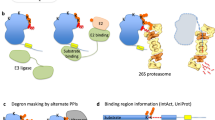
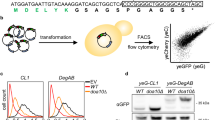
Abstract
Degrons are pivotal components of the ubiquitin–proteasome system, serving as the recognition determinants through which E3 ubiquitin ligases identify their substrates. Degrons have central roles in both protein quality control and intracellular signalling pathways, and mutations that dysregulate degron activity are associated with a wide range of diseases, including cancer, immunological disorders and neurodegeneration. The number of well-defined degrons remains sparse relative to the ~600 E3 ubiquitin ligases encoded in the human genome. Recent advances in high-throughput degron discovery technologies have accelerated progress in this area, expanding the number of N- and C-terminal degrons, internal degrons and ubiquitin-independent degrons defined experimentally at high resolution. In this Review, we discuss the latest insights into the molecular mechanisms through which degrons act, their functional importance and their relevance in human disease, and consider how bifunctional molecules harness degrons to enable targeted protein degradation for therapeutic benefit.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zheng, N. & Shabek, N. Ubiquitin ligases: structure, function, and regulation. Annu. Rev. Biochem. 86, 129–157 (2017).
Collins, G. A. & Goldberg, A. L. The logic of the 26S proteasome. Cell 169, 792–806 (2017).
Dikic, I. & Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 19, 349–364 (2018).
Deshaies, R. J. & Joazeiro, C. A. P. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 78, 399–434 (2009).
Oughtred, R. et al. The BioGRID database: a comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 30, 187–200 (2021).
Yau, R. & Rape, M. The increasing complexity of the ubiquitin code. Nat. Cell Biol. 18, 579–586 (2016).
Dikic, I., Wakatsuki, S. & Walters, K. J. Ubiquitin-binding domains—from structures to functions. Nat. Rev. Mol. Cell Biol. 10, 659–671 (2009).
Komander, D., Clague, M. J. & Urbé, S. Breaking the chains: structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol. 10, 550–563 (2009).
Zhang, Z. & Elledge, S. J. Harnessing GPT-4 for automated curation of E3–substrate relationships in the ubiquitin-proteasome system. Preprint at bioRxiv https://doi.org/10.1101/2024.10.20.619305 (2024).
Morreale, F. E. & Walden, H. Types of ubiquitin ligases. Cell 165, 248 (2016).
Harper, J. W. & Schulman, B. A. Cullin-RING ubiquitin ligase regulatory circuits: a quarter century beyond the F-box hypothesis. Annu. Rev. Biochem. 90, 403–429 (2021).
Skowyra, D., Craig, K. L., Tyers, M., Elledge, S. J. & Harper, J. W. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 91, 209–219 (1997).
Bachmair, A., Finley, D. & Varshavsky, A. In vivo half-life of a protein is a function of its amino-terminal residue. Science 234, 179–186 (1986). This study provides the first demonstration that degrons are transferable and that the nature of the amino acid at the N terminus affects protein turnover rate.
Varshavsky, A. The N‐end rule pathway and regulation by proteolysis. Protein Sci. 20, 1298–1345 (2011).
Winston, J. T. et al. The SCFβ–TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkBα and β-catenin and stimulates IkBα ubiquitination in vitro. Genes Dev. 13, 270–283 (1999). The studies by Winston et al. 1999 and Yaron et al. 1998 identify, to our knowledge, the first phosphodegron.
Yaron, A. et al. Identification of the receptor component of the IκBα–ubiquitin ligase. Nature 396, 590–594 (1998).
Glotzer, M., Murray, A. W. & Kirschner, M. W. Cyclin is degraded by the ubiquitin pathway. Nature 349, 132–138 (1991). This study shows that fusing a peptide derived from cyclin to a heterologous protein is sufficient to confer instability and represents the first report, to our knowledge, of an internal degron.
Timms, R. T. et al. A glycine-specific N-degron pathway mediates the quality control of protein N-myristoylation. Science 365, eaaw4912 (2019).
Koren, I. et al. The eukaryotic proteome is shaped by E3 ubiquitin ligases targeting C-terminal degrons. Cell 173, 1622–1635.e14 (2018).
Lin, H. C. et al. C-terminal end-directed protein elimination by CRL2 ubiquitin ligases. Mol. Cell 70, 602–613.e3 (2018). The studies by Koren et al. 2018 and Lin et al. 2018 develop the GPS–peptidome technology and represent the first systematic characterization of C-degrons in a mammalian system.
Kats, I. et al. Mapping degradation signals and pathways in a eukaryotic N-terminome. Mol. Cell 70, 488–501.e5 (2018).
Geffen, Y. et al. Mapping the landscape of a eukaryotic degronome. Mol. Cell 63, 1055–1065 (2016).
Zhang, Z. et al. Elucidation of E3 ubiquitin ligase specificity through proteome-wide internal degron mapping. Mol. Cell 83, 3377–3392.e6 (2023). This study performs the first human proteome-wide characterization of internal degrons, and discovers 14 additional E3–degron pairs that were previously unknown or had not been accurately defined.
Gu, X. et al. The midnolin–proteasome pathway catches proteins for ubiquitination-independent degradation. Science 381, eadh5021 (2023).
Makaros, Y. et al. Ubiquitin-independent proteasomal degradation driven by C-degron pathways. Mol. Cell 83, 1921–1935.e7 (2023). This study performs the first systematic characterization of ubiquitin-independent degrons in a mammalian system.
Szulc, N. A. et al. DEGRONOPEDIA: a web server for proteome-wide inspection of degrons. Nucleic Acids Res. 52, W221–W232 (2024).
Levine, B. & Kroemer, G. Biological functions of autophagy genes: a disease perspective. Cell 176, 11–42 (2019).
Liu, J. et al. Genetic fusions favor tumorigenesis through degron loss in oncogenes. Nat. Commun. 12, 6704 (2021).
Tokheim, C. et al. Systematic characterization of mutations altering protein degradation in human cancers. Mol. Cell 81, 1292–1308.e11 (2021).
Sherpa, D., Chrustowicz, J. & Schulman, B. A. How the ends signal the end: regulation by E3 ubiquitin ligases recognizing protein termini. Mol. Cell 82, 1424–1438 (2022).
Kim, Y. et al. Targeted kinase degradation via the KLHDC2 ubiquitin E3 ligase. Cell Chem. Biol. 30, 1414–1420.e5 (2023).
Röth, S. et al. Identification of KLHDC2 as an efficient proximity-induced degrader of K-RAS, STK33, β-catenin, and FoxP3. Cell Chem. Biol. 30, 1261–1276.e7 (2023).
