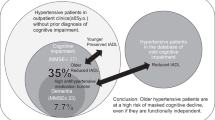
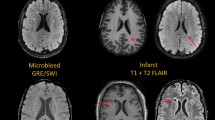
Abstract
Hypertension affects two-thirds of people aged >60 years and significantly increases the risk of both vascular cognitive impairment and Alzheimer’s disease. Hypertension compromises the structural and functional integrity of the cerebral microcirculation, promoting microvascular rarefaction, cerebromicrovascular endothelial dysfunction and neurovascular uncoupling, which impair cerebral blood supply. In addition, hypertension disrupts the blood–brain barrier, promoting neuroinflammation and exacerbation of amyloid pathologies. Ageing is characterized by multifaceted homeostatic dysfunction and impaired cellular stress resilience, which exacerbate the deleterious cerebromicrovascular effects of hypertension. Neuroradiological markers of hypertension-induced cerebral small vessel disease include white matter hyperintensities, lacunar infarcts and microhaemorrhages, all of which are associated with cognitive decline. Use of pharmaceutical and lifestyle interventions that reduce blood pressure, in combination with treatments that promote microvascular health, have the potential to prevent or delay the pathogenesis of vascular cognitive impairment and Alzheimer’s disease in patients with hypertension.
Key points
-
Hypertension is associated with ageing and significantly increases the risk of vascular cognitive impairment and Alzheimer’s disease.
-
In older individuals, hypertension leads to maladaptation of the cerebral circulation, resulting in dysregulation of cerebral blood flow, microvascular rarefaction, blood–brain barrier disruption, oxidative stress and impaired neurovascular coupling.
-
Hypertension causes pathological alterations in cerebral microvessels that damage microvascular structure, network architecture and function, and contribute to the genesis of cerebral microhaemorrhages, lacunar infarcts and white matter injury; these factors are associated with cognitive decline.
-
Potential mechanisms by which hypertension could exacerbate the progression of Alzheimer’s disease include increased oxidative microvascular damage, brain inflammation and blood–brain barrier disruption, as well as impaired glymphatic (also known as glial-lymphatic) clearance of amyloid-β.
-
Use of pharmaceutical and/or lifestyle interventions that reduce blood pressure in combination with treatments that promote microvascular health could potentially prevent or delay cognitive decline in patients with hypertension.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hurd, M. D., Martorell, P., Delavande, A., Mullen, K. J. & Langa, K. M. Monetary costs of dementia in the United States. N. Engl. J. Med. 368, 1326–1334 (2013).
Wimo, A. et al. The worldwide economic impact of dementia 2010. Alzheimers Dement. 9, 1–11.e3 (2013).
Iadecola, C. et al. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension 68, e67–e94 (2016).
Forette, F. et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet 352, 1347–1351 (1998).
Launer, L. J. et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol. Aging 21, 49–55 (2000).
Israeli-Korn, S. D. et al. Hypertension increases the probability of Alzheimer’s disease and of mild cognitive impairment in an Arab community in northern Israel. Neuroepidemiology 34, 99–105 (2010).
Petrovitch, H. et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia aging Study. Neurobiol. Aging 21, 57–62 (2000).
van Dijk, E. J. et al. The association between blood pressure, hypertension, and cerebral white matter lesions: cardiovascular determinants of dementia study. Hypertension 44, 625–630 (2004).
Baker, A. B., Resch, J. A. & Loewenson, R. B. Hypertension and cerebral atherosclerosis. Circulation 39, 701–710 (1969).
James, P. A. et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311, 507–520 (2014).
Fryar, C. D., Ostchega, Y., Hales, C. M., Zhang, G. & Kruszon-Moran, D. Hypertension Prevalence and Control among Adults: United States 2015-1026. NCHS data brief, no 289 (National Center for Health Statistics, 2017).
Muntner, P. et al. Potential U.S. Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. J. Am. Coll. Cardiol. 71, 109–118 (2018).
Iadecola, C. & Gottesman, R. F. Neurovascular and cognitive dysfunction in hypertension. Circ. Res. 124, 1025–1044 (2019).
Wilkie, F. L., Eisdorfer, C. & Nowlin, J. B. Memory and blood pressure in the aged. Exp. Aging Res. 2, 3–16 (1976).
Kennelly, S. P., Lawlor, B. A. & Kenny, R. A. Blood pressure and dementia — a comprehensive review. Ther. Adv. Neurol. Disord. 2, 241–260 (2009).
Whitmer, R. A., Sidney, S., Selby, J., Johnston, S. C. & Yaffe, K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64, 277–281 (2005).
Kivipelto, M. et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ 322, 1447–1451 (2001).
