. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem. Biol. 24, 507–514.e4 (2017).
Lei, X. et al. Enterovirus 71 inhibits pyroptosis through cleavage of Gasdermin D. J. Virol. https://doi.org/10.1128/JVI.01069-17 (2017).
Luchetti, G. et al. Shigella ubiquitin ligase IpaH7.8 targets gasdermin D for degradation to prevent pyroptosis and enable infection. Cell Host Microbe 29, 1521–1530.e10 (2021).
Gao, W. et al. TRIM21 regulates pyroptotic cell death by promoting gasdermin D oligomerization. Cell Death Differ. 29, 439–450 (2022).
Margheritis, E. et al. Gasdermin D cysteine residues synergistically control its palmitoylation-mediated membrane targeting and assembly. EMBO J. 43, 4274–4297 (2024).
Liu, Z. et al. Palmitoylation at a conserved cysteine residue facilitates gasdermin D-mediated pyroptosis and cytokine release. Proc. Natl Acad. Sci. USA 121, e2400883121 (2024).
Zhang, N. et al. A palmitoylation–depalmitoylation relay spatiotemporally controls GSDMD activation in pyroptosis. Nat. Cell Biol. 26, 757–769 (2024).
Balasubramanian, A. et al. The palmitoylation of gasdermin D directs its membrane translocation and pore formation during pyroptosis. Sci. Immunol. 9, eadn1452 (2024).
Hu, L. et al. Chemotherapy-induced pyroptosis is mediated by BAK/BAX–caspase-3–GSDME pathway and inhibited by 2-bromopalmitate. Cell Death Dis. 11, 281 (2020).
Yu, T. et al. NLRP3 Cys126 palmitoylation by ZDHHC7 promotes inflammasome activation. Cell Rep. 43, 114070 (2024).
Zheng, S. et al. ZDHHC5-mediated NLRP3 palmitoylation promotes NLRP3–NEK7 interaction and inflammasome activation. Mol. Cell 83, 4570–4585.e7 (2023).
Wang, L. et al. Palmitoylation prevents sustained inflammation by limiting NLRP3 inflammasome activation through chaperone-mediated autophagy. Mol. Cell 83, 281–297.e10 (2023).
Humphries, F. et al. Succination inactivates gasdermin D and blocks pyroptosis. Science 369, 1633–1637 (2020).
Hu, J. J. et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 21, 736–745 (2020).
Rathkey, J. K. et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci. Immunol. https://doi.org/10.1126/sciimmunol.aat2738 (2018). Together with Humphries et al. (2020) and Hu et al. (2020), this article identifies Cys-reactive drugs that modify Cys191 in GSDMD and block GSDMD pore-forming activity.
Chai, Q. et al. A bacterial phospholipid phosphatase inhibits host pyroptosis by hijacking ubiquitin. Science 378, eabq0132 (2022).
Santa Cruz Garcia, A. B., Schnur, K. P., Malik, A. B. & Mo, G. C. H. Gasdermin D pores are dynamically regulated by local phosphoinositide circuitry. Nat. Commun. 13, 52 (2022).
Jimenez, A. J. et al. ESCRT machinery is required for plasma membrane repair. Science 343, 1247136 (2014).
Ruhl, S. et al. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362, 956–960 (2018).
Evavold, C. L. et al. The pore-forming protein Gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48, 35–44.e6 (2018).
Zanoni, I. et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 352, 1232–1236 (2016).
Zhang, J. et al. Epithelial gasdermin D shapes the host–microbial interface by driving mucus layer formation. Sci. Immunol. 7, eabk2092 (2022).
Zhou, B. & Abbott, D. W. Gasdermin E permits interleukin-1β release in distinct sublytic and pyroptotic phases. Cell Rep. 35, 108998 (2021).
Gaidt, M. M. et al. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell 171, 1110–1124.e18 (2017).