Hickey, C. M. et al. Co-opting the E3 ligase KLHDC2 for targeted protein degradation by small molecules. Nat. Struct. Mol. Biol. 31, 311–322 (2024).
Chana, C. K. et al. Discovery and structural characterization of small molecule binders of the human CTLH E3 ligase subunit GID4. J. Med. Chem. 65, 12725–12746 (2022).
Henning, N. J. et al. Discovery of a covalent FEM1B recruiter for targeted protein degradation applications. J. Am. Chem. Soc. 144, 701–708 (2022).
Ichikawa, S. et al. The E3 ligase adapter cereblon targets the C-terminal cyclic imide degron. Nature 610, 775–782 (2022). This study shows that cereblon recognizes C-terminal cyclin imide degrons.
Heim, C., Spring, A. K., Kirchgäßner, S., Schwarzer, D. & Hartmann, M. D. Identification and structural basis of C-terminal cyclic imides as natural degrons for cereblon. Biochem. Biophys. Res. Commun. 637, 66–72 (2022).
Miyawaki, S. et al. The mouse Sry locus harbors a cryptic exon that is essential for male sex determination. Science 370, 121–124 (2020).
Zhang, P. et al. CRL2–KLHDC3 E3 ubiquitin ligase complex suppresses ferroptosis through promoting p14ARF degradation. Cell Death Differ. 29, 58–771 (2022).
Varshavsky, A. N-degron and C-degron pathways of protein degradation. Proc. Natl Acad. Sci. USA 116, 358–366 (2019).
Timms, R. T. & Koren, I. Tying up loose ends: the N-degron and C-degron pathways of protein degradation. Biochem. Soc. Trans. 48, 1557–1567 (2020).
Varshavsky, A. N-degron pathways. Proc. Natl Acad. Sci. USA 121, e2408697121 (2024).
Tran, A. The N-end rule pathway and Ubr1 enforce protein compartmentalization via P2-encoded cellular location signals. J. Cell Sci. 132, jcs231662 (2019).
Lee, K. E., Heo, J. E., Kim, J. M. & Hwang, C. S. N-terminal acetylation-targeted N-end rule proteolytic system: the Ac/N-end rule pathway. Mol. Cells 39, 169–178 (2016).
Aksnes, H., Ree, R. & Arnesen, T. Co-translational, post-translational, and non-catalytic roles of N-terminal acetyltransferases. Mol. Cell 73, 1097–1114 (2019).
Shemorry, A., Hwang, C. S. & Varshavsky, A. Control of protein quality and stoichiometries by N-terminal acetylation and the N-end rule pathway. Mol. Cell 50, 540–551 (2013).
Hwang, C.-S., Shemorry, A. & Varshavsky, A. N-terminal acetylation of cellular proteins creates specific degradation signals. Science 327, 973–977 (2010).
Chen, S.-J., Wu, X., Wadas, B., Oh, J.-H. & Varshavsky, A. An N-end rule pathway that recognizes proline and destroys gluconeogenic enzymes. Science 355, eaal3655 (2017).
Sherpa, D. et al. GID E3 ligase supramolecular chelate assembly configures multipronged ubiquitin targeting of an oligomeric metabolic enzyme. Mol. Cell 81, 2445–2459.e13 (2021).
Qiao, S. et al. Interconversion between anticipatory and active GID E3 ubiquitin ligase conformations via metabolically driven substrate receptor assembly. Mol. Cell 77, 150–163.e9 (2020).
Shin, J. S., Park, S. H., Kim, L., Heo, J. & Song, H. K. Crystal structure of yeast Gid10 in complex with Pro/N-degron. Biochem. Biophys. Res. Commun. 582, 86–92 (2021).
Melnykov, A., Chen, S. J. & Varshavsky, A. Gid10 as an alternative N-recognin of the Pro/N-degron pathway. Proc. Natl Acad. Sci. USA 116, 15914–15923 (2019).
Kong, K. Y. E. et al. Timer-based proteomic profiling of the ubiquitin-proteasome system reveals a substrate receptor of the GID ubiquitin ligase. Mol. Cell 81, 2460–2476.e11 (2021).
Dong, C. et al. Recognition of nonproline N-terminal residues by the Pro/N-degron pathway. Proc. Natl Acad. Sci. USA 117, 14158–14167 (2020).
Yi, S. A., Sepic, S., Schulman, B. A., Ordureau, A. & An, H. mTORC1–CTLH E3 ligase regulates the degradation of HMG-CoA synthase 1 through the Pro/N-degron pathway. Mol. Cell 84, 2166–2184.e9 (2024).
Li, Y. et al. CRL2ZER1/ZYG11B recognizes small N-terminal residues for degradation. Nat. Commun. 13, 7636 (2022).
Yan, X. et al. Molecular basis for recognition of Gly/N-degrons by CRL2ZYG11B and CRL2ZER1. Mol. Cell 81, 3262–3274.e3 (2021).
Robinson, K. S. et al. Enteroviral 3C protease activates the human NLRP1 inflammasome in airway epithelia. Science 370, eaay2002 (2020).
Rusnac, D.-V. et al. Recognition of the diglycine C-end degron by CRL2KLHDC2 ubiquitin ligase. Mol. Cell 72, 813–822.e4 (2018).
Scott, D. C. et al. E3 ligase autoinhibition by C-degron mimicry maintains C-degron substrate fidelity. Mol. Cell 83, 770–786.e9 (2023). This study shows that KLHDC2 distinguishes bona fide substrates from non-substrates through an autoinhibitory mechanism involving C-degron mimicry.
Scott, D. C. et al. Structural basis for C-degron selectivity across KLHDCX family E3 ubiquitin ligases. Nat. Commun. 15, 9899 (2024).
Thrun, A. et al. Convergence of mammalian RQC and C-end rule proteolytic pathways via alanine tailing. Mol. Cell 81, 2112–2122.e7 (2021).
Patil, P. R. et al. Mechanism and evolutionary origins of alanine-tail C-degron recognition by E3 ligases Pirh2 and CRL2-KLHDC10. Cell Rep. 42, 113100 (2023).
Wang, X. et al. Recognition of an Ala-rich C-degron by the E3 ligase Pirh2. Nat. Commun. 14, 2474 (2023).
Zhao, S. et al. Molecular basis for C-degron recognition by CRL2APPBP2 ubiquitin ligase. Proc. Natl Acad. Sci. USA 120, e2308870120 (2023).
Yan, X. et al. Molecular basis for ubiquitin ligase CRL2FEM1C-mediated recognition of C-degron. Nat. Chem. Biol. 17, 263–271 (2021).
Zhao, S. et al. Structural insights into SMCR8 C-degron recognition by FEM1B. Biochem. Biophys. Res. Commun. 557, 236–239 (2021).