Walker, K. A. et al. Association of midlife to late-life blood pressure patterns with incident dementia. JAMA 322, 535–545 (2019).
Skoog, I. et al. 15-year longitudinal study of blood pressure and dementia. Lancet 347, 1141–1145 (1996).
Qiu, C., von Strauss, E., Fastbom, J., Winblad, B. & Fratiglioni, L. Low blood pressure and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Arch. Neurol. 60, 223–228 (2003).
Li, G. et al. Age-varying association between blood pressure and risk of dementia in those aged 65 and older: a community-based prospective cohort study. J. Am. Geriatr. Soc. 55, 1161–1167 (2007).
Yoshitake, T. et al. Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: the Hisayama Study. Neurology 45, 1161–1168 (1995).
Posner, H. B. et al. The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology 58, 1175–1181 (2002).
Lopez, O. L. et al. Risk factors for mild cognitive impairment in the cardiovascular health study cognition study: part 2. Arch. Neurol. 60, 1394–1399 (2003).
Hestad, K., Engedal, K., Schirmer, H. & Strand, B. H. The effect of blood pressure on cognitive performance. an 8-year follow-up of the tromso study, comprising people aged 45–74 years. Front. Psychol. 11, 607 (2020).
Swan, G. E. et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology 51, 986–993 (1998).
Mills, K. T. et al. Global Disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 134, 441–450 (2016).
Chin, A. L., Negash, S. & Hamilton, R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 25, 187–195 (2011).
Ferri, C. P. et al. Global prevalence of dementia: a Delphi consensus study. Lancet 366, 2112–2117 (2005).
Levine, D. A. et al. Association between blood pressure and later-life cognition among black and white individuals. JAMA Neurol. 77, 810–819 (2020).
Mills, K. T., Stefanescu, A. & He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 16, 223–237 (2020).
Noh, J. et al. Prevalence of comorbidity among people with hypertension: The Korea National Health and Nutrition Examination Survey 2007–2013. Korean Circ. J. 46, 672–680 (2016).
Trauernicht, A. K., Sun, H., Patel, K. P. & Mayhan, W. G. Enalapril prevents impaired nitric oxide synthase-dependent dilatation of cerebral arterioles in diabetic rats. Stroke 34, 2698–2703 (2003).
Tarantini, S. et al. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood brain barrier disruption, neuroinflammation, amyloidogenic gene expression and cognitive decline in mice, mimicking the aging phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 73, 853–863 (2018).
Tucsek, Z. et al. Obesity in aging exacerbates blood brain barrier disruption, neuroinflammation and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J. Gerontol. A Biol. Sci. Med. Sci. 69, 1212–1226 (2014).
Valcarcel-Ares, M. N. et al. Obesity in aging exacerbates neuroinflammation, dysregulating synaptic function-related genes and altering eicosanoid synthesis in the mouse hippocampus: potential role in impaired synaptic plasticity and cognitive decline. J. Gerontol. A Biol. Sci. Med. Sci. 74, 290–298 (2018).
Viggiano, D. et al. Mechanisms of cognitive dysfunction in CKD. Nat. Rev. Nephrol. 16, 452–469 (2020).
Hooghiemstra, A. M. et al. Frequent cognitive impairment in patients with disorders along the heart-brain axis. Stroke 50, 3369–3375 (2019).
Toth, P., Tarantini, S., Csiszar, A. & Ungvari, Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am. J. Physiol. Heart Circ. Physiol. 312, H1–H20 (2017).
Fulop, G. A. et al. IGF-1 deficiency promotes pathological remodeling of cerebral arteries: a potential mechanism contributing to the pathogenesis of intracerebral hemorrhages in aging. J. Gerontol. A Biol. Sci. Med. Sci. 74, 446–454 (2018).
Toth, P. et al. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J. Cereb. Blood Flow. Metab. 33, 1732–1742 (2013).
Harder, D. R., Smeda, J. & Lombard, J. Enhanced myogenic depolarization in hypertensive cerebral arterial muscle. Circ. Res. 57, 319–322 (1985).
Iadecola, C., Park, L. & Capone, C. Threats to the mind: aging, amyloid, and hypertension. Stroke 40, S40–S44 (2009).
Toth, P. et al. Role of 20-HETE, TRP channels and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am. J. Physiol. Heart Circ. Physiol. 305, H1698–H1708 (2013).
Choi, S. K., Yeon, S. I., Kwon, Y., Byeon, S. & Lee, Y. H. Involvement of epithelial Na+ channel in the elevated myogenic response in posterior cerebral arteries from spontaneously hypertensive rats. Sci. Rep. 7, 45996 (2017).
