Abstract
In December 2019, a novel coronavirus was isolated from the respiratory epithelium of patients with unexplained pneumonia in Wuhan, China. This pathogen, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causes a pathogenic condition that has been termed coronavirus disease 2019 (COVID-19) and has reached pandemic proportions. As of 17 September 2020, more than 30 million confirmed SARS-CoV-2 infections have been reported in 204 different countries, claiming more than 1 million lives worldwide. Accumulating evidence suggests that SARS-CoV-2 infection can lead to a variety of clinical conditions, ranging from asymptomatic to life-threatening cases. In the early stages of the disease, most patients experience mild clinical symptoms, including a high fever and dry cough. However, 20% of patients rapidly progress to severe illness characterized by atypical interstitial bilateral pneumonia, acute respiratory distress syndrome and multiorgan dysfunction. Almost 10% of these critically ill patients subsequently die. Insights into the pathogenic mechanisms underlying SARS-CoV-2 infection and COVID-19 progression are emerging and highlight the critical role of the immunological hyper-response — characterized by widespread endothelial damage, complement-induced blood clotting and systemic microangiopathy — in disease exacerbation. These insights may aid the identification of new or existing therapeutic interventions to limit the progression of early disease and treat severe cases.
Key points
-
Although most patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) experience mild to moderate symptoms that resolve after 6–10 days, almost 20% of patients develop severe illness characterized by atypical interstitial bilateral pneumonia and acute respiratory distress syndrome, with a high fatality rate.
-
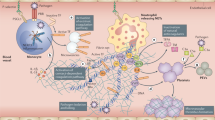
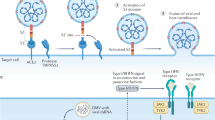
Accumulating evidence suggests that an unbalanced and unrestrained innate immune response, which comes at the expense of effective adaptive immunity, underpins the progression of coronavirus disease 2019 (COVID-19).
-
In severe cases of COVID-19, massive endothelial dysfunction, widespread coagulopathy and complement-induced thrombosis can lead to the development of systemic microangiopathy and thromboembolism; these complications can be life-threatening and ultimately lead to multi-organ failure.
-
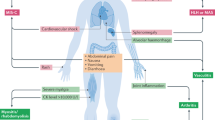
The kidney is one of the main targets of COVID-19 complications, and abnormal kidney function is associated with a significantly increased risk of death in severely ill patients.
-
Most repurposed anti-viral drugs have failed to improve clinical outcomes in COVID-19; by contrast, therapeutic interventions that target the host response, including the hyper-immune response, complement activation and systemic thrombosis, seem to be more promising approaches to the treatment of severe COVID-19.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Andersen, K. G., Rambaut, A., Lipkin, W. I., Holmes, E. C. & Garry, R. F. The proximal origin of SARS-CoV-2. Nat. Med. 6, 450–452 (2020).
Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020).
Shi, J. et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 368, 1016–1020 (2020).
Zhang, Y. Z. & Holmes, E. C. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell 181, 223–227 (2020).
Zhou, H. et al. A novel bat coronavirus closely related to SARS-CoV-2 contains natural insertions at the S1/S2 cleavage site of the spike protein. Curr. Biol. 30, 2196–2203.e3 (2020).
Zhou, Y. et al. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 6, 14–18 (2020).
Peng, Q. et al. Structural and biochemical characterization of nsp12-nsp7-nsp8 core polymerase complex from SARS-CoV-2. Cell Rep. 31, 107774 (2020).
Kirchdoerfer, R. N. & Ward, A. B. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat. Commun. 10, 2342 (2019).
Zumla, A., Chan, J. F. W., Azhar, E. I., Hui, D. S. C. & Yuen, K.-Y. Coronaviruses — drug discovery and therapeutic options. Nat. Rev. Drug Discov. 15, 327–347 (2016).
Hoffmann, M. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280.e8 (2020).
Shang, J. et al. Cell entry mechanisms of SARS-CoV-2. Proc. Natl Acad. Sci. USA 117, 11727–11734 (2020).
Wan, Y., Shang, J., Graham, R., Baric, R. S. & Li, F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. https://doi.org/10.1128/JVI.00127-20 (2020).
Jaimes, J. A., Millet, J. K. & Whittaker, G. R. Proteolytic cleavage of the SARS-CoV-2 spike protein and the role of the novel S1/S2 site. iScience 23, 101212 (2020).
Perico, L., Benigni, A. & Remuzzi, G. Should COVID-19 concern nephrologists? Why and to what extent? The emerging impasse of angiotensin blockade. Nephron 144, 213–221 (2020).
Li, Y. et al. The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 spike. iScience 23, 101160 (2020).
Xu, H. et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral. Sci. 12, 1–5 (2020).
Sungnak, W. et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 26, 681–687 (2020).
Ziegler, C. G. K. et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181, 1016–1035.e19 (2020).
Wölfel, R. et al. Virological assessment of hospitalized patients with COVID-2019. Nature 581, 465–469 (2020).
Li, Q. et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 382, 1199–1207 (2020).