Timms, R. T. et al. Defining E3 ligase–substrate relationships through multiplex CRISPR screening. Nat. Cell Biol. 25, 1535–1545 (2023). This study describes a scalable technology platform for multiplex E3–degron/substrate mapping.
Chen, X. et al. Mechanism of Ψ-Pro/C-degron recognition by the CRL2FEM1B ubiquitin ligase. Nat. Commun. 15, 3558 (2024).
Geiger, T. & Clarke, S. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J. Biol. Chem. 262, 785–794 (1987).
Voorter, C. E. M., De Haard-Hoekman, W. A., Van Den Oetelaar, P. J. M., Bloemendal, H. & De Jong, W. W. Spontaneous peptide bond cleavage in aging α-crystallin through a succinimide intermediate. J. Biol. Chem. 263, 19020–19023 (1988).
Tyler-Cross, R. & Schirch, V. Effects of amino acid sequence, buffers, and ionic strength on the rate and mechanism of deamidation of asparagine residues in small peptides. J. Biol. Chem. 266, 22549–22556 (1991).
Brennan, T. V. & Clarke, S. Effect of adjacent histidine and cysteine residues on the spontaneous degradation of asparaginyl‐ and aspartyl‐containing peptides. Int. J. Pept. Protein Res. 45, 547–553 (1995).
Paulus, H. Protein splicing and related forms of protein autoprocessing. Annu. Rev. Biochem. 69, 447–496 (2000).
Mills, K. V., Manning, J. S., Garcia, A. M. & Wuerdeman, L. A. Protein splicing of a pyrococcus abyssi intein with a C-terminal glutamine. J. Biol. Chem. 279, 20685–20691 (2004).
Zhao, Z. et al. PCMT1 generates the C-terminal cyclic imide degron on CRBN substrates. Preprint at bioRxiv https://doi.org/10.1101/2025.03.24.645050 (2025).
Pla‐Prats, C., Cavadini, S., Kempf, G. & Thomä, N. H. Recognition of the CCT5 di‐Glu degron by CRL4 DCAF12 is dependent on TRiC assembly. EMBO J. 42, e112253 (2023).
Ravichandran, R., Kodali, K., Peng, J. & Potts, P. R. Regulation of MAGE-A3/6 by the CRL4-DCAF12 ubiquitin ligase and nutrient availability. EMBO Rep. 20, e47352 (2019).
Hasenjäger, S., Bologna, A., Essen, L. O., Spadaccini, R. & Taxis, C. C-terminal sequence stability profiling in Saccharomyces cerevisiae reveals protective protein quality control pathways. J. Biol. Chem. 299, 105166 (2023).
Kong, K.-Y. E., Shankar, S., Rühle, F. & Khmelinskii, A. Orphan quality control by an SCF ubiquitin ligase directed to pervasive C-degrons. Nat. Commun. 14, 8363 (2023).
Muhar, M. F. et al. C-terminal amides mark proteins for degradation via SCF-FBXO31. Nature 638, 519–527 (2025).
Ravalin, M. et al. Specificity for latent C termini links the E3 ubiquitin ligase CHIP to caspases. Nat. Chem. Biol. 15, 786–794 (2019).
Ru, Y. et al. C-terminal glutamine acts as a C-degron targeted by E3 ubiquitin ligase TRIM7. Proc. Natl Acad. Sci. USA 119, e2203218119 (2022).
Liang, X. et al. A C-terminal glutamine recognition mechanism revealed by E3 ligase TRIM7 structures. Nat. Chem. Biol. 18, 1214–1223 (2022).
Kumar, M. et al. ELM — the eukaryotic linear motif resource — 2024 update. Nucleic Acids Res. 52, D442–D455 (2024).
Yada, M. et al. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 23, 2116–2125 (2004).
Welcker, M. et al. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc. Natl Acad. Sci. USA 101, 9085–9090 (2004).
Koepp, D. M. et al. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science 294, 173–177 (2001).
Strohmaier, H. et al. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 413, 316–322 (2001).
Fryer, C. J., White, J. B. & Jones, K. A. Mastermind recruits CycC:CDK8 to phosphorylate the notch ICD and coordinate activation with turnover. Mol. Cell 16, 509–520 (2004).
Jin, J. et al. SCFβ-TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes. Dev. 17, 3062–3074 (2003).
Liu, C. et al. β-Trcp couples β-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc. Natl Acad. Sci. USA 96, 6273–6278 (1999).
Hart, M. et al. The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr. Biol. 9, 207–211 (1999).
Busino, L. et al. Degradation of Cdc25A by β-TrCP during S phase and in response to DNA damage. Nature 426, 87–91 (2003).
Liu, C. et al. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108, 837–847 (2002).
Spencer, E., Jiang, J. & Chen, Z. J. Signal-induced ubiquitination of IkBα by the F-box protein Slimb/β-TrCP. Genes. Dev. 13, 284–294 (1999).
Peters, J.-M. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 7, 644–656 (2006).
Zhang, J. et al. Cyclin D–CDK4 kinase destabilizes PD-L1 via cullin 3–SPOP to control cancer immune surveillance. Nature 553, 91–95 (2018).
Kavsak, P. et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGFβ receptor for degradation. Mol. Cell 6, 1365–1375 (2000).
Willems, A. R. et al. Cdc53 targets phosphorylated G1 cyclins for degradation by the ubiquitin proteolytic pathway. Cell 86, 453–463 (1996).
Lanker, S., Valdivieso, M. H. & Wittenberg, C. Rapid degradation of the G1 cyclin Cln2 induced by CDK-dependent phosphorylation. Science 271, 1597–1601 (1996).
Barral, Y., Jentsch, S. & Mann, C. G1 cyclin turnover and nutrient uptake are controlled by a common pathway in yeast. Genes Dev. 9, 399–409 (1995).
Nash, P. et al. Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature 414, 514–521 (2001). This study shows that multisite phosphorylation of suboptimal Cdc4 phosphodegron motifs is crucial for establishing a regulatory threshold for temporal regulation of cell cycle progression.
Roff, M. et al. Role of IkBα ubiquitination in signal-induced activation of NFkB in vivo. J. Biol. Chem. 271, 7844–7850 (1996).
Papavassiliou, A. G., Treier, M., Chavrier, C. & Bohmann, D. Targeted degradation of c-Fos, but not v-Fos, by a phosphorylation-dependent signal on c-Jun. Science 258, 1941–1944 (1992).
Didonato, J. et al. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol. Cell Biol. 16, 1295–1304 (1996).
Vlach, J. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. EMBO J. 16, 5334–5344 (1997).
Carrano, A. C., Eytan, E., Hershko, A. & Pagano, M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1, 193–199 (1999).