Zheng, Z. et al. The lysosomal Rag–Ragulator complex licenses RIPK1 and caspase-8-mediated pyroptosis by yersinia. Science 372, eabg0269 (2021).
Neel, D. V. et al. Gasdermin-E mediates mitochondrial damage in axons and neurodegeneration. Neuron 111, 1222–1240.e9 (2023).
Zhou, B. et al. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 28, 1171–1185 (2018).
Xia, S. et al. Synthetic protein circuits for programmable control of mammalian cell death. Cell 187, 2785–2800.e16 (2024).
Yuan, J. & Ofengeim, D. A guide to cell death pathways. Nat. Rev. Mol. Cell Biol. 25, 379–395 (2024).
Fritsch, M. et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575, 683–687 (2019).
Pierini, R. et al. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ. 19, 1709–1721 (2012).
Sagulenko, V. et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ. 20, 1149–1160 (2013).
Antonopoulos, C. et al. Caspase-8 as an effector and regulator of NLRP3 inflammasome signaling. J. Biol. Chem. 290, 20167–20184 (2015).
Van Opdenbosch, N. et al. Caspase-1 engagement and TLR-induced c-FLIP expression suppress ASC/caspase-8-dependent apoptosis by inflammasome sensors NLRP1b and NLRC4. Cell Rep. 21, 3427–3444 (2017).
Rauch, I. et al. NAIP–NLRC4 inflammasomes coordinate intestinal epithelial cell expulsion with eicosanoid and IL-18 release via activation of caspase-1 and -8. Immunity 46, 649–659 (2017).
Tsuchiya, K. et al. Caspase-1 initiates apoptosis in the absence of gasdermin D. Nat. Commun. 10, 2091 (2019).
Wang, C. et al. NLRP3 inflammasome activation triggers gasdermin D-independent inflammation. Sci. Immunol. 6, eabj3859 (2021).
Pasparakis, M. & Vandenabeele, P. Necroptosis and its role in inflammation. Nature 517, 311–320 (2015).
Conos, S. A. et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc. Natl Acad. Sci. USA 114, E961–E969 (2017).
Gutierrez, K. D. et al. MLKL activation triggers NLRP3-mediated processing and release of IL-1β independently of gasdermin-D. J. Immunol. 198, 2156–2164 (2017).
Polykratis, A. et al. A20 prevents inflammasome-dependent arthritis by inhibiting macrophage necroptosis through its ZnF7 ubiquitin-binding domain. Nat. Cell Biol. 21, 731–742 (2019).
Kang, T. B., Yang, S. H., Toth, B., Kovalenko, A. & Wallach, D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 38, 27–40 (2013).
Zheng, M., Karki, R., Vogel, P. & Kanneganti, T. D. Caspase-6 is a key regulator of innate immunity, inflammasome activation, and host defense. Cell 181, 674–687.e13 (2020).
Lee, S. et al. AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature 597, 415–419 (2021).
Banerjee, I. et al. Gasdermin D restrains type I interferon response to cytosolic DNA by disrupting ionic homeostasis. Immunity 49, 413–426.e5 (2018).
Zhu, Q., Zheng, M., Balakrishnan, A., Karki, R. & Kanneganti, T. D. Gasdermin D promotes AIM2 inflammasome activation and is required for host protection against Francisella novicida. J. Immunol. 201, 3662–3668 (2018).
Goncalves, A. V. et al. Gasdermin-D and caspase-7 are the key caspase-1/8 substrates downstream of the NAIP5/NLRC4 inflammasome required for restriction of Legionella pneumophila. PLoS Pathog. 15, e1007886 (2019).
Cerqueira, D. M. et al. Guanylate-binding protein 5 licenses caspase-11 for gasdermin-D mediated host resistance to Brucella abortus infection. PLoS Pathog. 14, e1007519 (2018).
Chen, K. W. et al. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci. Immunol. 3, eaar6676 (2018).