Hou, Y. J. et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 182, 429–446.e14 (2020).
Shi, Y. et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 27, 1451–1454 (2020).
Cao, X. COVID-19: immunopathology and its implications for therapy. Nat.Rev. Immunol. 20, 269–270 (2020).
Ackermann, M. et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 383, 120–128 (2020).
Aguiar, D., Lobrinus, J. A., Schibler, M., Fracasso, T. & Lardi, C. Inside the lungs of COVID-19 disease. Int. J. Legal Med. 34, 1271–1274 (2020).
Pernazza, A. et al. Early histologic findings of pulmonary SARS-CoV-2 infection detected in a surgical specimen. Virchows Arch. https://doi.org/10.1007/s00428-020-02829-1 (2020).
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020).
Xu, Z. et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 8, 420–422 (2020).
Fan, E. et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir. Med. 8, 816–821 (2020).
Marini, J. J. & Gattinoni, L. Management of COVID-19 respiratory distress. JAMA 323, 2329–2330 (2020).
Patel, A. et al. New onset anosmia and ageusia in adult patients diagnosed with SARS-CoV-2. Clin. Microbiol. Infect. 26, 1236–1241 (2020).
Romero-Sánchez, C. M. et al. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology 95, e1060–e1070 (2020).
Dockery, D. M., Rowe, S. G., Murphy, M. A. & Krzystolik, M. G. The ocular manifestations and transmission of COVID-19: recommendations for prevention. J. Emerg. Med. 59, 137–140 (2020).
Suresh Kumar, V. C. et al. Novelty in the gut: a systematic review and meta-analysis of the gastrointestinal manifestations of COVID-19. BMJ Open Gastroenterol. 7, e000417 (2020).
J J Jin, X. et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 69, 1002–1009 (2020).
Shi, S. et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 5, 802–810 (2020).
Zang, R. et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 5, eabc3582 (2020).
Lamers, M. M. et al. SARS-CoV-2 productively infects human gut enterocytes. Science 369, 50–54 (2020).
Grajewski, R. S. et al. Letter to the editor: a missing link between SARS-CoV-2 and the eye?: ACE2 expression on the ocular surface. J. Med. Virol. https://doi.org/10.1002/jmv.26136 (2020).
Lucas, C. et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584, 463–469 (2020).
Giamarellos-Bourboulis, E. J. et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 27, 992–1000.e3 (2020).
Mathew, D. et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 369, eabc8511 (2020).
McKechnie, J. L. & Blish, C. A. The innate immune system: fighting on the front lines or fanning the flames of COVID-19? Cell Host Microbe 27, 863–869 (2020).
Vabret, N. et al. Immunology of COVID-19: current state of the science. Immunity 52, 910–941 (2020).
Blanco-Melo, D. et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181, 1036–1045.e9 (2020).
Park, A. & Iwasaki, A. Type I. and type III interferons — induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe 27, 870–878 (2020).
Thoms, M. et al. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science 369, 1249–1255 (2020).
Jiang, H. et al. SARS-CoV-2 Orf9b suppresses type I interferon responses by targeting TOM70. Cell. Mol. Immunol. 17, 998–1000 (2020).
Vazquez, C. & Horner, S. M. MAVS coordination of antiviral innate immunity. J. Virol. 89, 6974–6977 (2015).
Gordon, D. E. et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583, 459–468 (2020).
Hadjadj, J. et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369, 718–724 (2020).
Trouillet-Assant, S. et al. Type I IFN immunoprofiling in COVID-19 patients. J. Allergy Clin. Immunol. 146, 206–208.e2 (2020).
Mehta, P. et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395, 1033–1034 (2020).
Yang, Y. et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J. Allergy Clin. Immunol. 146, 119–127.e4 (2020).
Usmani, G. N., Woda, B. A. & Newburger, P. E. Advances in understanding the pathogenesis of HLH. Br. J. Haematol. 161, 609–622 (2013).
McGonagle, D., Sharif, K., O’Regan, A. & Bridgewood, C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 19, 102537 (2020).
Sinha, P., Matthay, M. A. & Calfee, C. S. Is a “cytokine storm” relevant to COVID-19? JAMA Intern. Med. 180, 1152–1154 (2020).
Pedersen, S. F. & Ho, Y.-C. SARS-CoV-2: a storm is raging. J. Clin. Invest. 130, 2202–2205 (2020).
Wang, C. et al. Aveolar macrophage activation and cytokine storm in the pathogenesis of severe COVID-19. EBioMedicine 57, 102833 (2020).
Liao, M. et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 26, 842–844 (2020).
B Bost, P. et al. Host-viral infection maps reveal signatures of severe COVID-19 patients. Cell 181, 1475–1488.e12 (2020).
Shen, B. et al. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell 182, 59–72.e15 (2020).
Giordano, S., Kramer, P., Darley-Usmar, V. M., & White C. R. in Apolipoprotein Mimetics in the Management of Human Disease (eds Anantharamaiah, G., Goldberg, D.)(Adis, Springer International Publishing, 2015).
