Abstract
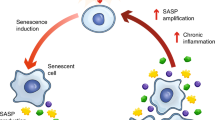
Cellular senescence is a ubiquitous process with roles in tissue remodelling, including wound repair and embryogenesis. However, prolonged senescence can be maladaptive, leading to cancer development and age-related diseases. Cellular senescence involves cell-cycle arrest and the release of inflammatory cytokines with autocrine, paracrine and endocrine activities. Senescent cells also exhibit morphological alterations, including flattened cell bodies, vacuolization and granularity in the cytoplasm and abnormal organelles. Several biomarkers of cellular senescence have been identified, including SA-βgal, p16 and p21; however, few markers have high sensitivity and specificity. In addition to driving ageing, senescence of immune and parenchymal cells contributes to the development of a variety of diseases and metabolic disorders. In the kidney, senescence might have beneficial roles during development and recovery from injury, but can also contribute to the progression of acute kidney injury and chronic kidney disease. Therapies that target senescence, including senolytic and senomorphic drugs, stem cell therapies and other interventions, have been shown to extend lifespan and reduce tissue injury in various animal models. Early clinical trials confirm that senotherapeutic approaches could be beneficial in human disease. However, larger clinical trials are needed to translate these approaches to patient care.
Key points
-
Cellular senescence regulates physiological and homeostatic processes, particularly during embryonic development and wound healing, but can also be a pathological process that contributes to ageing, various diseases and metabolic disorders.
-
Senescent cells are characterized by morphological alterations including large, flat bodies and organelle abnormalities, as well as loss of physiological functions, an inability to proliferate and the senescence-associated secretory phenotype.
-
SABG, p21 and p16 are the most commonly used senescence markers but have limitations; novel non-invasive approaches are needed to detect cellular senescence with high sensitivity and specificity in vitro.
-
Cellular senescence is involved in the pathogenesis of chronic kidney disease and acute kidney injury, but also seems to have a protective role in the early stages of acute kidney injury.
-
Senescence-targeting interventions, including senolytic drugs conjugated to antibodies against β2-microglobulin, chimeric antigen receptor T cells and anti-ageing vaccines, show promise for clinical application.
-
Clinical trials are needed to assess the safety and efficacy of senotherapeutic approaches, optimize treatment regimens and develop more individualized and standardized treatment strategies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37, 614–636 (1965).
Di Leonardo, A., Linke, S. P., Clarkin, K. & Wahl, G. M. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 8, 2540–2551 (1994).
Gire, V. & Dulic, V. Senescence from G2 arrest, revisited. Cell Cycle 14, 297–304 (2015).
Sun, D. & Buttitta, L. States of G0 and the proliferation-quiescence decision in cells, tissues and during development. Int. J. Dev. Biol. 61, 357–366 (2017).
Herranz, N. & Gil, J. Mitochondria and senescence: new actors for an old play. EMBO J. 35, 701–702 (2016).
Di Micco, R., Krizhanovsky, V., Baker, D. & d’Adda di Fagagna, F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 22, 75–95 (2021).
Rossiello, F., Herbig, U., Longhese, M. P., Fumagalli, M. & d’Adda di Fagagna, F. Irreparable telomeric DNA damage and persistent DDR signalling as a shared causative mechanism of cellular senescence and ageing. Curr. Opin. Genet. Dev. 26, 89–95 (2014).
Kumari, R. & Jat, P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front. Cell Dev. Biol. 9, 645593 (2021).
Petrova, N. V., Velichko, A. K., Razin, S. V. & Kantidze, O. L. Small molecule compounds that induce cellular senescence. Aging Cell 15, 999–1017 (2016).
Dulić, V., Beney, G. E., Frebourg, G., Drullinger, L. F. & Stein, G. H. Uncoupling between phenotypic senescence and cell cycle arrest in aging p21-deficient fibroblasts. Mol. Cell Biol. 20, 6741–6754 (2000).
Basisty, N. et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 18, e3000599 (2020).
Terlecki-Zaniewicz, L. et al. Small extracellular vesicles and their miRNA cargo are anti-apoptotic members of the senescence-associated secretory phenotype. Aging 10, 1103–1132 (2018).
Özcan, S. et al. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging 8, 1316–1329 (2016).