Diaz-Otero, J. M. et al. Mineralocorticoid receptor antagonism improves parenchymal arteriole dilation via a TRPV4-dependent mechanism and prevents cognitive dysfunction in hypertension. Am. J. Physiol. Heart Circ. Physiol. 315, H1304–H1315 (2018).
Jarajapu, Y. P. & Knot, H. J. Relative contribution of Rho kinase and protein kinase C to myogenic tone in rat cerebral arteries in hypertension. Am. J. Physiol. Heart Circ. Physiol. 289, H1917–H1922 (2005).
Tomoto, T., Sugawara, J., Nogami, Y., Aonuma, K. & Maeda, S. The influence of central arterial compliance on cerebrovascular hemodynamics: insights from endurance training intervention. J. Appl. Physiol. 119, 445–451 (2015).
Diaz-Otero, J. M., Garver, H., Fink, G. D., Jackson, W. F. & Dorrance, A. M. Aging is associated with changes to the biomechanical properties of the posterior cerebral artery and parenchymal arterioles. Am. J. Physiol. Heart Circ. Physiol. 310, H365–H375 (2016).
Webb, A. J. et al. Increased cerebral arterial pulsatility in patients with leukoaraiosis: arterial stiffness enhances transmission of aortic pulsatility. Stroke 43, 2631–2636 (2012).
Mitchell, G. F. et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility — Reykjavik study. Brain 134, 3398–3407 (2011).
Brown, I. A. M. et al. Vascular smooth muscle remodeling in conductive and resistance arteries in hypertension. Arterioscler. Thromb. Vasc. Biol. 38, 1969–1985 (2018).
Springo, Z. et al. Aging impairs myogenic adaptation to pulsatile pressure in mouse cerebral arteries. J. Cereb. Blood Flow. Metab. 35, 527–530 (2015).
Ascenzi, F. et al. Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell 18, e12954 (2019).
Tarantini, S. et al. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell 16, 469–479 (2017).
Toth, P. et al. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J. Cereb. Blood Flow. Metab. 34, 1887–1897 (2014).
Sonntag, W. E. et al. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front. Aging Neurosci. 5, 27 (2013).
Tarantini, S. et al. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age 38, 273–289 (2016).
Angelini, A. et al. Insulin-like growth factor-1 (IGF-1): relation with cognitive functioning and neuroimaging marker of brain damage in a sample of hypertensive elderly subjects. Arch. Gerontol. Geriatr. 49 (Suppl 1), 5–12 (2009).
Johnsen, S. P. et al. Insulin-like growth factor (IGF) I, -II, and IGF binding protein-3 and risk of ischemic stroke. J. Clin. Endocrinol. Metab. 90, 5937–5941 (2005).
Park, L., Anrather, J., Girouard, H., Zhou, P. & Iadecola, C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J. Cereb. Blood Flow. Metab. 27, 1908–1918 (2007).
Springo, Z. et al. Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. J. Gerontol. A Biol. Sci. Med. Sci 70, 1355–1359 (2015).
Toth, P. et al. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell 14, 400–408 (2015).
Ungvari, Z. et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response. Am. J. Physiol. Heart Circ. Physiol. 301, H363–H372 (2011).
Ungvari, Z. et al. Nrf2 dysfunction and impaired cellular resilience to oxidative stressors in the aged vasculature: from increased cellular senescence to the pathogenesis of age-related vascular diseases. Geroscience 41, 727–738 (2019).
Fulop, G. A. et al. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience 40, 513–521 (2018).
Bailey-Downs, L. C. et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J. Gerontol. A Biol. Sci. Med. Sci. 67, 313–329 (2012).
Csiszar, A. et al. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am. J. Physiol. Heart Circ. Physiol. 307, H292–H306 (2014).
Ungvari, Z. et al. Age-associated vascular oxidative stress, Nrf2 dysfunction and NF-kB activation in the non-human primate Macaca mulatta. J. Gerontol. A Biol. Sci. Med. Sci. 66, 866–875 (2011).
Valcarcel-Ares, M. N. et al. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J. Gerontol. A Biol. Sci. Med. Sci. 67, 821–829 (2012).
Gorelick, P. B. et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42, 2672–2713 (2011).
Girouard, H., Park, L., Anrather, J., Zhou, P. & Iadecola, C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler. Thromb. Vasc. Biol. 27, 303–309 (2007).
Suzuki, K., Masawa, N., Sakata, N. & Takatama, M. Pathologic evidence of microvascular rarefaction in the brain of renal hypertensive rats. J. Stroke Cerebrovasc. Dis. 12, 8–16 (2003).
Jimenez-Balado, J. et al. Prevalence of hippocampal enlarged perivascular spaces in a sample of patients with hypertension and their relation with vascular risk factors and cognitive function. J. Neurol. Neurosurg. Psychiatry 89, 651–656 (2018).