Li, J. et al. Virus-host interactome and proteomic survey of PMBCs from COVID-19 patients reveal potential virulence factors influencing SARS-CoV-2 pathogenesis. Med https://doi.org/10.1016/j.medj.2020.07.002 (2020).
Z Zhou, Z. et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe 27, 883–890.e2 (2020).
Wang, D. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323, 1061–1069 (2020).
Liu, J. et al. Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. Preprint at medRxiv https://doi.org/10.1101/2020.02.10.20021584 (2020).
Carissimo, G. et al. Whole blood immunophenotyping uncovers immature neutrophil-to-VD2 T-cell ratio as an early prognostic marker for severe COVID-19. Preprint at bioRxiv https://doi.org/10.1101/2020.06.11.147389 (2020).
Barnes, B. J. et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 217, e20200652 (2020).
Chen, G. et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 130, 2620–2629 (2020).
Yang, X. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 8, 475–481 (2020).
Tan, L. et al. Validation of predictors of disease severity and outcomes in COVID-19 patients: a descriptive and retrospective study. Med https://doi.org/10.1016/j.medj.2020.05.002 (2020).
Zheng, M. et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 17, 533–535 (2020).
Diao, B. et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front. Immunol. 11, 827 (2020).
Chen, Y. et al. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. Preprint at medRxiv https://doi.org/10.1101/2020.03.27.20045427 (2020).
Bellesi, S. et al. Increased CD95 (Fas) and PD-1 expression in peripheral blood T lymphocytes in COVID-19 patients. Br. J.Haematol. https://doi.org/10.1111/bjh.17034 (2020).
Qin, C. et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 71, 762–768 (2020).
Bermejo-Martin, J. F. et al. Lymphopenic community acquired pneumonia as signature of severe COVID-19 infection. J. Infect. 80, e23–e24 (2020).
Bermejo-Martin, J. F. et al. Lymphopenic community acquired pneumonia (L-CAP), an immunological phenotype associated with higher risk of mortality. EBioMedicine 24, 231–236 (2017).
Menéndez, R. et al. simultaneous depression of immunological synapse and endothelial injury is associated with organ dysfunction in community-acquired pneumonia. J. Clin. Med. 8, 1404 (2019).
Grifoni, A. et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 181, 1489–1501.e15 (2020).
Peng, Y. et al. Broad and strong memory CD4 + and CD8 + T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. https://doi.org/10.1038/s41590-020-0782-6 (2020).
Le Bert, N. et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 584, 457–462 (2020).
Weiskopf, D. et al. Phenotype and kinetics of SARS-CoV-2–specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 5, eabd2071 (2020).
Braun, J. et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 217, e20200206 (2020).
Cassotta, A. et al. Deciphering and predicting CD4+ T cell immunodominance of influenza virus hemagglutinin. J. Exp. Med. 217, e20200206 (2020).
Mateus, J. et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 26, 845–848 (2020).
Long, Q. X. et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 26, 845–848 (2020).
Wang, Y.-T. et al. Spiking pandemic potential: structural and immunological aspects of SARS-CoV-2. Trends Microbiol. 28, 605–618 (2020).
Yuan, M. et al. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science 368, 630–633 (2020).
Wu, Y. et al. Identification of human single-domain antibodies against SARS-CoV-2. Cell Host Microbe 27, 891–898.e5 (2020).
Chandrashekar, A. et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 369, 812–817 (2020).
Deng, W. et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science 369, 818–823 (2020).
Zost, S. J. et al. Potently neutralizing human antibodies that block SARS-CoV-2 receptor binding and protect animals. Preprint at bioRxiv https://doi.org/10.1101/2020.05.22.111005 (2020).
Long, Q.-X. et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 26, 1200–1204 (2020).
Seow, J. et al. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection. Preprint at medRxiv https://doi.org/10.1101/2020.07.09.20148429 (2020).
Ibarrondo, F. J. et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N. Engl. J. Med. 83, 1085–1087 (2020).
Gudbjartsson, D. F. et al. Humoral immune response to SARS-CoV-2 in Iceland. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2026116 (2020).
Seydoux, E. et al. Analysis of a SARS-CoV-2-infected individual reveals development of potent neutralizing antibodies with limited somatic mutation. Immunity 53, 98–105.e5 (2020).
Juno, J. A. et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat. Med. 26, 1428–1434 (2020).
Robbiani, D. F. et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 584, 437–442 (2020).
Kuri-Cervantes, L. et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 5, eabd7114 (2020).
Wilk, A. J. et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 26, 1070–1076 (2020).
Woodruff, M. et al. Critically ill SARS-CoV-2 patients display lupus-like hallmarks of extrafollicular B cell activation. Preprint at medRxiv https://doi.org/10.1101/2020.04.29.20083717 (2020).
Amrun, S. N. et al. Linear B-cell epitopes in the spike and nucleocapsid proteins as markers of SARS-CoV-2 exposure and disease severity. EBioMedicine 58, 102911 (2020).
Wu, F. et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. Preprint at medRxiv https://doi.org/10.1101/2020.03.30.20047365 (2020).