Wang, J., Deobald, K. & Re, F. Gasdermin D protects from melioidosis through pyroptosis and direct killing of bacteria. J. Immunol. 202, 3468–3473 (2019).
Yang, X. et al. Bacterial endotoxin activates the coagulation cascade through gasdermin D-dependent phosphatidylserine exposure. Immunity 51, 983–996.e6 (2019).
Wu, C. et al. Inflammasome activation triggers blood clotting and host death through pyroptosis. Immunity 50, 1401–1411.e4 (2019).
Wei, C. et al. Brain endothelial GSDMD activation mediates inflammatory BBB breakdown. Nature 629, 893–900 (2024).
Zhu, S. et al. Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature 546, 667–670 (2017).
Liu, T. et al. NOD-like receptor family, pyrin domain containing 3 (NLRP3) contributes to inflammation, pyroptosis, and mucin production in human airway epithelium on rhinovirus infection. J. Allergy Clin. Immunol. 144, 777–787.e9 (2019).
Rodrigues, T. S. et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 218, e20201707 (2021).
Junqueira, C. et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature 606, 576–584 (2022).
Rosli, S. et al. Gasdermin D promotes hyperinflammation and immunopathology during severe influenza A virus infection. Cell Death Dis. 14, 727 (2023).
Speaks, S. et al. Gasdermin D promotes influenza virus-induced mortality through neutrophil amplification of inflammation. Nat. Commun. 15, 2751 (2024).
Dubois, H. et al. Nlrp3 inflammasome activation and gasdermin D-driven pyroptosis are immunopathogenic upon gastrointestinal norovirus infection. PLoS Pathog. 15, e1007709 (2019).
Zhao, Z. et al. Zika virus causes placental pyroptosis and associated adverse fetal outcomes by activating GSDME. eLife 11, e73792 (2022).
Dong, S. et al. Gasdermin E is required for induction of pyroptosis and severe disease during enterovirus 71 infection. J. Biol. Chem. 298, 101850 (2022).
Wan, X. et al. H7N9 virus infection triggers lethal cytokine storm by activating gasdermin E-mediated pyroptosis of lung alveolar epithelial cells. Natl Sci. Rev. 9, nwab137 (2022).
Wei, Z. et al. Baicalin inhibits influenza A (H1N1)-induced pyroptosis of lung alveolar epithelial cells via caspase-3/GSDME pathway. J. Med. Virol. 95, e28790 (2023).
Batista, S. J. et al. Gasdermin-D-dependent IL-1‒release from microglia promotes protective immunity during chronic Toxoplasma gondii infection. Nat. Commun. 11, 3687 (2020).
Sateriale, A. et al. The intestinal parasite Cryptosporidium is controlled by an enterocyte intrinsic inflammasome that depends on NLRP6. Proc. Natl. Acad. Sci. USA 118, e2007807118 (2021).
de Sa, K. S. G. et al. Gasdermin-D activation promotes NLRP3 activation and host resistance to Leishmania infection. Nat. Commun. 14, 1049 (2023).
Ding, X. et al. Inflammasome-mediated GSDMD activation facilitates escape of Candida albicans from macrophages. Nat. Commun. 12, 6699 (2021).
Soderman, J., Berglind, L. & Almer, S. Gene expression–genotype analysis implicates GSDMA, GSDMB, and LRRC3C as contributors to inflammatory bowel disease susceptibility. Biomed. Res. Int. 2015, 834805 (2015).
Moreno-Moral, A. et al. Changes in macrophage transcriptome associate with systemic sclerosis and mediate GSDMA contribution to disease risk. Ann. Rheum. Dis. 77, 596–601 (2018).
Terao, C. et al. Transethnic meta-analysis identifies GSDMA and PRDM1 as susceptibility genes to systemic sclerosis. Ann. Rheum. Dis. 76, 1150–1158 (2017).
Yu, J. et al. Polymorphisms in GSDMA and GSDMB are associated with asthma susceptibility, atopy and BHR. Pediatr. Pulmonol. 46, 701–708 (2011).