Carnevale, D. et al. Role of neuroinflammation in hypertension-induced brain amyloid pathology. Neurobiol. Aging 33, 205.e19–e29 (2012).
Carnevale, D. et al. Hypertension induces brain β-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension 60, 188–197 (2012).
Zhang, M., Mao, Y., Ramirez, S. H., Tuma, R. F. & Chabrashvili, T. Angiotensin II induced cerebral microvascular inflammation and increased blood-brain barrier permeability via oxidative stress. Neuroscience 171, 852–858 (2010).
Jorgensen, D. R. et al. A population neuroscience approach to the study of cerebral small vessel disease in midlife and late life: an invited review. Am. J. Physiol. Heart Circ. Physiol. 314, H1117–H1136 (2018).
Alber, J. et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): knowledge gaps and opportunities. Alzheimers Dement. 5, 107–117 (2019).
Wardlaw, J. M., Valdes Hernandez, M. C. & Munoz-Maniega, S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J. Am. Heart Assoc. 4, 001140 (2015).
de Leeuw, F. E. et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain 125, 765–772 (2002).
Guevarra, A. C. et al. Age moderates associations of hypertension, white matter hyperintensities, and cognition. J. Alzheimers Dis. 75, 1351–1360 (2020).
Weaver, N. A. et al. Cerebral amyloid burden is associated with white matter hyperintensity location in specific posterior white matter regions. Neurobiol. Aging 84, 225–234 (2019).
Tsao, C. W. et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology 81, 984–991 (2013).
Vermeer, S. E., Longstreth, W. T. Jr. & Koudstaal, P. J. Silent brain infarcts: a systematic review. Lancet Neurol. 6, 611–619 (2007).
Geerlings, M. I. et al. Association of white matter lesions and lacunar infarcts with executive functioning: the SMART-MR study. Am. J. Epidemiol. 170, 1147–1155 (2009).
Zlokovic, B. V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201 (2008).
Kerkhofs, D. et al. Pharmacological depletion of microglia and perivascular macrophages prevents vascular cognitive impairment in Ang II-induced hypertension. Theranostics 10, 9512–9527 (2020).
Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R. & Zlokovic, B. V. Blood-brain barrier: from physiology to disease and back. Physiol. Rev. 99, 21–78 (2019).
Xu, L., Nirwane, A. & Yao, Y. Basement membrane and blood-brain barrier. Stroke Vasc. Neurol. 4, 78–82 (2019).
Bailey, E. L. et al. Cerebral small vessel endothelial structural changes predate hypertension in stroke-prone spontaneously hypertensive rats: a blinded, controlled immunohistochemical study of 5- to 21-week-old rats. Neuropathol. Appl. Neurobiol. 37, 711–726 (2011).
Fan, Y. et al. Tight junction disruption of blood-brain barrier in white matter lesions in chronic hypertensive rats. Neuroreport 26, 1039–1043 (2015).
Setiadi, A., Korim, W. S., Elsaafien, K. & Yao, S. T. The role of the blood-brain barrier in hypertension. Exp. Physiol. 103, 337–342 (2018).
Yang, Y. et al. Vascular tight junction disruption and angiogenesis in spontaneously hypertensive rat with neuroinflammatory white matter injury. Neurobiol. Dis. 114, 95–110 (2018).
Santisteban, M. M. et al. Endothelium-macrophage crosstalk mediates blood-brain barrier dysfunction in hypertension. Hypertension 76, 795–807 (2020).
Yang, Y., Estrada, E. Y., Thompson, J. F., Liu, W. & Rosenberg, G. A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow. Metab. 27, 697–709 (2007).
Ueno, M. et al. Blood-brain barrier disruption in the hypothalamus of young adult spontaneously hypertensive rats. Histochem. Cell Biol. 122, 131–137 (2004).
Roggendorf, W., Opitz, H. & Schuppan, D. Altered expression of collagen type VI in brain vessels of patients with chronic hypertension. A comparison with the distribution of collagen IV and procollagen III. Acta Neuropathol. 77, 55–60 (1988).
Bell, R. D. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427 (2010).
Winkler, E. A., Bell, R. D. & Zlokovic, B. V. Central nervous system pericytes in health and disease. Nat. Neurosci. 14, 1398–1405 (2011).
Sure, V. N. et al. A novel high-throughput assay for respiration in isolated brain microvessels reveals impaired mitochondrial function in the aged mice. Geroscience 40, 365–375 (2018).
Van Skike, C. E. et al. Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer’s disease and vascular cognitive impairment. Am. J. Physiol. Heart Circ. Physiol. 314, H693–H703 (2018).