Kim, S. H. et al. Upregulation of chicken p15INK4b at senescence and in the developing brain. J. Cell Sci. 119, 2435–2443 (2006).
Favetta, L. A. et al. The oxidative stress adaptor p66Shc is required for permanent embryo arrest in vitro. BMC Dev. Biol. 7, 132 (2007).
Storer, M. et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155, 1119–1130 (2013).
Muñoz-Espín, D. et al. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013).
Da Silva-Álvarez, S. et al. Developmentally-programmed cellular senescence is conserved and widespread in zebrafish. Aging 12, 17895–17901 (2020).
Rawlings, T. M. et al. Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids. Elife 10, e69603 (2021).
Jun, J. I. & Lau, L. F. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol. 12, 676–685 (2010).
Wang, X. et al. Induction of fibroblast senescence during mouse corneal wound healing. Invest. Ophthalmol. Vis. Sci. 60, 3669–3679 (2019).
Younis, L. T., Abu Hassan, M. I., Taiyeb Ali, T. B. & Bustami, T. J. 3D TECA hydrogel reduces cellular senescence and enhances fibroblasts migration in wound healing. Asian J. Pharm. Sci. 13, 317–325 (2018).
Bang, M. et al. Tenovin-1 induces senescence and decreases wound-healing activity in cultured rat primary astrocytes. Biomol. Ther. 27, 283–289 (2019).
Blokland, K. E. C. et al. Senescence of IPF lung fibroblasts disrupt alveolar epithelial cell proliferation and promote migration in wound healing. Pharmaceutics 12, 389 (2020).
Xu, M. et al. Hydrogen peroxide-induced senescence reduces the wound healing-promoting effects of mesenchymal stem cell-derived exosomes partially via miR-146a. Aging Dis. 12, 102–115 (2021).
Bitar, M. S., Abdel-Halim, S. M. & Al-Mulla, F. Caveolin-1/PTRF upregulation constitutes a mechanism for mediating p53-induced cellular senescence: implications for evidence-based therapy of delayed wound healing in diabetes. Am. J. Physiol. Endocrinol. Metab. 305, E951–E963 (2013).
Wilkinson, H. N. et al. Elevated local senescence in diabetic wound healing is linked to pathological repair via CXCR2. J. Invest. Dermatol. 139, 1171–1181 (2019).
Bartkova, J. et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434, 864–870 (2005).
Trost, T. M. et al. Premature senescence is a primary fail-safe mechanism of ERBB2-driven tumorigenesis in breast carcinoma cells. Cancer Res. 65, 840–849 (2005).
Alimonti, A. et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J. Clin. Invest. 120, 681–693 (2010).
Tsujimoto, I., Yoshida, A., Yoneda-Kato, N. & Kato, J. Y. Depletion of CSN5 inhibits Ras-mediated tumorigenesis by inducing premature senescence in p53-null cells. FEBS Lett. 586, 4326–4331 (2012).
Yasaei, H. et al. Carcinogen-specific mutational and epigenetic alterations in INK4A, INK4B and p53 tumour-suppressor genes drive induced senescence bypass in normal diploid mammalian cells. Oncogene 32, 171–179 (2013).
Guo, X. et al. TAp63 induces senescence and suppresses tumorigenesis in vivo. Nat. Cell Biol. 11, 1451–1457 (2009).
Jin, X. et al. Inactivation of heat shock factor Hsf4 induces cellular senescence and suppresses tumorigenesis in vivo. Mol. Cancer Res. 10, 523–534 (2012).
Osanai, M. et al. Occludin-mediated premature senescence is a fail-safe mechanism against tumorigenesis in breast carcinoma cells. Cancer Sci. 98, 1027–1034 (2007).
Chen, Y. et al. HBP1-mediated regulation of p21 protein through the Mdm2/p53 and TCF4/EZH2 pathways and its impact on cell senescence and tumorigenesis. J. Biol. Chem. 291, 12688–12705 (2016).
Xiong, J., Jiang, P., Zhong, L. & Wang, Y. The novel tumor suppressor gene ZNF24 induces THCA cells senescence by regulating wnt signaling pathway, resulting in inhibition of THCA tumorigenesis and invasion. Front. Oncol. 11, 646511 (2021).