Moffatt, M. F. et al. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 363, 1211–1221 (2010).
French, F. M. F. C. A candidate gene for familial Mediterranean fever. Nat. Genet. 17, 25–31 (1997).
Feldmann, J. et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am. J. Hum. Genet. 71, 198–203 (2002).
Chae, J. J. et al. Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1β activation and severe autoinflammation in mice. Immunity 34, 755–768 (2011).
Bertoni, A. et al. A novel knock-in mouse model of cryopyrin-associated periodic syndromes with development of amyloidosis: therapeutic efficacy of proton pump inhibitors. J. Allergy Clin. Immunol. 145, 368–378.e13 (2020).
Ma, X. et al. Prussian blue nanozyme as a pyroptosis inhibitor alleviates neurodegeneration. Adv. Mater. 34, e2106723 (2022).
Van Schoor, E. et al. Increased pyroptosis activation in white matter microglia is associated with neuronal loss in ALS motor cortex. Acta Neuropathol. 144, 393–411 (2022).
McKenzie, B. A. et al. Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proc. Natl Acad. Sci. USA 115, E6065–E6074 (2018).
Li, S. et al. Gasdermin D in peripheral myeloid cells drives neuroinflammation in experimental autoimmune encephalomyelitis. J. Exp. Med. 216, 2562–2581 (2019).
Li, J. et al. Blocking GSDMD processing in innate immune cells but not in hepatocytes protects hepatic ischemia–reperfusion injury. Cell Death Dis. 11, 244 (2020).
Wu, X. Y., Chen, Y. J., Liu, C. A., Gong, J. H. & Xu, X. S. STING induces liver ischemia–reperfusion injury by promoting calcium-dependent caspase 1-GSDMD processing in macrophages. Oxid. Med. Cell Longev. 2022, 8123157 (2022).
Shi, H. et al. GSDMD-mediated cardiomyocyte pyroptosis promotes myocardial I/R injury. Circ. Res. 129, 383–396 (2021).
Zhong, L. et al. Novel GSDMD inhibitor GI-Y1 protects heart against pyroptosis and ischemia/reperfusion injury by blocking pyroptotic pore formation. Basic. Res. Cardiol. 118, 40 (2023).
Zhang, D. et al. Gasdermin D serves as a key executioner of pyroptosis in experimental cerebral ischemia and reperfusion model both in vivo and in vitro. J. Neurosci. Res. 97, 645–660 (2019).
Yang, F. et al. Silencing long non-coding RNA Kcnq1ot1 alleviates pyroptosis and fibrosis in diabetic cardiomyopathy. Cell Death Dis. 9, 1000 (2018).
Wang, Y. et al. TLR4/NF-κB signaling induces GSDMD-related pyroptosis in tubular cells in diabetic kidney disease. Front. Endocrinol. 10, 603 (2019).
Xu, B. et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J. Hepatol. 68, 773–782 (2018).
Khanova, E. et al. Pyroptosis by caspase11/4–gasdermin-D pathway in alcoholic hepatitis in mice and patients. Hepatology 67, 1737–1753 (2018).
Lu, Y. et al. Caspase-11 signaling enhances graft-versus-host disease. Nat. Commun. 10, 4044 (2019).
Xiao, J. et al. Radiation causes tissue damage by dysregulating inflammasome–gasdermin D signaling in both host and transplanted cells. PLoS Biol. 18, e3000807 (2020).
Tan, G., Huang, C., Chen, J., Chen, B. & Zhi, F. Gasdermin-E-mediated pyroptosis participates in the pathogenesis of Crohn’s disease by promoting intestinal inflammation. Cell Rep. 35, 109265 (2021).
Zhai, Z. et al. Attenuation of rheumatoid arthritis through the inhibition of tumor necrosis factor-induced caspase 3/gasdermin E-mediated pyroptosis. Arthritis Rheumatol. 74, 427–440 (2022).