Wilhelm, I., Nyul-Toth, A., Kozma, M., Farkas, A. E. & Krizbai, I. A. Role of pattern recognition receptors of the neurovascular unit in inflamm-aging. Am. J. Physiol. Heart Circ. Physiol 313, H1000–H1012 (2017).
Kiss, T. et al. Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience 42, 429–444 (2020).
Mayhan, W. G. & Heistad, D. D. Role of veins and cerebral venous pressure in disruption of the blood-brain barrier. Circ. Res. 59, 216–220 (1986).
Fulop, G. A. et al. Cerebral venous congestion promotes blood-brain barrier disruption and neuroinflammation, impairing cognitive function in mice. Geroscience 41, 575–589 (2019).
Tucsek, Z. et al. Hypertension-induced synapse loss and impairment in synaptic plasticity in the mouse hippocampus mimics the aging phenotype: implications for the pathogenesis of vascular cognitive impairment. Geroscience 39, 385–406 (2017).
Davalos, D. et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat. Commun. 3, 1227 (2012).
Sadekova, N. et al. Arterial stiffness induced by carotid calcification leads to cerebral gliosis mediated by oxidative stress. J. Hypertens. 36, 286–298 (2018).
Bowman, G. L. et al. Blood-brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimers Dement. 14, 1640–1650 (2018).
Singh, M. V., Chapleau, M. W., Harwani, S. C. & Abboud, F. M. The immune system and hypertension. Immunol. Res. 59, 243–253 (2014).
Carnevale, D. & Lembo, G. ‘Alzheimer-like’ pathology in a murine model of arterial hypertension. Biochem. Soc. Trans. 39, 939–944 (2011).
Ungvari, Z., Tarantini, S., Kirkpatrick, A. C., Csiszar, A. & Prodan, C. I. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am. J. Physiol. Heart Circ. Physiol. 312, H1128–H1143 (2017).
Yates, P. A. et al. Cerebral microhemorrhage and brain β-amyloid in aging and Alzheimer disease. Neurology 77, 48–54 (2011).
Petrea, R. E. et al. Mid to late life hypertension trends and cerebral small vessel disease in the framingham heart study. Hypertension 76, 707–714 (2020).
Lau, W. L. et al. Chronic kidney disease increases cerebral microbleeds in mouse and man. Transl. Stroke Res. 11, 122–134 (2020).
Ungvari, Z., Tarantini, S., Kirkpatrick, A. C., Csiszar, A. & Prodan, C. I. Cerebral microhemorrhages: mechanisms, consequences and prevention. Am. J. Physiol. Heart Circ. Physiol. 312, H1128–H1143 (2017).
Ungvari, Z. et al. Repeated Valsalva maneuvers promote symptomatic manifestations of cerebral microhemorrhages: implications for the pathogenesis of vascular cognitive impairment in older adults. Geroscience 40, 485–496 (2018).
Choi, Y. & Lee, M. K. Neuroimaging findings of brain MRI and CT in patients with COVID-19: a systematic review and meta-analysis. Eur. J. Radiol. 133, 109393 (2020).
Haroon, K. H., Patro, S. N., Hussain, S., Zafar, A. & Muhammad, A. Multiple microbleeds: a serious neurological manifestation in a critically ill COVID-19 patient. Case Rep. Neurol. 12, 373–377 (2020).
Kirschenbaum, D. et al. Intracerebral endotheliitis and microbleeds are neuropathological features of COVID-19. Neuropathol. Appl. Neurobiol. 47, 454–459 (2020).
Bosch, A. J. et al. Retinal capillary rarefaction in patients with untreated mild-moderate hypertension. BMC Cardiovasc. Disord. 17, 300 (2017).
Hoenig, M. R., Bianchi, C., Rosenzweig, A. & Sellke, F. W. The cardiac microvasculature in hypertension, cardiac hypertrophy and diastolic heart failure. Curr. Vasc. Pharmacol. 6, 292–300 (2008).
Kubis, N. et al. Decreased arteriolar density in endothelial nitric oxide synthase knockout mice is due to hypertension, not to the constitutive defect in endothelial nitric oxide synthase enzyme. J. Hypertens. 20, 273–280 (2002).
Williams, S. A. et al. Capillary hypertension and abnormal pressure dynamics in patients with essential hypertension. Clin. Sci. 79, 5–8 (1990).
Antonios, T. F., Singer, D. R., Markandu, N. D., Mortimer, P. S. & MacGregor, G. A. Structural skin capillary rarefaction in essential hypertension. Hypertension 33, 998–1001 (1999).
Ungvari, Z. et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 15, 555–565 (2018).