Tront, J. S., Hoffman, B. & Liebermann, D. A. Gadd45a suppresses Ras-driven mammary tumorigenesis by activation of c-Jun NH2-terminal kinase and p38 stress signaling resulting in apoptosis and senescence. Cancer Res. 66, 8448–8454 (2006).
Georgilis, A. et al. PTBP1-mediated alternative splicing regulates the inflammatory secretome and the pro-tumorigenic effects of senescent cells. Cancer Cell 34, 85–102 (2018).
Yang, G. et al. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc. Natl Acad. Sci. USA 103, 16472–16477 (2006).
Alimirah, F. et al. Cellular senescence promotes skin carcinogenesis through p38MAPK and p44/42MAPK signaling. Cancer Res. 80, 3606–3619 (2020).
Alspach, E. et al. p38MAPK plays a crucial role in stromal-mediated tumorigenesis. Cancer Discov. 4, 716–729 (2014).
Ruhland, M. K. et al. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat. Commun. 7, 11762 (2016).
Okamura, K., Suzuki, T. & Nohara, K. Gestational arsenite exposure augments hepatic tumors of C3H mice by promoting senescence in F1 and F2 offspring via different pathways. Toxicol. Appl. Pharmacol. 408, 115259 (2020).
Flanagan, K. C. et al. c-Myb and C/EBPβ regulate OPN and other senescence-associated secretory phenotype factors. Oncotarget 9, 21–36 (2018).
Muñoz-Galván, S. et al. Tumor cell-secreted PLD increases tumor stemness by senescence-mediated communication with microenvironment. Oncogene 38, 1309–1323 (2019).
Guccini, I. et al. Senescence reprogramming by TIMP1 deficiency promotes prostate cancer metastasis. Cancer Cell 39, 68–82 (2021).
Gonçalves, S. et al. COX2 regulates senescence secretome composition and senescence surveillance through PGE2. Cell Rep. 34, 108860 (2021).
Vindrieux, D. et al. Repression of PLA2R1 by c-MYC and HIF-2alpha promotes cancer growth. Oncotarget 5, 1004–1013 (2014).
Yan, W. et al. Mice deficient in poly(C)-binding protein 4 are susceptible to spontaneous tumors through increased expression of ZFP871 that targets p53 for degradation. Genes Dev. 30, 522–534 (2016).
Xie, H. et al. Cell-cycle arrest and senescence in TP53-wild type renal carcinoma by enhancer RNA-P53-bound enhancer regions 2 (p53BER2) in a p53-dependent pathway. Cell Death Dis. 12, 1 (2021).
Zhang, Q. et al. Higher expression of XPF is a critical factor in intrinsic chemotherapy resistance of human renal cell carcinoma. Int. J. Cancer 139, 2827–2837 (2016).
Hu, J. et al. Endoglin is essential for the maintenance of self-renewal and chemoresistance in renal cancer stem cells. Stem Cell Rep. 9, 464–477 (2017).
Kamal, Y., Cheng, C., Frost, H. R. & Amos, C. I. Predictors of disease aggressiveness influence outcome from immunotherapy treatment in renal clear cell carcinoma. Oncoimmunology 8, e1500106 (2019).
Sarkisian, C. J. et al. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat. Cell Biol. 9, 493–505 (2007).
Ou, H. L. et al. Cellular senescence in cancer: from mechanisms to detection. Mol. Oncol. 15, 2634–2671 (2021).
Rubelt, F. et al. Onset of immune senescence defined by unbiased pyrosequencing of human immunoglobulin mRNA repertoires. PLoS One 7, e49774 (2012).
Zhang, H., Puleston, D. J. & Simon, A. K. Autophagy and immune senescence. Trends Mol. Med. 22, 671–686 (2016).
Sato, Y. & Yanagita, M. Immunology of the ageing kidney. Nat. Rev. Nephrol. 15, 625–640 (2019).
Chiu, Y. L. et al. Emergence of T cell immunosenescence in diabetic chronic kidney disease. Immun. Ageing 17, 31 (2020).
Lioulios, G., Fylaktou, A., Papagianni, A. & Stangou, M. T cell markers recount the course of immunosenescence in healthy individuals and chronic kidney disease. Clin. Immunol. 225, 108685 (2021).
Trzonkowski, P. et al. Immunosenescence increases the rate of acceptance of kidney allotransplants in elderly recipients through exhaustion of CD4+ T-cells. Mech. Ageing Dev. 131, 96–104 (2010).