Liu, L. et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight 4, e123158 (2019).
Yu, H. et al. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur. Respir. J. 56, 2001526 (2020).
Hotez, P. J., Corry, D. B. & Bottazzi, M. E. COVID-19 vaccine design: the Janus face of immune enhancement. Nat. Rev. Immunol. 20, 347–348 (2020).
Olson, J. D. D-dimer: an overview of hemostasis and fibrinolysis, assays, and clinical applications. Adv. Clin. Chem. 69, 1–46 (2015).
Yau, J. W., Teoh, H. & Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 15, 130 (2015).
Reitsma, S., Slaaf, D. W., Vink, H., van Zandvoort, M. A. M. J. & oude Egbrink, M. G. A. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 454, 345–359 (2007).
Teuwen, L. A., Geldhof, V., Pasut, A. & Carmeliet, P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 20, 389–391 (2020).
Varga, Z. et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 395, 1417–1418 (2020).
Oxley, T. J. et al. Large-vessel stroke as a presenting feature of covid-19 in the Young. N. Engl. J. Med. 382, e60 (2020).
Cheng. M. European Doctors Warn Rare Kids’ Syndrome May Have Virus Tie. (Associated Press, 2020).
Bunyavanich, S., Do, A. & Vicencio, A. Nasal gene expression of angiotensin-converting Enzyme 2 in children and adults. JAMA 323, 2427–2429 (2020).
Riphagen, S., Gomez, X., Gonzalez-Martinez, C., Wilkinson, N. & Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 395, 1607–1608 (2020).
Diorio, C. et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J. Clin. Invest. https://doi.org/10.1172/JCI140970 (2020).
Cheung, E. W. et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA 324, 294–296 (2020).
Verdoni, L. et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 395, 1771–1778 (2020).
Zahra, B. et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation https://doi.org/10.1161/CIRCULATIONAHA.120.048360 (2020).
Monteil, V. et al. Inhibition of SARS-CoV-2 Infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 181, 905–913.e7 (2020).
Verdecchia, P., Cavallini, C., Spanevello, A. & Angeli, F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 76, 14–20 (2020).
Garvin, M. R. et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. eLife 9, e59177 (2020).
Schmaier, A. H. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J. Thromb. Haemost. 14, 28–39 (2016).
Schmaier, A. H. A novel antithrombotic mechanism mediated by the receptors of the kallikrein/kinin and renin–angiotensin systems. Front. Med. 3, 61 (2016).
Stavrou, E. X. et al. Reduced thrombosis in Klkb1−/− mice is mediated by increased Mas receptor, prostacyclin, Sirt1, and KLF4 and decreased tissue factor. Blood 125, 710–719 (2015).
Sodhi, C. P. et al. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am. J. Physiol. Lung Cell Mol. Physiol. 314, L17–L31 (2018).
Chung, M. K. et al. SARS-CoV-2 and ACE2: the biology and clinical data settling the ARB and ACEI controversy. EBioMedicine 58, 102907 (2020).
Fang, C. et al. Angiotensin 1-7 and Mas decrease thrombosis in Bdkrb2−/− mice by increasing NO and prostacyclin to reduce platelet spreading and glycoprotein VI activation. Blood 121, 3023–3032 (2013).
Veerdonk, F. L. van de et al. Outcomes associated with use of a kinin B2 receptor antagonist among patients with COVID-19. JAMA Netw. Open 3, e2017708–e2017708 (2020).
van de Veerdonk, F. L. et al. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. eLife 9, e57555 (2020).
Roche, J. A. & Roche, R. A hypothesized role for dysregulated bradykinin signaling in COVID-19 respiratory complications. FASEB J. 34, 7265–7269 (2020).
Buzhdygan, T. P. et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in vitro models of the human blood–brain barrier. Preprint at bioRxiv https://doi.org/10.1101/2020.06.15.150912 (2020).
Martini, R. The compelling arguments for the need of microvascular investigation in COVID-19 critical patients. Clin. Hemorheol. Microcirc. 75, 27–34 (2020).
Noris, M., Benigni, A. & Remuzzi, G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 98, 314–322 (2020).
Song, W. C. & FitzGerald, G. A. COVID-19, microangiopathy, hemostatic activation, and complement. J. Clin. Invest. 130, 3950–3953 (2020).
Gralinski, L. E. et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio 9, e01753–18 (2018).
Ip, W. K. E. et al. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 191, 1697–1704 (2005).
Jiang, Y. et al. Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg. Microbes Infect. 7, 77 (2018).
Gao, T. et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. Preprint at medRxiv https://doi.org/10.1101/2020.03.29.20041962 (2020).
Yang, Y. H. et al. Autoantibodies against human epithelial cells and endothelial cells after severe acute respiratory syndrome (SARS)-associated coronavirus infection. J. Med. Virol. 77, 1–7 (2005).
Messner, C. B. et al. Ultra-high-throughput clinical proteomics reveals classifiers of COVID-19 infection. Cell Syst. 11, 11–24.e4 (2020).