Kiss, T. et al. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for the prevention of vascular cognitive impairment. Geroscience 41, 619–630 (2019).
Banki, E. et al. Age-related decline of autocrine pituitary adenylate cyclase-activating polypeptide impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J. Gerontol. A Biol. Sci. Med. Sci. 70, 665–674 (2015).
Reglodi, D. et al. PACAP deficiency as a model of aging. Geroscience 40, 437–452 (2018).
Ungvari, Z. et al. Aging-induced dysregulation of dicer1-dependent microRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J. Gerontol. A Biol. Sci. Med. Sci. 68, 877–891 (2013).
Ungvari, Z. et al. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity. J. Gerontol. A Biol. Sci. Med. Sci. 68, 1443–1457 (2013).
Toth, P. et al. Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am. J. Physiol. Heart Circ. Physiol. 309, H1837–H1845 (2015).
Tarantini, S. et al. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J. Cereb. Blood Flow. Metab. 35, 1871–1881 (2015).
Tarantini, S. et al. Treatment with the poly(ADP-ribose) polymerase inhibitor PJ-34 improves cerebromicrovascular endothelial function, neurovascular coupling responses and cognitive performance in aged mice, supporting the NAD+ depletion hypothesis of neurovascular aging. Geroscience 41, 533–542 (2019).
Tong, X. K., Lecrux, C., Rosa-Neto, P. & Hamel, E. Age-dependent rescue by simvastatin of Alzheimer’s disease cerebrovascular and memory deficits. J. Neurosci. 32, 4705–4715 (2012).
Nicolakakis, N. et al. Complete rescue of cerebrovascular function in aged Alzheimer’s disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J. Neurosci. 28, 9287–9296 (2008).
Faraco, G. et al. Hypertension enhances Aβ-induced neurovascular dysfunction, promotes β-secretase activity, and leads to amyloidogenic processing of APP. J. Cereb. Blood Flow. Metab. 36, 241–252 (2016).
Girouard, H., Park, L., Anrather, J., Zhou, P. & Iadecola, C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through nox-2-derived radicals. Arterioscler. Thromb. Vasc. Biol. 26, 826–832 (2006).
Kazama, K. et al. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ. Res. 95, 1019–1026 (2004).
Kazama, K., Wang, G., Frys, K., Anrather, J. & Iadecola, C. Angiotensin II attenuates functional hyperemia in the mouse somatosensory cortex. Am. J. Physiol. Heart Circ. Physiol. 285, H1890–H1899 (2003).
Wong, R. et al. Assessment of cerebral blood flow in adult patients with aortic coarctation. Cardiol. Young 27, 1606–1613 (2017).
Muhire, G. et al. Arterial stiffness due to carotid calcification disrupts cerebral blood flow regulation and leads to cognitive deficits. J. Am. Heart Assoc. 8, e011630 (2019).
Faraco, G. et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J. Clin. Invest. 126, 4674–4689 (2016).
Huang, A., Sun, D. & Koller, A. Endothelial dysfunction augments myogenic arteriolar constriction in hypertension. Hypertension 22, 913–921 (1993).
Ungvari, Z. et al. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation 108, 1253–1258 (2003).
Ungvari, Z., Csiszar, A., Kaminski, P. M., Wolin, M. S. & Koller, A. Chronic high pressure-induced arterial oxidative stress: Involvement of protein kinase C-dependent NAD(P)H oxidase and local renin-angiotensin system. Am. J. Pathol. 165, 219–226 (2004).
Koh, K. K. et al. Comparison of effects of losartan, irbesartan, and candesartan on flow-mediated brachial artery dilation and on inflammatory and thrombolytic markers in patients with systemic hypertension. Am. J. Cardiol. 93, 1432–1435, A1410 (2004).
Calcinaghi, N. et al. Multimodal imaging in rats reveals impaired neurovascular coupling in sustained hypertension. Stroke 44, 1957–1964 (2013).
Wiedenhoeft, T. et al. Fusogenic liposomes effectively deliver resveratrol to the cerebral microcirculation and improve endothelium-dependent neurovascular coupling responses in aged mice. Geroscience 41, 711–725 (2019).
Toth, P. et al. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am. J. Physiol. Heart Circ. Physiol. 306, H299–H308 (2014).
Liang, E. S. et al. PARP-1 (Poly[ADP-Ribose] Polymerase 1) inhibition protects from Ang II (Angiotensin II)-induced abdominal aortic aneurysm in mice. Hypertension 72, 1189–1199 (2018).
Tarantini, S. et al. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell 17, e12731 (2018).
Dikalov, S. I. et al. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid. Redox Signal. 20, 281–294 (2014).