Aberle, H., Bauer, A., Stappert, J., Kispert, A. & Kemler, R. β-Catenin is a target for the ubiquitin–proteasome pathway. EMBO J. 16, 3797–3804 (1997).
Orford, K., Crockett, C., Jensen, J. P., Weissman, A. M. & Byers, S. W. Serine phosphorylation-regulated ubiquitination and degradation of β-catenin. J. Biol. Chem. 272, 24735–24738 (1997).
Rada, P. et al. SCF/β-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol. Cell Biol. 31, 1121–1133 (2011).
Ding, Q. et al. Degradation of Mcl-1 by β-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol. Cell Biol. 27, 4006–4017 (2007).
Westbrook, T. F. et al. SCFβ-TRCP controls oncogenic transformation and neural differentiation through REST degradation. Nature 452, 370–374 (2008).
Nardone, C. et al. A central role for regulated protein stability in the control of TFE3 and MITF by nutrients. Mol. Cell 83, 57–73.e9 (2023).
Welcker, M. & Clurman, B. E. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat. Rev. Cancer 8, 83–93 (2008).
Nateri, A. S., Riera-Sans, L., Da Costa, C. & Behrens, A. The ubiquitin ligase SCFFbw7 antagonizes apoptotic JNK signaling. Science 303, 1374–1378 (2004).
Tetzlaff, M. T. et al. Defective cardiovascular development and elevated cyclin E and notch proteins in mice lacking the Fbw7 F-box protein. Proc. Natl Acad. Sci. USA 101, 3338–3345 (2004).
Yu, F., White, S. B., Zhao, Q. & Lee, F. S. HIF-1α binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc. Natl Acad. Sci. USA 98, 9630–9635 (2001).
Ivan, M. et al. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 (2001).
Lee, J. M. et al. EZH2 generates a methyl degron that is recognized by the DCAF1/DDB1/CUL4 E3 ubiquitin ligase complex. Mol. Cell 48, 572–586 (2012).
Sakaguchi, K. et al. Damage-mediated phosphorylation of human p53 threonine 18 through a cascade mediated by a casein 1-like kinase. J. Biol. Chem. 275, 9278–9283 (2000).
Wei, W. et al. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature 428, 194–198 (2004).
Irniger, S., Piatti, S., Michaelis, C. & Nasmyth, K. Genes involved in sister chromatid separation are needed for b-type cyclin proteolysis in budding yeast. Cell 81, 269–278 (1995).
McGarry, T. J. & Kirschner, M. W. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 93, 1043–1053 (1998).
Fang, G., Yu, H. & Kirschner, M. W. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol. Cell 2, 163–171 (1998).
Rape, M., Reddy, S. K. & Kirschner, M. W. The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell 124, 89–103 (2006).
D’Angiolella, V. et al. SCFCyclin F controls centrosome homeostasis and mitotic fidelity through CP110 degradation. Nature 466, 138–142 (2010).
Sauvé, V. et al. Mechanism of parkin activation by phosphorylation. Nat. Struct. Mol. Biol. 25, 623–630 (2018).
Ganoth, D. et al. The cell-cycle regulatory protein Cks1 is required for SCFSkp2-mediated ubiquitinylation of p27. Nat. Cell Biol. 3, 321–324 (2001).
Spruck, C. et al. A CDK-independent function of mammalian Cks1. Mol. Cell 7, 639–650 (2001).
Hao, B. et al. Structural basis of the Cks1-dependent recognition of p27Kip1 by the SCFSkp2 ubiquitin ligase. Mol. Cell 20, 9–19 (2005).
Havens, C. G. & Walter, J. C. Docking of a specialized PIP box onto chromatin-bound PCNA creates a degron for the ubiquitin ligase CRL4Cdt2. Mol. Cell 35, 93–104 (2009).
Havens, C. G. & Walter, J. C. Mechanism of CRL4Cdt2, a PCNA-dependent E3 ubiquitin ligase. Genes Dev. 25, 1568–1582 (2011).
Abbas, T. et al. PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex. Genes Dev. 22, 2496–2506 (2008).
Hessa, T. et al. Protein targeting and degradation are coupled for elimination of mislocalized proteins. Nature 475, 394–397 (2011). This study shows that BAG6 promotes the degradation of mislocalized proteins in the cytosol by recognizing exposed hydrophobic targeting signals.
Murakami, Y. et al. Ornithine decarboxylase is degraded by the 26S proteasome without ubiquitination. Nature 360, 597–9 (1992).
Rosenberg‐Hasson, Y., Bercovich, Z., Ciechanover, A. & Kahana, C. Degradation of ornithine decarboxylase in mammalian cells is ATP dependent but ubiquitin independent. Eur. J. Biochem. 185, 469–474 (1989).
Bercovich, Z., Rosenberg-Hasson, Y., Ciechanover, A. & Kahana, C. Degradation of ornithine decarboxylase in reticulocyte lysate is ATP-dependent but ubiquitin-independent. J. Biol. Chem. 264, 15949–15952 (1989). The studies by Murakami et al. 1992 and Berkovich et al. 1989 indicate that alongside the ubiquitin-dependent proteolytic pathway, reticulocyte lysates and mammalian cells also contain a ubiquitin-independent, proteasome-dependent pathway.
Matsufuji, S. et al. Autoregulatory frameshifting in decoding mammalian ornithine decarboxylase antizyme. Cell 80, 51–60 (1995).
Rom, E. & Kahana, C. Polyamines regulate the expression of ornithine decarboxylase antizyme in vitro by inducing ribosomal frame-shifting. Proc. Natl Acad. Sci. USA 91, 3959–3963 (1994).
Li, X. & Coffino, P. Degradation of ornithine decarboxylase: exposure of the C-terminal target by a polyamine-inducible inhibitory protein. Mol. Cell Biol. 13, 2377–2383 (1993).
Ghoda, L., Van Daalen Wetters, T., Macrae, M., Ascherman, D. & Coffino, P. Prevention of rapid intracellular degradation of ODC by a carboxyl-terminal truncation. Science 243, 1493–1495 (1989).
Zhang, M., Pickart, C. M. & Coffino, P. Determinants of proteasome recognition of ornithine decarboxylase, a ubiquitin-independent substrate. EMBO J. 22, 1488–1496 (2003).
Asher, G., Tsvetkov, P., Kahana, C. & Shaul, Y. A mechanism of ubiquitin-independent proteasomal degradation of the tumor suppressors p53 and p73. Genes Dev. 19, 316–321 (2005).
Wiggins, C. M. et al. BIMEL, an intrinsically disordered protein, is degraded by 20S proteasomes in the absence of poly-ubiquitylation. J. Cell Sci. 124, 969–977 (2011).
Adamovich, Y. et al. The protein level of PGC-1α, a key metabolic regulator, is controlled by NADH-NQO1. Mol. Cell Biol. 33, 2603–2613 (2013).