Risitano, A. M. et al. Complement as a target in COVID-19? Nat. Rev. Immunol. 20, 343–344 (2020).
McGonagle, D., O’Donnell, J. S., Sharif, K., Emery, P. & Bridgewood, C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2, e437–e445 (2020).
Fox, S. E. et al. Pulmonary and cardiac pathology in Covid-19: the first autopsy series from New Orleans. Preprint at medRxiv https://doi.org/10.1101/2020.04.06.20050575 (2020).
Carsana, L. et al. Pulmonary post-mortem findings in a large series of COVID-19 cases from Northern Italy. Preprint at medRxiv https://doi.org/10.1101/2020.04.19.20054262 (2020).
Tang, N., Li, D., Wang, X. & Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 18, 844–847 (2020).
Danzi, G. B., Loffi, M., Galeazzi, G. & Gherbesi, E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur. Heart J. 41, 1858 (2020).
Llitjos, J. F. et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 18, 1743–1746 (2020).
Zuckier, L. S., Moadel, R. M., Haramati, L. B. & Freeman, L. Diagnostic evaluation of pulmonary embolism during the COVID-19 pandemic. J. Nucl. Med. 61, 630–631 (2020).
Beun, R., Kusadasi, N., Sikma, M., Westerink, J. & Huisman, A. Thromboembolic events and apparent heparin resistance in patients infected with SARS-CoV-2. Int. J. Lab. Hematol. 42, 19–20 (2020).
Levi, M. & van der Poll, T. Coagulation and sepsis. Thromb. Res. 149, 38–44 (2017).
Radermecker, C. et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J. Exp. Med. 217, e20201012 (2020).
Cheng, Y. et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 97, 829–838 (2020).
Gabarre, P. et al. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 46, 1339–1348 (2020).
Chan, L. et al. AKI in hospitalized patients with COVID-19. J. Am. Soc. Nephrol. https://doi.org/10.1681/ASN.2020050615 (2020).
Ali, H. et al. Survival rate in acute kidney injury superimposed COVID-19 patients: a systematic review and meta-analysis. Ren. Fail. 42, 393–397 (2020).
Lim, J.-H. et al. Fatal outcomes of COVID-19 in patients with severe acute kidney injury. J. Clin. Med. 9, 1718 (2020).
Division of Nephrology, Columbia University Vagelos College of Physicians. Disaster Response to the COVID-19 Pandemic for Patients with Kidney Disease in New York City. J. Am. Soc. Nephrol. 31, 1371–1379 (2020).
Alberici, F. et al. Management of patients on dialysis and with kidney transplantation during the SARS-CoV-2 (COVID-19) pandemic in Brescia, Italy. Kidney Int. Rep. 5, 580–585 (2020).
Su, H. et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 98, 219–227 (2020).
Magro, C. et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 220, 1–13 (2020).
Diao, B. et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Preprint at medRxiv https://doi.org/10.1101/2020.03.04.20031120 (2020).
Larsen, C. P., Bourne, T. D., Wilson, J. D., Saqqa, O. & Sharshir, M. A. collapsing glomerulopathy in a patient with COVID-19. Kidney Int. Rep. 5, 935–939 (2020).
Magoon, S. et al. COVID-19–related glomerulopathy: a report of 2 cases of collapsing focal segmental glomerulosclerosis. Kidney Med. 2, 488–492 (2020).
Hepokoski, M. L., Malhotra, A., Singh, P. & Crotty Alexander, L. E. Ventilator-induced kidney injury: are novel biomarkers the key to prevention? Nephron 140, 90–93 (2018).
Durvasula, R., Wellington, T., McNamara, E. & Watnick, S. COVID-19 and kidney failure in the acute care setting: our experience from Seattle. Am. J. Kidney Dis. 76, 4–6 (2020).
Naicker, S. et al. The novel coronavirus 2019 epidemic and kidneys. Kidney Int. 97, 824–828 (2020).
Farkash, E. A., Wilson, A. M. & Jentzen, J. M. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J. Am. Soc. Nephrol. 31, 1683–1687 (2020).
Abbate, M., Rottoli, D. & Gianatti, A. COVID-19 attacks the kidney: ultrastructural evidence for the presence of virus in the glomerular epithelium. Nephron 144, 341–342 (2020).
Xu, D. et al. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 46, 1114–1116 (2020).
Ye, M. et al. Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: implications for albuminuria in diabetes. J. Am. Soc. Nephrol. 17, 3067–3075 (2006).
Samavati, L. & Uhal, B. D. ACE2, much more than just a receptor for SARS-COV-2. Front. Cell. Infect. Microbiol. 10, 317 (2020).
Puelles, V. G. et al. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 383, 590–592 (2020).
Miller, S. E. & Goldsmith, C. S. Caution in identifying coronaviruses by electron microscopy. J. Am. Soc. Nephrol. 31, 2223–2224 (2020).
Wu, H. et al. AKI and collapsing glomerulopathy associated with COVID-19 and APOL1 high-risk genotype. J. Am. Soc. Nephrol. 31, 1688–1695 (2020).