Xia, W. et al. Gasdermin E deficiency attenuates acute kidney injury by inhibiting pyroptosis and inflammation. Cell Death Dis. 12, 139 (2021).
Saeki, N., Kuwahara, Y., Sasaki, H., Satoh, H. & Shiroishi, T. Gasdermin (Gsdm) localizing to mouse chromosome 11 is predominantly expressed in upper gastrointestinal tract but significantly suppressed in human gastric cancer cells. Mamm. Genome 11, 718–724 (2000).
Molina-Crespo, A. et al. Intracellular delivery of an antibody targeting gasdermin-B reduces HER2 breast cancer aggressiveness. Clin. Cancer Res. 25, 4846–4858 (2019).
Hergueta-Redondo, M. et al. Gasdermin-B promotes invasion and metastasis in breast cancer cells. PLoS ONE 9, e90099 (2014).
Cui, Y., Zhou, Z., Chai, Y. & Zhang, Y. Upregulated GSDMB in clear cell renal cell carcinoma is associated with immune infiltrates and poor prognosis. J. Immunol. Res. 2021, 7753553 (2021).
He, H. et al. USP24–GSDMB complex promotes bladder cancer proliferation via activation of the STAT3 pathway. Int. J. Biol. Sci. 17, 2417–2429 (2021).
Miguchi, M. et al. Gasdermin C is upregulated by inactivation of transforming growth factor β receptor type II in the presence of mutated Apc, promoting colorectal cancer proliferation. PLoS ONE 11, e0166422 (2016).
Cui, Y. Q., Meng, F., Zhan, W. L., Dai, Z. T. & Liao, X. High expression of GSDMC is associated with poor survival in kidney clear cell cancer. Biomed. Res. Int. 2021, 5282894 (2021).
Yan, C., Niu, Y., Li, F., Zhao, W. & Ma, L. System analysis based on the pyroptosis-related genes identifies GSDMC as a novel therapy target for pancreatic adenocarcinoma. J. Transl. Med. 20, 455 (2022).
Wang, W. J. et al. Downregulation of gasdermin D promotes gastric cancer proliferation by regulating cell cycle-related proteins. J. Dig. Dis. 19, 74–83 (2018).
Peng, J. et al. CD147 expression is associated with tumor proliferation in bladder cancer via GSDMD. Biomed. Res. Int. 2020, 7638975 (2020).
Wang, S. et al. Gasdermin C sensitizes tumor cells to PARP inhibitor therapy in cancer models. J. Clin. Invest. 134, e166841 (2024).
Wang, Q. et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature 579, 421–426 (2020).
Yuan, R. et al. Cucurbitacin B inhibits non-small cell lung cancer in vivo and in vitro by triggering TLR4/NLRP3/GSDMD-dependent pyroptosis. Pharmacol. Res. 170, 105748 (2021).
Johnson, D. C. et al. DPP8/DPP9 inhibitor-induced pyroptosis for treatment of acute myeloid leukemia. Nat. Med. 24, 1151–1156 (2018).
Fontana, P. et al. Small-molecule GSDMD agonism in tumors stimulates antitumor immunity without toxicity. Cell 197, 6165–6181.e22 (2024).
Lu, H. et al. Molecular targeted therapies elicit concurrent apoptotic and GSDME-dependent pyroptotic tumor cell death. Clin. Cancer Res. 24, 6066–6077 (2018).
Zhang, C. C. et al. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis 24, 312–325 (2019).
Zhang, Z. et al. Caspase-3-mediated GSDME induced pyroptosis in breast cancer cells through the ROS/JNK signalling pathway. J. Cell Mol. Med. 25, 8159–8168 (2021).
Jiang, Y. et al. Gasdermin D restricts anti-tumor immunity during PD-L1 checkpoint blockade. Cell Rep. 41, 111553 (2022).
Ren, F. et al. ER stress induces caspase-2–tBID–GSDME-dependent cell death in neurons lytically infected with herpes simplex virus type 2. EMBO J. 42, e113118 (2023).