Rodriguez, I. J. et al. Immunosenescence study of T cells: a systematic review. Front. Immunol. 11, 604591 (2020).
Alonso-Arias, R. et al. NKG2D expression in CD4+ T lymphocytes as a marker of senescence in the aged immune system. Age 33, 591–-605 (2011).
Pellicanò, M. et al. Evidence for less marked potential signs of T-cell immunosenescence in centenarian offspring than in the general age-matched population. J. Gerontol. A Biol. Sci. Med. Sci. 69, 495–504 (2014).
Song, Y. et al. T-cell immunoglobulin and ITIM domain contributes to CD8+ T-cell immunosenescence. Aging Cell 17, e12716 (2018).
Listì, F. et al. A study of serum immunoglobulin levels in elderly persons that provides new insights into B cell immunosenescence. Ann. NY Acad. Sci. 1089, 487–495 (2006).
de Bourcy, C. F. et al. Phylogenetic analysis of the human antibody repertoire reveals quantitative signatures of immune senescence and aging. Proc. Natl Acad. Sci. USA 114, 1105–1110 (2017).
Lescale, C. et al. Reduced EBF expression underlies loss of B-cell potential of hematopoietic progenitors with age. Aging Cell 9, 410–419 (2010).
Ratliff, M., Alter, S., Frasca, D., Blomberg, B. B. & Riley, R. L. In senescence, age-associated B cells secrete TNFα and inhibit survival of B-cell precursors. Aging Cell 12, 303–311 (2013).
Lutz, C. T. & Quinn, L. S. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging 4, 535–546 (2012).
Sadhu, S. et al. Radiation-induced macrophage senescence impairs resolution programs and drives cardiovascular inflammation. J. Immunol. 207, 1812–1823 (2021).
Li, H. et al. Using ROS as a second messenger, NADPH oxidase 2 mediates macrophage senescence via interaction with NF-κB during Pseudomonas aeruginosa infection. Oxid. Med. Cell Longev. 2018, 9741838 (2018).
Zhao, Q. et al. Activating transcription factor 3 involved in Pseudomonas aeruginosa PAO1-induced macrophage senescence. Mol. Immunol. 133, 122–127 (2021).
Wang, H. et al. BRD4 contributes to LPS-induced macrophage senescence and promotes progression of atherosclerosis-associated lipid uptake. Aging 12, 9240–9259 (2020).
Behmoaras, J. & Gil, J. Similarities and interplay between senescent cells and macrophages. J. Cell Biol. 220, e202010162 (2021).
Hall, B. M. et al. p16Ink4a and senescence-associated β-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging 9, 1867–1884 (2017).
Tonnessen-Murray, C. A. et al. Chemotherapy-induced senescent cancer cells engulf other cells to enhance their survival. J. Cell Biol. 218, 3827–3844 (2019).
Hickson, L. J., Eirin, A. & Lerman, L. O. Challenges and opportunities for stem cell therapy in patients with chronic kidney disease. Kidney Int. 89, 767–778 (2016).
Malaise, O. et al. Mesenchymal stem cell senescence alleviates their intrinsic and seno-suppressive paracrine properties contributing to osteoarthritis development. Aging 11, 9128–9146 (2019).
Chen, P. M. et al. c-Maf regulates pluripotency genes, proliferation/self-renewal, and lineage commitment in ROS-mediated senescence of human mesenchymal stem cells. Oncotarget 6, 35404–35418 (2015).
Fan, C. et al. TGF‑β induces periodontal ligament stem cell senescence through increase of ROS production. Mol. Med. Rep. 20, 3123–3130 (2019).
Gu, S., Ran, S., Liu, B. & Liang, J. miR-152 induces human dental pulp stem cell senescence by inhibiting SIRT7 expression. FEBS Lett. 590, 1123–1131 (2016).
Xiao, Y. Z. et al. Reducing hypothalamic stem cell senescence protects against aging-associated physiological decline. Cell Metab. 31, 534–548 (2020).
Cho, J. et al. Ewing sarcoma gene Ews regulates hematopoietic stem cell senescence. Blood 117, 1156–1166 (2011).
Pi, C. et al. Nicotinamide phosphoribosyltransferase postpones rat bone marrow mesenchymal stem cell senescence by mediating NAD+-Sirt1 signaling. Aging 11, 3505–3522 (2019).