Chen, X., Barton, L. F., Chi, Y., Clurman, B. E. & Roberts, J. M. Ubiquitin-independent degradation of cell-cycle inhibitors by the REGγ proteasome. Mol. Cell 26, 843–852 (2007).
Chen, X. et al. N-acetylation and ubiquitin-independent proteasomal degradation of p21Cip1. Mol. Cell 16, 839–847 (2004).
Li, X. et al. Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGγ-proteasome pathway. Mol. Cell 26, 831–842 (2007).
Bossis, G., Ferrara, P., Acquaviva, C., Jariel-Encontre, I. & Piechaczyk, M. c-Fos proto-oncoprotein is degraded by the proteasome independently of its own ubiquitinylation in vivo. Mol. Cell Biol. 23, 7425–7436 (2003).
Basbous, J., Chalbos, D., Hipskind, R., Jariel-Encontre, I. & Piechaczyk, M. Ubiquitin-independent proteasomal degradation of Fra-1 is antagonized by Erk1/2 pathway-mediated phosphorylation of a unique C-terminal destabilizer. Mol. Cell Biol. 27, 3936–3950 (2007).
Forsthoefel, A. M., Peña, M. M. O., Xing, Y. Y., Rafique, Z. & Berger, F. G. Structural determinants for the intracellular degradation of human thymidylate synthase. Biochemistry 43, 1972–1979 (2004).
Ukmar-Godec, T. et al. Proteasomal degradation of the intrinsically disordered protein tau at single-residue resolution. Sci. Adv. 6, eaba3916 (2020).
Du, J. et al. Stuxnet facilitates the degradation of polycomb protein during development. Dev. Cell 37, 507–519 (2016).
Nardone, C. et al. Structural basis for the midnolin-proteasome pathway and its role in suppressing myeloma. Preprint at bioRxiv https://doi.org/10.1101/2025.02.22.639686 (2025).
Bahrami, S. & Drabløs, F. Gene regulation in the immediate-early response process. Adv. Biol. Regul. 62, 37–49 (2016).
Collins, G. A. & Goldberg, A. L. Proteins containing ubiquitin-like (Ubl) domains not only bind to 26S proteasomes but also induce their activation. Proc. Natl Acad. Sci. USA 117, 4664–4674 (2020).
Hipp, M. S., Kalveram, B., Raasi, S., Groettrup, M. & Schmidtke, G. FAT10, a ubiquitin-independent signal for proteasomal degradation. Mol. Cell Biol. 25, 3483–3491 (2005).
Rani, N., Aichem, A., Schmidtke, G., Kreft, S. G. & Groettrup, M. FAT10 and NUB1L bind to the VWA domain of Rpn10 and Rpn1 to enable proteasome-mediated proteolysis. Nat. Commun. 3, 749 (2012).
Schmidtke, G., Kalveram, B. & Groettrup, M. Degradation of FAT10 by the 26S proteasome is independent of ubiquitylation but relies on NUB1L. FEBS Lett. 583, 591–594 (2009).
Raasi, S., Schmidtke, G., De Giuli, R. & Groettrup, M. A ubiquitin-like protein which is synergistically inducible by interferon-γ and tumor necrosis factor-α. Eur. J. Immunol. 29, 4030–4036 (1999).
Aichem, A. & Groettrup, M. The ubiquitin-like modifier FAT10 – much more than a proteasome-targeting signal. J. Cell Sci. 133, jcs246041 (2020).
Aichem, A. et al. The proteomic analysis of endogenous FAT10 substrates identifies p62/SQSTM1 as a substrate of FAT10ylation. J. Cell Sci. 125, 4576–4585 (2012).
Padovani, C., Jevtić, P. & Rapé, M. Quality control of protein complex composition. Mol. Cell 82, 1439–1450 (2022).
Juszkiewicz, S. & Hegde, R. S. Quality control of orphaned proteins. Mol. Cell 71, 443–457 (2018).
Mena, E. L. et al. Dimerization quality control ensures neuronal development and survival. Science 362, eaap8236 (2018).
Mena, E. L. et al. Structural basis for dimerization quality control. Nature 586, 452–456 (2020). The studies by Mena et al. 2018 and Mena et al. 2020 delineate an example of how a conditionally exposed degron allows quality control of complex assembly.
Yanagitani, K., Juszkiewicz, S. & Hegde, R. S. UBE2O is a quality control factor for orphans of multiprotein complexes. Science 357, 472–475 (2017).
Mark, K. G. et al. Orphan quality control shapes network dynamics and gene expression. Cell 186, 3460–3475.e23 (2023).
Yagita, Y., Zavodszky, E., Peak-Chew, S.-Y. & Hegde, R. S. Mechanism of orphan subunit recognition during assembly quality control. Cell 186, 3443–3459.e24 (2023).
Carrillo Roas, S. et al. Convergence of orphan quality control pathways at a ubiquitin chain-elongating ligase. Mol. Cell 85, 815–828.e10 (2025).
Rodrigo-Brenni, M. C., Gutierrez, E. & Hegde, R. S. Cytosolic quality control of mislocalized proteins requires RNF126 recruitment to Bag6. Mol. Cell 55, 227–237 (2014).
Müller, M. B. D., Kasturi, P., Jayaraj, G. G. & Hartl, F. U. Mechanisms of readthrough mitigation reveal principles of GCN1-mediated translational quality control. Cell 186, 3227–3244.e20 (2023).
Kesner, J. S. et al. Noncoding translation mitigation. Nature 617, 395–402 (2023).
Hu, X. et al. RNF126-mediated reubiquitination is required for proteasomal degradation of p97-extracted membrane proteins. Mol. Cell 79, 320–331.e9 (2020).
Haakonsen, D. L. et al. Stress response silencing by an E3 ligase mutated in neurodegeneration. Nature 626, 874–880 (2024).
Itakura, E. et al. Ubiquilins chaperone and triage mitochondrial membrane proteins for degradation. Mol. Cell 63, 21–33 (2016).
Finger, Y. et al. Proteasomal degradation induced by DPP9‐mediated processing competes with mitochondrial protein import. EMBO J. 39, e103889 (2020).
Shimshon, A. et al. Dipeptidyl peptidases and E3 ligases of N-degron pathways cooperate to regulate protein stability. J. Cell Biol. 223, e202311035 (2024).
Salghetti, S. E. Destruction of Myc by ubiquitin-mediated proteolysis: cancer-associated and transforming mutations stabilize Myc. EMBO J. 18, 717–726 (1999).
Gregory, M. A. & Hann, S. R. c-Myc proteolysis by the ubiquitin-proteasome pathway: stabilization of c-Myc in Burkitt’s lymphoma cells. Mol. Cell Biol. 20, 2423–2435 (2000).