Xiao, C. et al. Tisp40 induces tubular epithelial cell GSDMD-mediated pyroptosis in renal ischemia–reperfusion injury via NF-κB signaling. Front. Physiol. 11, 906 (2020).
Delmaghani, S. et al. Hypervulnerability to sound exposure through impaired adaptive proliferation of peroxisomes. Cell 163, 894–906 (2015).
Kayagaki, N. et al. IRF2 transcriptionally induces GSDMD expression for pyroptosis. Sci. Signal. 12, eaax4917 (2019).
Kayagaki, N. et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 591, 131–136 (2021). This publication uncovers the key role for NINJ1 in pyroptotic plasma membrane rupture.
Pourmal, S. et al. Autoinhibition of dimeric NINJ1 prevents plasma membrane rupture. Nature 637, 446–452 (2025).
Degen, M. et al. Structural basis of NINJ1-mediated plasma membrane rupture in cell death. Nature 618, 1065–1071 (2023).
David, L. et al. NINJ1 mediates plasma membrane rupture by cutting and releasing membrane disks. Cell 187, 2224–2235.e16 (2024).
Sahoo, B., Mou, Z., Liu, W., Dubyak, G. & Dai, X. How NINJ1 mediates plasma membrane rupture and why NINJ2 cannot. Cell 188, 292–302.e11 (2025).
Kayagaki, N. et al. Inhibiting membrane rupture with NINJ1 antibodies limits tissue injury. Nature 618, 1072–1077 (2023).
Cardoso, C. C. et al. Ninjurin 1 asp110ala single nucleotide polymorphism is associated with protection in leprosy nerve damage. J. Neuroimmunol. 190, 131–138 (2007).
Graca, C. R. et al. NINJURIN1 single nucleotide polymorphism and nerve damage in leprosy. Infect. Genet. Evol. 12, 597–600 (2012).
Tamura, M. et al. Members of a novel gene family, Gsdm, are expressed exclusively in the epithelium of the skin and gastrointestinal tract in a highly tissue-specific manner. Genomics 89, 618–629 (2007).
Delmaghani, S. et al. Mutations in the gene encoding pejvakin, a newly identified protein of the afferent auditory pathway, cause DFNB59 auditory neuropathy. Nat. Genet. 38, 770–778 (2006).
Collin, R. W. et al. Involvement of DFNB59 mutations in autosomal recessive nonsyndromic hearing impairment. Hum. Mutat. 28, 718–723 (2007).
Kazmierczak, M. et al. Pejvakin, a candidate stereociliary rootlet protein, regulates hair cell function in a cell-autonomous manner. J. Neurosci. 37, 3447–3464 (2017).
Katoh, M. & Katoh, M. Identification and characterization of human DFNA5L, mouse Dfna5l, and rat Dfna5l genes in silico. Int. J. Oncol. 25, 765–770 (2004).
van Camp, G. et al. Localization of a gene for non-syndromic hearing loss (DFNA5) to chromosome 7p15. Hum. Mol. Genet. 4, 2159–2163 (1995).
Watabe, K. et al. Structure, expression and chromosome mapping of MLZE, a novel gene which is preferentially expressed in metastatic melanoma cells. Jpn J. Cancer Res. 92, 140–151 (2001).
Acknowledgements
This work was partly supported by the National Natural Science Foundation of China (32425023, 32400723, 32122034), the National Key R&D Program of China (2020YFA0509600), the Shanghai Pilot Program for Basic Research-Chinese Academy of Sciences, Shanghai Branch (JCYJ-SHFY-2021-009), the Strategic Priority Research Program of Chinese Academy of Sciences (XDB0570000), the Innovative Research Team of High-Level Local Universities in Shanghai (SHSMU-ZDCX20211002), the National Postdoctoral Program for Innovative Talents (BX20240395), the Chinese Postdoctoral Science Foundation (2024M753367) and the Shanghai Sailing Program (24YF2754200). X.L. is a SANS Exploration Scholar. The authors apologize to colleagues whose work could not be cited owing to space limitations.