Son, M. J., Kwon, Y., Son, T. & Cho, Y. S. Restoration of mitochondrial NAD+ levels delays stem cell senescence and facilitates reprogramming of aged somatic cells. Stem Cell 34, 2840–2851 (2016).
Zhai, X. Y. et al. Knockdown of SIRT6 enables human bone marrow mesenchymal stem cell senescence. Rejuvenation Res. 19, 373–384 (2016).
Liu, F. et al. NANOG attenuates hair follicle-derived mesenchymal stem cell senescence by upregulating PBX1 and activating AKT signaling. Oxid. Med. Cell Longev. 2019, 4286213 (2019).
Iwata, T. et al. Functional regulatory mechanisms underlying bone marrow mesenchymal stem cell senescence during cell passages. Cell Biochem. Biophys. 79, 321–336 (2021).
Gannon, H. S., Donehower, L. A., Lyle, S. & Jones, S. N. Mdm2-p53 signaling regulates epidermal stem cell senescence and premature aging phenotypes in mouse skin. Dev. Biol. 353, 1–9 (2011).
Dong, X. Y. et al. Downregulation of ROR2 promotes dental pulp stem cell senescence by inhibiting STK4-FOXO1/SMS1 axis in sphingomyelin biosynthesis. Aging Cell 20, e13430 (2021).
Cho, A. et al. An endogenous anti-aging factor, sonic hedgehog, suppresses endometrial stem cell aging through SERPINB2. Mol. Ther. 27, 1286–1298 (2019).
Zhang, D. et al. Autophagy inhibits the mesenchymal stem cell aging induced by D-galactose through ROS/JNK/p38 signalling. Clin. Exp. Pharmacol. Physiol. 47, 466–477 (2020).
Chang, T. C., Hsu, M. F. & Wu, K. K. High glucose induces bone marrow-derived mesenchymal stem cell senescence by upregulating autophagy. PLoS ONE 10, e0126537 (2015).
Conley, S. M. et al. Human obesity induces dysfunction and early senescence in adipose tissue-derived mesenchymal stromal/stem cells. Front. Cell Dev. Biol. 8, 197 (2020).
Hickson, L. J. et al. Diabetic kidney disease alters the transcriptome and function of human adipose-derived mesenchymal stromal cells but maintains immunomodulatory and paracrine activities important for renal repair. Diabetes 70, 1561–1574 (2021).
Davalli, P., Mitic, T., Caporali, A., Lauriola, A. & D’Arca, D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid. Med. Cell Longev. 2016, 3565127 (2016).
Zhang, C. F. et al. Suppression of autophagy dysregulates the antioxidant response and causes premature senescence of melanocytes. J. Invest. Dermatol. 135, 1348–1357 (2015).
Song, X. et al. Autophagy deficient keratinocytes display increased DNA damage, senescence and aberrant lipid composition after oxidative stress in vitro and in vivo. Redox Biol. 11, 219–230 (2017).
Xing, S. et al. Lactose induced redox-dependent senescence and activated Nrf2 pathway. Int. J. Clin. Exp. Pathol. 12, 2034–2045 (2019).
Su, S. et al. Lowering endogenous cathepsin d abundance results in reactive oxygen species accumulation and cell senescence. Mol. Cell Proteom. 16, 1217–1232 (2017).
Volonte, D. & Galbiati, F. Inhibition of thioredoxin reductase 1 by caveolin 1 promotes stress-induced premature senescence. EMBO Rep. 10, 1334–1340 (2009).
Dasari, A., Bartholomew, J. N., Volonte, D. & Galbiati, F. Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res. 66, 10805–10814 (2006).
Uraoka, M. et al. Loss of Bcl-2 during the senescence exacerbates the impaired angiogenic functions in endothelial cells by deteriorating the mitochondrial redox state. Hypertension 58, 254–263 (2011).
Sakai, T., Kurokawa, R., Hirano, S. I. & Imai, J. Hydrogen indirectly suppresses increases in hydrogen peroxide in cytoplasmic hydroxyl radical-induced cells and suppresses cellular senescence. Int. J. Mol. Sci. 20, 456 (2019).