Bahram, F., von der Lehr, N., Cetinkaya, C. & Larsson, L.-G. c-Myc hot spot mutations in lymphomas result in inefficient ubiquitination and decreased proteasome-mediated turnover. Blood 95, 2104–2110 (2000).
Thompson, B. J. et al. The SCFFBW7 ubiquitin ligase complex as a tumor suppressor in T cell leukemia. J. Exp. Med. 204, 1825–1835 (2007).
Weng, A. P. et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306, 269–271 (2004).
Dhamija, S. et al. A pan-cancer analysis reveals nonstop extension mutations causing SMAD4 tumour suppressor degradation. Nat. Cell Biol. 22, 999–1010 (2020).
Senft, D., Qi, J. & Ronai, Z. A. Ubiquitin ligases in oncogenic transformation and cancer therapy. Nat. Rev. Cancer 18, 69–88 (2018).
Leach, F. S. et al. p53 mutation and MDM2 amplification in human soft tissue sarcomas. Cancer Res. 53, 2231–2234 (1993).
Reifenberger, G., Liu, L., Ichimura, K., Schmidt, E. E. & Collins, V. P. Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res. 53, 2736–2739 (1993).
Rajagopalan, H. et al. Inactivation of hCDC4 can cause chromosomal instability. Nature 428, 77–81 (2004).
Mao, J.-H. et al. Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature 432, 775–779 (2004).
Chen, Z. et al. Disease-associated KBTBD4 mutations in medulloblastoma elicit neomorphic ubiquitylation activity to promote CoREST degradation. Cell Death Differ. 29, 1955–1969 (2022).
Popovic, D., Vucic, D. & Dikic, I. Ubiquitination in disease pathogenesis and treatment. Nat. Med. 20, 1242–1253 (2014).
Meyer-Schwesinger, C. The ubiquitin–proteasome system in kidney physiology and disease. Nat. Rev. Nephrol. 15, 393–411 (2019).
Rotin, D. & Kumar, S. Physiological functions of the HECT family of ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 10, 398–409 (2009).
Rape, M. Ubiquitylation at the crossroads of development and disease. Nat. Rev. Mol. Cell Biol. 19, 59–70 (2018).
Zheng, Q. et al. Dysregulation of ubiquitin-proteasome system in neurodegenerative diseases. Front. Aging Neurosci. 8, 303 (2016).
George, A. J., Hoffiz, Y. C., Charles, A. J., Zhu, Y. & Mabb, A. M. A comprehensive atlas of E3 ubiquitin ligase mutations in neurological disorders. Front. Genet. 9, 29 (2018).
Clausen, L. et al. A mutational atlas for parkin proteostasis. Nat. Commun. 15, 1541 (2024).
Shimura, H. et al. Ubiquitination of a new form of α-synuclein by parkin from human brain: implications for Parkinson’s disease. Science 293, 263–269 (2001).
Kitada, T. et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 (1998).
Lin, Z. et al. Stabilizing mutations of KLHL24 ubiquitin ligase cause loss of keratin 14 and human skin fragility. Nat. Genet. 48, 1508–1516 (2016).
Knies, K. et al. Biallelic mutations in the ubiquitin ligase RFWD3 cause Fanconi anemia. J. Clin. Investig. 127, 3013–3027 (2017).
Ahel, J. et al. Moyamoya disease factor RNF213 is a giant E3 ligase with a dynein-like core and a distinct ubiquitin-transfer mechanism. eLife 9, e56185 (2020).
Locke, M., Tinsley, C. L., Benson, M. A. & Blake, D. J. TRIM32 is an E3 ubiquitin ligase for dysbindin. Hum. Mol. Genet. 18, 2344–2358 (2009).
Frosk, P. et al. Limb-girdle muscular dystrophy type 2H associated with mutation in TRIM32, a putative E3–ubiquitin-ligase gene. Am. J. Hum. Genet. 70, 663–672 (2002).
Bodine, S. C. & Baehr, L. M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 307, E469–E484 (2014).
Scheffner, M., Huibregtse, J. M., Vierstra, R. D. & Howley, P. M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75, 495–505 (1993).
Scheffner, M., Werness, B. A., Huibregtse, J. M., Levine, A. J. & Howley, P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63, 1129–1136 (1990).
Margottin, F. et al. A novel human WD protein, h-βTrCP, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell 1, 565–574 (1998).
Douglas, J. L. et al. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a βTrCP-dependent mechanism. J. Virol. 83, 7931–7947 (2009).
Wąchalska, M. et al. The herpesvirus UL49.5 protein hijacks a cellular C-degron pathway to drive TAP transporter degradation. Proc. Natl Acad. Sci. USA 121, e2309841121 (2024).
Rohde, J. R., Breitkreutz, A., Chenal, A., Sansonetti, P. J. & Parsot, C. Type III secretion effectors of the IpaH family are E3 ubiquitin ligases. Cell Host Microbe 1, 77–83 (2007).
Singer, A. U. et al. Structure of the Shigella T3SS effector IpaH defines a new class of E3 ubiquitin ligases. Nat. Struct. Mol. Biol. 15, 1293–1301 (2008).
Zhu, Y. et al. Structure of a Shigella effector reveals a new class of ubiquitin ligases. Nat. Struct. Mol. Biol. 15, 1302–1308 (2008).
Ravid, T. & Hochstrasser, M. Diversity of degradation signals in the ubiquitin–proteasome system. Nat. Rev. Mol. Cell Biol. 9, 679–689 (2008).
Chen, X. et al. Molecular basis for arginine C-terminal degron recognition by Cul2FEM1 E3 ligase. Nat. Chem. Biol. 17, 254–262 (2021).
Manford, A. G. et al. Structural basis and regulation of the reductive stress response. Cell 184, 5375–5390.e16 (2021).
Fischer, E. S. et al. Structure of the DDB1–CRBN E3 ubiquitin ligase in complex with thalidomide. Nature 512, 49–53 (2014).
Van Nguyen, T. et al. Glutamine triggers acetylation-dependent degradation of glutamine synthetase via the thalidomide receptor cereblon. Mol. Cell 61, 809–820 (2016).
Mancias, J. D. et al. Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis. eLife 4, e10308 (2015).
Davey, N. E. & Morgan, D. O. Building a regulatory network with short linear sequence motifs: lessons from the degrons of the anaphase-promoting complex. Mol. Cell 64, 12–23 (2016).
Gan, W. et al. SPOP promotes ubiquitination and degradation of the ERG oncoprotein to suppress prostate cancer progression. Mol. Cell 59, 917–930 (2015).
Inuzuka, H. et al. SCFFBW7 regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 471, 104–109 (2011).