Iadecola, C. & Gottesman, R. F. Cerebrovascular alterations in alzheimer disease. Circ. Res. 123, 406–408 (2018).
Kim, H. J. et al. Assessment of extent and role of tau in subcortical vascular cognitive impairment using 18F-AV1451 positron emission tomography imaging. JAMA Neurol. 75, 999–1007 (2018).
Zlokovic, B. V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 12, 723–738 (2011).
Niwa, K. et al. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am. J. Physiol. Heart Circ. Physiol. 283, H315–H323 (2002).
Nyul-Toth, A. et al. Increases in hypertension-induced cerebral microhemorrhages exacerbate gait dysfunction in a mouse model of Alzheimer’s disease. Geroscience 42, 1685–1698 (2020).
Rasmussen, M. K., Mestre, H. & Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 17, 1016–1024 (2018).
Mortensen, K. N. et al. Impaired glymphatic transport in spontaneously hypertensive rats. J. Neurosci. 39, 6365–6377 (2019).
Rouch, L. et al. Antihypertensive drugs, prevention of cognitive decline and dementia: a systematic review of observational studies, randomized controlled trials and meta-analyses, with discussion of potential mechanisms. CNS Drugs 29, 113–130 (2015).
Peters, R. et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 7, 683–689 (2008).
Menezes, S. T. et al. Hypertension, prehypertension, and hypertension control: association with decline in cognitive performance in the ELSA-Brasil cohort. Hypertension 77, 672–681 (2020).
Tzourio, C. et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch. Intern. Med. 163, 1069–1075 (2003).
Barthold, D., Joyce, G., Wharton, W., Kehoe, P. & Zissimopoulos, J. The association of multiple anti-hypertensive medication classes with Alzheimer’s disease incidence across sex, race, and ethnicity. PLoS One 13, e0206705 (2018).
Hughes, D. et al. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis. JAMA 323, 1934–1944 (2020).
Maxwell, C. J., Hogan, D. B. & Ebly, E. M. Calcium-channel blockers and cognitive function in elderly people: results from the Canadian study of health and aging. CMAJ 161, 501–506 (1999).
Tu, K. et al. Antihypertensive drug prescribing and persistence among new elderly users: implications for persistence improvement interventions. Can. J. Cardiol. 30, 647–652 (2014).
Quitterer, U. & AbdAlla, S. Improvements of symptoms of Alzheimer’s disease by inhibition of the angiotensin system. Pharmacol. Res. 154, 104230 (2020).
Hachinski, V. et al. Preventing dementia by preventing stroke: The Berlin Manifesto. Alzheimers Dement. 15, 961–984 (2019).
Friberg, L. & Rosenqvist, M. Less dementia with oral anticoagulation in atrial fibrillation. Eur. Heart J. 39, 453–460 (2018).
SPRINT MIND Investigators for the SPRINT Research Group. et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 321, 553–561 (2019).
Lv, Y. B. et al. A U-shaped association between blood pressure and cognitive impairment in chinese elderly. J. Am. Med. Dir. Assoc. 18, 193.e7–193.e13 (2017).
Waldstein, S. R., Giggey, P. P., Thayer, J. F. & Zonderman, A. B. Nonlinear relations of blood pressure to cognitive function: the baltimore longitudinal study of aging. Hypertension 45, 374–379 (2005).
Nilsson, S. E. et al. Low systolic blood pressure is associated with impaired cognitive function in the oldest old: longitudinal observations in a population-based sample 80 years and older. Aging Clin. Exp. Res. 19, 41–47 (2007).
Noriega de la Colina, A. et al. Diurnal blood pressure loads are associated with lower cognitive performances in controlled-hypertensive elderly individuals. J. Hypertens. 37, 2168–2179 (2019).
Xie, Z., Gao, M., Togashi, H., Saito, H. & Koyama, T. Improvement in the capillarity of the left ventricular wall of stroke-prone spontaneously hypertensive rats following angiotensin II receptor blockade. Clin. Exp. Hypertens. 21, 441–452 (1999).
Whitson, J. A. et al. SS-31 and NMN: Two paths to improve metabolism and function in aged hearts. Aging Cell 19, e13213 (2020).
Lee, G. H. et al. Anthocyanins attenuate endothelial dysfunction through regulation of uncoupling of nitric oxide synthase in aged rats. Aging Cell 19, e13279 (2020).
Walaszczyk, A. et al. Pharmacological clearance of senescent cells improves survival and recovery in aged mice following acute myocardial infarction. Aging Cell 18, e12945 (2019).
Lewis-McDougall, F. C. et al. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell 18, e12931 (2019).
Yang, D. et al. Histone methyltransferase Smyd3 is a new regulator for vascular senescence. Aging Cell 19, e13212 (2020).