Kudose, S. et al. Kidney biopsy findings in patients with COVID-19. J. Am. Soc. Nephrol. 31, 1959–1968 (2020).
Santoriello, D. et al. Postmortem kidney pathology findings in patients with COVID-19. J. Am. Soc. Nephrol. 31, 2158–2167 (2020).
Sharma, P. et al. COVID-19-associated kidney injury: a case series of kidney biopsy findings. J. Am. Soc. Nephrol. 31, 1948–1958 (2020).
Hanley, B. et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe https://doi.org/10.1016/S2666-5247(20)30115-4 (2020).
Ma, Y. et al. 2019 novel coronavirus disease in hemodialysis (HD) patients: Report from one HD center in Wuhan, China. Preprint at medRxiv https://doi.org/10.1101/2020.02.24.20027201 (2020).
Wang, R. et al. COVID-19 in hemodialysis patients: a report of 5 cases. Am. J. Kidney Dis. 76, 141–143 (2020).
Kikuchi, K. et al. COVID-19 in dialysis patients in Japan: current status and guidance on preventive measures. Ther. Apher. Dial. 24, 361–365 (2020).
Corbett, R. W. et al. Epidemiology of COVID-19 in an urban dialysis center. J. Am. Soc. Nephrol. 31, 1815–1823 (2020).
World Health Organization. Draft landscape of COVID-19 candidate vaccines https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (2020).
Begum, J. et al. Challenges and prospects of COVID-19 vaccine development based on the progress made in SARS and MERS vaccine development. Transbound Emerg. Dis. https://doi.org/10.1111/tbed.13804 (2020).
Shannon, A. et al. Remdesivir and SARS-CoV-2: Structural requirements at both nsp12 RdRp and nsp14 exonuclease active-sites. Antivir. Res. 178, 104793 (2020).
Liu, Z. et al. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J. Med. Virol. 92, 595–601 (2020).
Wang, Y. et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395, 1569–1578 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04280705 (2020).
Gilead. Gilead’s Investigational Antiviral Remdesivir Receives U.S. Food and Drug Administration Emergency Use Authorization for the Treatment of COVID-19. https://www.gilead.com/news-and-press/press-room/press-releases/2020/5/gileads-investigational-antiviral-remdesivir-receives-us-food-and-drug-administration-emergency-use-authorization-for-the-treatment-of-covid19 (2020).
Wang, Q. et al. Structural basis for RNA replication by the SARS-CoV-2 polymerase. Cell 82, 417–428.e13 (2020).
Shen, Y., Radhakrishnan, M. L. & Tidor, B. Molecular mechanisms and design principles for promiscuous inhibitors to avoid drug resistance: lessons learned from HIV-1 protease inhibition. Proteins 83, 351–372 (2015).
Cao, B. et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N. Engl. J. Med. 382, 1787–1799 (2020).
Zhang, L. et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 368, 409–412 (2020).
Gandolfini, I. et al. COVID-19 in kidney transplant recipients. Am. J. Transpl. 20, 1941–1943 (2020).
Bartiromo, M. et al. Threatening drug-drug interaction in a kidney transplant patient with Coronavirus Disease 2019 (COVID-19). Transpl. Infect. Dis. 22, e13286 (2020).
Vincent, M. J. et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2, 69 (2005).
Cavalcanti, A. B. et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2019014 (2020).
Boulware, D. R. et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N. Engl. J. Med. 383, 517–525 (2020).
Magagnoli, J. et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. Med https://doi.org/10.1016/j.medj.2020.06.001 (2020).
Borba, M. G. S. et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw. Open 3, e208857 (2020).
Kleynberg, R. L. & Schiller, G. J. Secondary hemophagocytic lymphohistiocytosis in adults: an update on diagnosis and therapy. Clin. Adv. Hematol. Oncol. 10, 726–732 (2012).
Cruz-Topete, D. & Cidlowski, J. A. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation 22, 20–32 (2015).
Russell, C. D., Millar, J. E. & Baillie, J. K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 395, 473–475 (2020).
Wu, C. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 180, 1–11 (2020).
Russell, B. et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19 — a systematic review of current evidence. Ecancermedicalscience 14, 1022 (2020).
Shang, L., Zhao, J., Hu, Y., Du, R. & Cao, B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 395, 683–684 (2020).
Blum, C. A. et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 385, 1511–1518 (2015).
Torres, A. et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 313, 677–686 (2015).
Lee, K. Y., Rhim, J.-W. & Kang, J.-H. Early preemptive immunomodulators (corticosteroids) for severe pneumonia patients infected with SARS-CoV-2. Clin. Exp. Pediatr. 63, 117–118 (2020).
Fadel, R. et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciaa601 (2020).
The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) working group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA https://doi.org/10.1001/jama.2020.17023 (2020).
RECOVERY Collaborative Group, Horby, P. et al. Dexamethasone in hospitalized patients with covid-19 — preliminary report. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2021436 (2020).
Jacob, K. A. et al. Intraoperative high-dose dexamethasone and severe AKI after cardiac surgery. J. Am. Soc. Nephrol. 26, 2947–2951 (2015).