Wei, W., Jin, J., Schlisio, S., Harper, J. W. & Kaelin, W. G. The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell 8, 25–33 (2005).
An, J. et al. Truncated ERG oncoproteins from TMPRSS2–ERG fusions are resistant to SPOP-mediated proteasome degradation. Mol. Cell 59, 904–916 (2015).
Ferretti, L. P. et al. Cullin3-KLHL15 ubiquitin ligase mediates CtIP protein turnover to fine-tune DNA-end resection. Nat. Commun. 7, 12628 (2016).
Langan, R. A. et al. De novo design of bioactive protein switches. Nature 572, 205–210 (2019).
Ng, A. H. et al. Modular and tunable biological feedback control using a de novo protein switch. Nature 572, 265–269 (2019).
Gao, X. J., Chong, L. S., Kim, M. S. & Elowitz, M. B. Programmable protein circuits in living cells. Science 361, 1252–1258 (2018).
Chassin, H. et al. A modular degron library for synthetic circuits in mammalian cells. Nat. Commun. 10, 2013 (2019).
Santner, A. & Estelle, M. Recent advances and emerging trends in plant hormone signalling. Nature 459, 1071–1078 (2009).
Zhang, L., Ward, J. D., Cheng, Z. & Dernburg, A. F. The auxin-inducible degradation (AID) system enables versatile conditional protein depletion in C. elegans. Development 142, 4374–4384 (2015).
Yesbolatova, A. et al. The auxin-inducible degron 2 technology provides sharp degradation control in yeast, mammalian cells, and mice. Nat. Commun. 11, 5701 (2020).
Holland, A. J., Fachinetti, D., Han, J. S. & Cleveland, D. W. Inducible, reversible system for the rapid and complete degradation of proteins in mammalian cells. Proc. Natl Acad. Sci. USA 109, E3350–E3357 (2012).
Nishimura, K., Fukagawa, T., Takisawa, H., Kakimoto, T. & Kanemaki, M. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat. Methods 6, 917–922 (2009).
Békés, M., Langley, D. R. & Crews, C. M. PROTAC targeted protein degraders: the past is prologue. Nat. Rev. Drug Discov. 21, 181–200 (2022).
Tsai, J. M., Nowak, R. P., Ebert, B. L. & Fischer, E. S. Targeted protein degradation: from mechanisms to clinic. Nat. Rev. Mol. Cell Biol. 25, 740–757 (2024).
Schneider, M. et al. The PROTACtable genome. Nat. Rev. Drug Discov. 20, 789–797 (2021).
Liu, Y. et al. Expanding PROTACtable genome universe of E3 ligases. Nat. Commun. 14, 6509 (2023).
Burroughs, A. M., Iyer, L. M. & Aravind, L. Structure and evolution of ubiquitin and ubiquitin-related domains. Methods Mol. Biol. 832, 15–63 (2012).
Battesti, A. & Gottesman, S. Roles of adaptor proteins in regulation of bacterial proteolysis. Curr. Opin. Microbiol. 16, 140–147 (2013).
Dixit, A. et al. Perturb-Seq: dissecting molecular circuits with scalable single-cell RNA profiling of pooled genetic screens. Cell 167, 1853–1866.e17 (2016).
Replogle, J. M. et al. Mapping information-rich genotype-phenotype landscapes with genome-scale Perturb-seq. Cell 185, 2559–2575.e28 (2022).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Klein, A. M. et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 161, 1187–1201 (2015).
Chaikovsky, A. C. et al. The AMBRA1 E3 ligase adaptor regulates the stability of cyclin D. Nature 592, 794–798 (2021).
Haupt, Y., Maya, R., Kazaz, A. & Oren, M. Mdm2 promotes the rapid degradation of p53. Nature 387, 296–299 (1997).
Gudjonsson, T. et al. TRIP12 and UBR5 suppress spreading of chromatin ubiquitylation at damaged chromosomes. Cell 150, 697–709 (2012).
Lafranchi, L. et al. APC/CCdh1 controls CtIP stability during the cell cycle and in response to DNA damage. EMBO J. 33, 2860–2879 (2014).
Sahasrabuddhe, A. A., Dimri, M., Bommi, P. V. & Dimri, G. P. βTrCP regulates BMI1 protein turnover via ubiquitination and degradation. Cell Cycle 10, 1322–1330 (2011).
Subramanian, A., Andronache, A., Li, Y. C. & Wade, M. Inhibition of MARCH5 ubiquitin ligase abrogates MCL1-dependent resistance to BH3 mimetics via NOXA. Oncotarget 7, 15986–16002 (2016).
Marti, A., Wirbelauer, C., Scheffner, M. & Krek, W. Interaction between ubiquitin-protein ligase SCFSKP2 and E2F-1 underlies the regulation of E2F-1 degradation. Nat. Cell Biol. 1, 14–19 (1999).
Vitari, A. C. et al. COP1 is a tumour suppressor that causes degradation of ETS transcription factors. Nature 474, 403–406 (2011).
Kim, Y., Starostina, N. G. & Kipreos, E. T. The CRL4Cdt2 ubiquitin ligase targets the degradation of p21Cip1 to control replication licensing. Genes Dev. 22, 2507–2519 (2008).
Wang, Z. et al. SCF β-TRCP promotes cell growth by targeting PR-Set7/Set8 for degradation. Nat. Commun. 6, 10185 (2015).
Pineda, C. T. et al. Degradation of AMPK by a cancer-specific ubiquitin ligase. Cell 160, 715–728 (2015).
Menzies, S. A. et al. The sterol-responsive RNF145 E3 ubiquitin ligase mediates the degradation of HMG-CoA reductase together with gp78 and hrd1. eLife 7, e40009 (2018).
Liu, C. C. et al. Cul3-KLHL20 ubiquitin ligase governs the turnover of ULK1 and VPS34 complexes to control autophagy termination. Mol. Cell 61, 84–97 (2016).
Ullah, K. et al. The E3 ubiquitin ligase STUB1 attenuates cell senescence by promoting the ubiquitination and degradation of the core circadian regulator BMAL1. J. Biol. Chem. 295, 4696–4708 (2020).
Zhu, H. et al. SPOP E3 ubiquitin ligase adaptor promotes cellular senescence by degrading the SENP7 deSUMOylase. Cell Rep. 13, 1183–1193 (2015).
Johmura, Y. et al. SCF Fbxo22-KDM4A targets methylated p53 for degradation and regulates senescence. Nat. Commun. 7, 10574 (2016).
Cao, Y. & Zhang, L. A Smurf1 tale: function and regulation of an ubiquitin ligase in multiple cellular networks. Cell. Mol. Life Sci. 70, 2305–2317 (2013).