Ogrodnik, M., Salmonowicz, H. & Gladyshev, V. N. Integrating cellular senescence with the concept of damage accumulation in aging: relevance for clearance of senescent cells. Aging Cell 18, e12841 (2019).
Marin-Aguilar, F. et al. NLRP3 inflammasome suppression improves longevity and prevents cardiac aging in male mice. Aging Cell 19, e13050 (2020).
Tarantini, S. et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 24, 101192 (2019).
Sardu, C. et al. Effects of metformin therapy on coronary endothelial dysfunction in patients with prediabetes with stable angina and nonobstructive coronary artery stenosis: the CODYCE multicenter prospective study. Diabetes Care 42, 1946–1955 (2019).
Van Skike, C. E. et al. mTOR drives cerebrovascular, synaptic, and cognitive dysfunction in normative aging. Aging Cell 19, e13057 (2020).
Toth, P. et al. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell (2015).
PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 358, 1033–1041 (2001).
Cortes-Canteli, M. & Iadecola, C. Alzheimer’s disease and vascular aging: JACC focus seminar. J. Am. Coll. Cardiol. 75, 942–951 (2020).
Acknowledgements
The authors’ work was supported by grants from the American Heart Association, the Oklahoma Center for the Advancement of Science and Technology, the Presbyterian Health Foundation and the Department of Veterans Affairs (award Number CX000340).
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks Prasad Katakam, Anja Meissner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Lacunar infarcts
-
Small infarcts (2–20 mm in diameter) in the deep cerebral white matter, basal ganglia, or pons that are presumed to result from the occlusion of a single small perforating artery supplying the subcortical areas of the brain.
- White matter lesions
-
Areas of abnormal myelination in the brain that are best visualized as hyperintensities on T2-weighted and fluid-attenuated inversion recovery (FLAIR) MRI sequences.
- Abstract reasoning
-
A cognitive domain that is closely related to fluid intelligence. The ability to quickly reason with information to solve new, unfamiliar problems, independent of any prior knowledge.
- Executive function
-
A set of mental skills that include working memory, flexible thinking and self-control.
- Digit Symbol Substitution Test
-
A paper-and-pencil cognitive test that requires matching of symbols to numbers.
- Mini-Mental State Examination
-
(MMSE). A test of cognitive function that is widely used for elderly people. The MMSE includes tests of orientation, attention, memory, language and visuo-spatial skills.
- Lipohyalinosis
-
Cerebral small vessel disease affecting the small arteries and arterioles in the brain. Lipohyalinosis is characterized by vessel wall thickening and a resultant reduction in luminal diameter.
- Lacunes
-
Small subcortical infarcts (<15 mm in diameter) in the territory of the deep penetrating arteries. These lesions may present with specific lacunar syndromes or they may be asymptomatic.
- Gliosis
-
An inflammatory process leading to scars in the central nervous system that involves the production of a dense fibrous network of neuroglia in areas of damage.
- Astrocytic endfeet
-
Processes that physically connect the astrocyte cell body to the outside of capillary walls.
- Pathogen-associated molecular patterns
-
Small molecular motifs that are recognized by Toll-like receptors. PAMPS activate innate immune responses that protect the host from infection.
- Transverse aortic coarctation
-
Narrowing of the transverse aortic arch.
- Neuropil
-
A dense network of interwoven nerve fibres and their branches and synapses together with glial filaments.
- Senolytics
-
A class of small molecules that selectively induce death of senescent cells. Senolytics are being developed with the aim of delaying, preventing, alleviating or reversing age-related diseases and improving human health.
Rights and permissions
About this article
Cite this article
Ungvari, Z., Toth, P., Tarantini, S. et al. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol 17, 639–654 (2021). https://doi.org/10.1038/s41581-021-00430-6
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41581-021-00430-6
This article is cited by
-
Effects of the Mediterranean–DASH Intervention for Neurodegenerative Delay (MIND) diet and forest bathing in improving cognitive and metabolic health among hypertensive older adults: a feasibility randomised controlled trial
BMC Nutrition (2026)
-
When Alzheimer’s pathology meets cardiometabolic risk: intrinsic subcortical–cortical connectivity signatures of retroactive interference in aging
Alzheimer's Research & Therapy (2026)
-
Advances in Alzheimer’s disease: mechanistic insights and therapeutic targets
Science China Life Sciences (2026)
-
Exploring the association between hemoglobin glycation index and cognitive function in older adults with hypertension: a cross-sectional study
BMC Geriatrics (2025)
-
Machine learning identification of influencing factors of global Nation-Level hypertension prevalence
BMC Public Health (2025)