Ponticelli, C. & Locatelli, F. Glucocorticoids in the treatment of glomerular diseases: pitfalls and pearls. Clin. J. Am. Soc. Nephrol. 13, 815–822 (2018).
Dusheiko, G. Side effects of alpha interferon in chronic hepatitis C. Hepatology 26, 112S–121S (1997).
Alattar, R. et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J. Med. Virol. https://doi.org/10.1002/jmv.25964 (2020).
Guaraldi, G. et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2, e474–e484 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04341870 (2020).
Furlow, B. COVACTA trial raises questions about tocilizumab’s benefit in COVID-19. Lancet Rheumatol. 2, e592 (2020).
Dimopoulos, G. et al. Favorable anakinra responses in severe Covid-19 patients with secondary hemophagocytic lymphohistiocytosis. Cell HostMicrobe 28, 117–123.e1 (2020).
Ahn, E. et al. Role of PD-1 during effector CD8 T cell differentiation. Proc. Natl Acad. Sci. USA 115, 4749–4754 (2018).
Jin, H.-T. et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl Acad. Sci. USA 107, 14733–14738 (2010).
Kamphorst, A. O. et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc. Natl Acad. Sci. USA 114, 4993–4998 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04413838 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04356508 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04268537 (2020).
Wei, S. C. et al. Combination anti-CTLA-4 plus anti–PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc. Natl Acad. Sci. USA 116, 22699–22709 (2019).
Goldstein, A. L. & Goldstein, A. L. From lab to bedside: emerging clinical applications of thymosin α1. Expert Opin. Biol. Ther. 9, 593–608 (2009).
Liu, Y. et al. Thymosin alpha 1 (Tα1) reduces the mortality of severe COVID-19 by restoration of lymphocytopenia and reversion of exhausted T cells. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciaa630 (2020).
Shi, C. et al. The potential of low molecular weight heparin to mitigate cytokine storm in severe COVID-19 patients: a retrospective clinical study. Clin. Transl Sci. https://doi.org/10.1111/cts.12880 (2020).
Stein, B. L. Coagulopathy associated with COVID-19. (NEJM Journal Watch, 2020).
Thachil, J. et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 8, 1023–1026 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04473053 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04352400 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04390594 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04418128 (2020).
Yamamoto, M. et al. Identification of nafamostat as a potent inhibitor of Middle East respiratory syndrome coronavirus s protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob. Agents Chemother. 60, 6532–6539 (2016).
Ansarin, K. et al. Effect of bromhexine on clinical outcomes and mortality in COVID-19 patients: a randomized clinical trial. Bioimpacts 10, 209–215 (2020).
Liu, X. et al. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta Pharm. Sin. B 10, 1205–1215 (2020).
Porfidia, A. & Pola, R. Venous thromboembolism and heparin use in COVID-19 patients: juggling between pragmatic choices, suggestions of medical societies. J. Thromb. Thrombolysis 50, 68–71 (2020).
Mastaglio, S. et al. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin. Immunol. 215, 108450 (2020).
O’Brien, K. B., Morrison, T. E., Dundore, D. Y., Heise, M. T. & Schultz-Cherry, S. A protective role for complement C3 protein during pandemic 2009 H1N1 and H5N1 influenza A virus infection. PLoS ONE 6, e17377 (2011).
Ricklin, D., Mastellos, D. C., Reis, E. S. & Lambris, J. D. The renaissance of complement therapeutics. Nat. Rev. Nephrol. 14, 26–47 (2018).
Keshari, R. S. et al. Complement C5 inhibition blocks the cytokine storm and consumptive coagulopathy by decreasing lipopolysaccharide (LPS) release in E. coli sepsis. Blood 126, 765–765 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04288713 (2020).
Woodruff, T. M., Nandakumar, K. S. & Tedesco, F. Inhibiting the C5–C5a receptor axis. Mol. Immunol. 48, 1631–1642 (2011).
Williams, A. L. et al. C5 inhibition prevents renal failure in a mouse model of lethal C3 glomerulopathy. Kidney Int. 91, 1386–1397 (2017).
Noris, M. & Remuzzi, G. Terminal complement effectors in atypical hemolytic uremic syndrome: C5a, C5b-9, or a bit of both? Kidney Int. 96, 13–15 (2019).
Gattinoni, L. et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 46, 1099–1102 (2020).
Gattinoni, L., Chiumello, D. & Rossi, S. COVID-19 pneumonia: ARDS or not? Crit. Care 24, 154 (2020).
Copin, M.-C. et al. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 46, 1124–1126 (2020).
Aloisi, S., Beasley, D., Borter, G., Escritt, T. & Kelland K. Special report: as virus advances, doctors rethink rush to ventilate. Tildes https://www.reuters.com/article/us-health-coronavirus-ventilators-specia/special-report-as-virus-advances-doctors-rethink-rush-to-ventilate-idUSKCN2251PE (2020).
Robba, C. et al. Distinct phenotypes require distinct respiratory management strategies in severe COVID-19. Respir. Physiol. Neurobiol. 279, 103455 (2020).