Gupta-Rossi, N. et al. Functional interaction between SEL-10, an F-box protein, and the nuclear form of activated Notch1 receptor. J. Biol. Chem. 276, 34371–34378 (2001).
Zhang, Y. et al. DCAF 13 promotes pluripotency by negatively regulating SUV 39H1 stability during early embryonic development. EMBO J. 37, e98981 (2018).
Schwamborn, J. C., Berezikov, E. & Knoblich, J. A. The TRIM-NHL protein TRIM32 activates MicroRNAs and prevents self-renewal in mouse neural progenitors. Cell 136, 913–925 (2009).
Urbán, N. et al. Return to quiescence of mouse neural stem cells by degradation of a proactivation protein. Science 353, 292–295 (2016).
Spencer, E., Jiang, J. & Chen, Z. J. Signal-induced ubiquitination of IκBα by the F-box protein Slimb/β-TrCP. Genes. Dev. 13, 284–94 (1999).
Zhong, B. et al. The ubiquitin ligase RNF5 regulates antiviral responses by mediating degradation of the adaptor protein MITA. Immunity 30, 284–294 (2009).
Sun, A. X. et al. Potassium channel dysfunction in human neuronal models of Angelman syndrome. Science 366, 1486–1492 (2019).
Chung, K. K. K. et al. Parkin ubiquitinates the α-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat. Med. 7, 1144–1150 (2001).
Wang, X. et al. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell 128, 129–139 (2007).
Maxwell, P. H. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999).
McMahon, M., Itoh, K., Yamamoto, M. & Hayes, J. D. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 278, 21592–21600 (2003).
Manford, A. G. et al. A cellular mechanism to detect and alleviate reductive stress. Cell 183, 46–61.e21 (2020).
Evans, R. et al. Protein complex prediction with AlphaFold-multimer. Preprint at bioRxiv https://doi.org/10.1101/2021.10.04.463034 (2022).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Watson, J. L. et al. De novo design of protein structure and function with RFdiffusion. Nature 620, 1089–1100 (2023).
Mashahreh, B. et al. Conserved degronome features governing quality control associated proteolysis. Nat. Commun. 13, 7588 (2022).
Khmelinskii, A. et al. Tandem fluorescent protein timers for in vivo analysis of protein dynamics. Nat. Biotechnol. 30, 708–714 (2012).
Maurer, M. J. et al. Degradation signals for ubiquitin-proteasome dependent cytosolic protein quality control (CytoQC) in yeast. G3 6, 1853–1866 (2016).
Acknowledgements
We thank W. Xu (Harvard), C. Woo (Harvard), E. Shrock and C. Nardone (Harvard Medical School) and members of the Elledge lab for helpful suggestions. Z.Z. is a Croucher Ph.D. Scholar. E.L.M. is supported by a US National Institutes of Health (NIH) K99 award (no. K99AG081456). R.T.T. is a Pemberton-Trinity Fellow and is supported by the European Research Council (ERC-2024-STG 101160971). I.K. is supported by the European Research Council (ERC-2020-STG 947709), the Israel Science Foundation (ISF grant nos. 2380/21 and 3096/21) and the United States–Israel Binational Science Foundation (grant no. 2021029). This work was supported by NIH Aging grant no. AG11085 (to S.J.E.). S.J.E. is a member of the Ludwig Center at Harvard and an Investigator with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
Z.Z., I.K., R.T.T. and E.L.M. wrote the manuscript, supervised by S.J.E. Z.Z. and I.K. prepared the figures with input from S.J.E. All authors discussed, reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Peter Kaiser and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Chaperone
-
A class of proteins that promote folding, assembly, or disassembly of other proteins. They help prevent misfolding and aggregation, especially under stress conditions. In certain contexts, they can promote the degradation of aberrant substrates by recruiting E3s.
- C-terminome stability profiling
-
A systems biology approach to assess the influence of C-terminal sequences on protein stability.
- Global protein stability (GPS)–peptidome system
-
An adaptation of the GPS platform that focuses on measuring the degradation profiles of short peptide sequences to identify degron motifs.
- GPS
-
(Global protein stability). A reporter-based genetic screening method for quantifying the relative stabilities of thousands of proteins in mammalian cells by generating GFP-fusion lentiviral libraries.
- GPT-4
-
A large language model developed by OpenAI, designed to perform advanced natural language processing and reasoning tasks. GPT-4 can synthesize information, extract structured knowledge from unstructured text and assist in the automated curation of biological data, offering new possibilities for accelerating discovery in life sciences.
- Immediate early gene
-
Immediate early genes encode transcription factors from the Fos, EGR and NR4A families. Although the proteins encoded are rapidly and consistently activated across nearly all mammalian cell types, they drive the expression of late-response genes in a cell-type-specific manner, shaping the appropriate response to the initial stimulus.
- Molecular degraders
-
Small molecules that induce selective degradation of target proteins by recruiting them to an E3.
- p97 AAA ATPase
-
A hexameric ATPase that extracts ubiquitinated substrates from membranes or complexes for proteasomal degradation.
- Proteasome
-
The proteasome is a large protein complex responsible for degrading unwanted or damaged proteins tagged with ubiquitin, maintaining protein quality and regulating cellular processes. It consists of a 20S core particle, which carries out proteolysis, and one or two 19S regulatory particles that recognize ubiquitinated proteins, unfold them and feed them into the 20S core for degradation.
- Ribosome quality control
-
A surveillance pathway that detects and resolves stalled ribosomes. It ensures the degradation of incomplete nascent polypeptides and the recycling of ribosomal subunits to maintain translation fidelity and protein homeostasis.
- Thalidomide class of drugs
-
Immunomodulatory imide drugs, including thalidomide, lenalidomide and pomalidomide, act as molecular glues by promoting neosubstrate recognition by the CUL4CRBN E3 complex.
- Ubiquilin
-
A family of ubiquitin-like (Ubl) and ubiquitin-associated (Uba) domain-containing adaptor proteins that can target proteins to the proteasome for both ubiquitin-dependent and ubiquitin-independent degradation.
- Ubiquitin-like (Ubl) domain
-
Ubiquitin-like (Ubl) domains are protein domains that share structural similarity with ubiquitin. Small proteins such as SUMO, NEDD8 and FAT10 are composed mainly of a Ubl domain and can be covalently conjugated to target proteins, thereby modifying their stability, activity or localization; larger proteins such as the ubiquilins contain a Ubl domain in addition to other protein domains, with the Ubl domain thought to enable interactions with the proteasome or other cellular machinery.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Z., Mena, E.L., Timms, R.T. et al. Degrons: defining the rules of protein degradation. Nat Rev Mol Cell Biol (2025). https://doi.org/10.1038/s41580-025-00870-z
Accepted:
Published:
DOI: https://doi.org/10.1038/s41580-025-00870-z