Author information
Authors and Affiliations
Contributions
X.L. and Y.B. researched data for the article, contributed substantially to discussion of the content and wrote the article. X.L. and Y.P. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Fiachra Humphries and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Bacterial effector protein
-
A bacteria-encoded toxic protein that is directly injected into host cells through bacterial secretion systems to interfere with host cell functions, especially hijacking antimicrobial immunity to facilitate bacterial proliferation and pathogenesis.
- cGAS–STING pathway
-
An innate immune signalling pathway responsible for recognition of cytosolic aberrant DNA by cyclic GMP–AMP synthase (cGAS) to produce 2′3′-cyclic GMP–AMP (cGAMP) as a second messenger to activate downstream signalling through endoplasmic reticulum (ER)-resident protein stimulator of interferon genes (STING), resulting in the induction of type I interferon and pro-inflammatory cytokines.
- Cold tumours
-
Cold tumours are immunosuppressive tumours that are unresponsive to immune surveillance or immune checkpoint blockade (ICB) therapy.
- Endosomal sorting complexes required for transport
-
(ESCRT). A molecular machinery that drives the invagination of endosomes to form multivesicular bodies that are critical for sorting and transporting membrane proteins.
- Familial Mediterranean fever
-
A genetic inflammatory disease caused by constitutively active mutations in the pyrin-encoding gene (MEFV) and characterized by recurrent fevers as well as pain in the abdomen, chest and joints.
- Goblet cells
-
A group of specialized epithelial cells on the mucosal surface that produce and secrete mucus to maintain mucosal homeostasis.
- Hot tumours
-
Hot tumours can trigger antitumour immune responses and effectively respond to immune checkpoint blockade (ICB) therapy.
- Immune checkpoint blockade
-
(ICB). An antitumour immunotherapy that blocks immunosuppressive checkpoints in cytotoxic T lymphocytes (CTLs) to increase CTL-mediated killing of cancer cells.
- Immune synapses
-
A specialized and highly-ordered cell–cell contact interface between immune cells and their target cells, where cell–cell communications take place.
- Immunologically silent
-
It is characterized by non-immunogenicity as no immune responses are provoked.
- Inflammatory bowel disease
-
A group of chronic inflammatory disorders in the gastrointestinal tract that can be divided into ulcerative colitis with colorectal inflammation and Crohn’s disease characterized by small intestinal inflammation.
- Neonatal-onset multisystem inflammatory disease
-
A rare genetic inflammatory disease caused by gain-of-function mutations in NLRP3 that occurs in infancy and is characterized by an urticaria-like rash, recurrent fever, skeletal dysplasia and pathological changes in the central nervous system including meningitis, intellectual delay and loss of vision or hearing.
- NETosis
-
A form of neutrophil death characterized by the release of neutrophil nuclear DNA and granule proteins forming neutrophil extracellular traps (NETs) to capture and kill the invading pathogens.
- Poly(ADP-ribosyl)ation
-
(PARylation). A type of post-translational protein modification catalysed by poly(ADP-ribose) polymerases that transfer ADP-ribose polymers onto target proteins using NAD+ as a substrate.
- Rheumatoid arthritis
-
A chronic inflammatory autoimmune disease that manifests as pain, swelling and even deformity in joints.
- Triple-negative breast cancer
-
The most aggressive subtype of breast cancer with the worst prognosis, characterized by a lack of three biomarkers, namely, oestrogen receptor, progesterone receptor and epidermal growth factor receptor 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bai, Y., Pan, Y. & Liu, X. Mechanistic insights into gasdermin-mediated pyroptosis. Nat Rev Mol Cell Biol 26, 501–521 (2025). https://doi.org/10.1038/s41580-025-00837-0
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41580-025-00837-0