Casadevall, A. & Pirofski, L. The convalescent sera option for containing COVID-19. J. Clin. Invest. 130, 1545–1548 (2020).
Duan, K. et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl Acad. Sci. USA 117, 9490–9496 (2020).
Shen, C. et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 323, 1582–1589 (2020).
Ye, M. et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J. Med. Virol. https://doi.org/10.1002/jmv.25882 (2020).
Zeng, Q. L. et al. Effect of convalescent plasma therapy on viral shedding and survival in COVID-19 patients. J. Infect. Dis. 222, 38–43 (2020).
Li, L. et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA 324, 460–470 (2020).
Agarwal, A. et al. Convalescent plasma in the management of moderate COVID-19 in India: An open-label parallel-arm phase II multicentre randomized controlled trial (PLACID Trial). Preprint at medRxiv https://doi.org/10.1101/2020.09.03.20187252 (2020).
Chi, X. et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science 369, 650–655 (2020).
Wec, A. Z. et al. Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science 369, 731–736 (2020).
Hansen, J. et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 369, 1010–1014 (2020).
Ju, B. et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 584, 115–119 (2020).
Pinto, D. et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 583, 290–295 (2020).
Wang, C. et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 11, 1–6 (2020).
Liu, Y. et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect. Dis. 20, 656–657 (2020).
Kirby, T. Evidence mounts on the disproportionate effect of COVID-19 on ethnic minorities. Lancet Respir. Med. 8, 547–548 (2020).
Acknowledgements
The authors thank Kerstin Mierke, Istituto di Ricerche Mario Negri IRCCS, Italy, for help with English language editing of the manuscript before submission and Antonella Piccinelli, Istituto di Ricerche Mario Negri IRCCS, Italy, for preparing the figures before submission. L.P. is a recipient of the Career Development Program from Fondazione Aiuti per la Ricerca sulle Malattie Rare (ARMR), Bergamo, Italy. We are also grateful to Fondazione Aiuti per la Ricerca sulle Malattie Rare (ARMR) for research support for our studies on COVID-19 pathogenesis. L.F.P.N. and L.R. are supported by core funds at the Singapore Immunology Network (SIgN) through the Biomedical Medical Research Council (BMRC), A*STAR.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks C. Garlanda, C. Ince and A. Salama for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Betacoronavirus
-
One of four genera (α, β, γ and δ) of enveloped, positive-strand RNA viruses that infects humans and mammals, causing respiratory diseases that can be mild or severe.
- RNA-dependent RNA polymerase
-
(RdRp). An enzyme encoded by viral RNA that catalyses the synthesis of new RNA strands complementary to the initial RNA template included in the viral capsid.
- Incubation period
-
The number of days between the initial exposure to a pathogen and the day in which the infected individual experiences the first symptoms of the disease.
- Anosmia
-
Complete loss of the ability to detect one or more smells; anosmia can be temporary, as in the case of COVID-19, or permanent such as occurs in certain neurological conditions.
- Ageusia
-
Complete loss of taste on the tongue, particularly the inability to perceive sweetness, sourness, bitterness and saltiness; ageusia can be temporary, as in the case of COVID-19, or permanent such as occurs in certain neurological conditions.
- Pattern recognition receptors
-
(PRRs). Host sensors that detect pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) generated during infections or injury.
- Cytokine storm
-
Also called hypercytokinaemia. An excessive and uncontrolled release of pro-inflammatory molecules by the cellular components of the innate immune system in response to a number of infectious and non-infectious aetiologies that can lead to multi-organ damage and failure.
- VH gene
-
The heavy-chain-variable region (VH) gene gene is located along with diversity (D), joining (J) and constant (C) genes at three primary loci in the human genome and undergoes somatic rearrangements for the biosynthesis of the heavy (H) or light (L) chain of IgG.
- Glucuronidases
-
A class of enzyme that includes beta-glucuronidases — a glycosidases that catalyse the breakdown of complex carbohydrates by hydrolysing β-d-glucuronic acid residues from the non-reducing end of glycosaminoglycans, such as heparan sulfate.
Rights and permissions
About this article
Cite this article
Perico, L., Benigni, A., Casiraghi, F. et al. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol 17, 46–64 (2021). https://doi.org/10.1038/s41581-020-00357-4
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41581-020-00357-4
This article is cited by
-
SARS-CoV-2 nucleocapsid protein forms complexes with soluble complement regulatory proteins that can bind to the virion
Scientific Reports (2026)
-
Mesenchymal stromal cell secretome reduces lung injury and thrombo-inflammation induced by SARS-CoV-2 spike protein
Stem Cell Research & Therapy (2025)
-
Management of COVID-19 associated coagulopathy in critically ill patients and the risk of acquired von willebrand syndrome
Scientific Reports (2025)
-
Immune-coagulation dynamics in severe COVID-19 revealed by autoantibody profiling and multi-omics integration
Scientific Reports (2025)
-
Long COVID and the kidney
Nature Reviews Nephrology (2025)