Xu, X. et al. Oxidative stress-induced miRNAs modulate AKT signaling and promote cellular senescence in uterine leiomyoma. J. Mol. Med. 96, 1095–1106 (2018).
Kurz, D. J. et al. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J. Cell Sci. 117, 2417–2426 (2004).
Barascu, A. et al. Oxydative stress alters nuclear shape through lamins dysregulation: a route to senescence. Nucleus 3, 411–417 (2012).
Chandrasekaran, A. et al. Redox and mTOR-dependent regulation of plasma lamellar calcium influx controls the senescence-associated secretory phenotype. Exp. Biol. Med. 245, 1560–1570 (2020).
McCarthy, D. A., Clark, R. R., Bartling, T. R., Trebak, M. & Melendez, J. A. Redox control of the senescence regulator interleukin-1α and the secretory phenotype. J. Biol. Chem. 288, 32149–32159 (2013).
Mancini, O. K. et al. Oxidative stress-induced senescence mediates inflammatory and fibrotic phenotypes in fibroblasts from systemic sclerosis patients. Rheumatology 61, 1265–1275 (2021).
Bourdens, M. et al. Short exposure to cold atmospheric plasma induces senescence in human skin fibroblasts and adipose mesenchymal stromal cells. Sci. Rep. 9, 8671 (2019).
Tsai, C. H. et al. Up-regulation of cofilin-1 in cell senescence associates with morphological change and p27kip1-mediated growth delay. Aging Cell 20, e13288 (2021).
Oliva, J. L., Caino, M. C., Senderowicz, A. M. & Kazanietz, M. G. S-Phase-specific activation of PKC alpha induces senescence in non-small cell lung cancer cells. J. Biol. Chem. 283, 5466–5476 (2008).
Freund, A., Laberge, R. M., Demaria, M. & Campisi, J. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell 23, 2066–2075 (2012).
Yang, H. et al. Reactive oxygen species and nitric oxide induce senescence of rudimentary leaves and the expression profiles of the related genes in Litchi chinensis. Hortic. Res. 5, 23 (2018).
Gewirtz, D. A., Holt, S. E. & Elmore, L. W. Accelerated senescence: an emerging role in tumor cell response to chemotherapy and radiation. Biochem. Pharmacol. 76, 947–957 (2008).
Liu, J. et al. Senescence as a consequence of ginsenoside rg1 response on k562 human leukemia cell line. Asian Pac. J. Cancer Prev. 13, 6191–6196 (2012).
Druelle, C. et al. ATF6α regulates morphological changes associated with senescence in human fibroblasts. Oncotarget 7, 67699–67715 (2016).
Chen, Q. M. et al. Involvement of Rb family proteins, focal adhesion proteins and protein synthesis in senescent morphogenesis induced by hydrogen peroxide. J. Cell Sci. 113, 4087–4097 (2000).
Rivas-Chacón, L. D. M. et al. Role of oxidative stress in the senescence pattern of auditory cells in age-related hearing loss. Antioxidants 10, 1497 (2021).
Shaerzadeh, F. et al. Microglia senescence occurs in both substantia nigra and ventral tegmental area. Glia 68, 2228–2245 (2020).
Ridzuan, N., Al Abbar, A., Yip, W. K., Maqbool, M. & Ramasamy, R. Characterization and expression of senescence marker in prolonged passages of rat bone marrow-derived mesenchymal stem cells. Stem Cell Int. 2016, 8487264 (2016).
Berkenkamp, B. et al. In vivo and in vitro analysis of age-associated changes and somatic cellular senescence in renal epithelial cells. PLoS ONE 9, e88071 (2014).
Cohen, C. et al. Glomerular endothelial cell senescence drives age-related kidney disease through PAI-1. EMBO Mol. Med. 13, e14146 (2021).
Karin, O. & Alon, U. Senescent cell accumulation mechanisms inferred from parabiosis. Geroscience 43, 329–341 (2021).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Wang, A. S. & Dreesen, O. Biomarkers of cellular senescence and skin aging. Front. Genet. 9, 247 (2018).
Netti, G. S. et al. Role of complement in regulating inflammation processes in renal and prostate cancers. Cells 10, 2426 (2021).
Kawagoe, Y. et al. CXCL5-CXCR2 signaling is a senescence-associated secretory phenotype in preimplantation embryos. Aging Cell 19, e13240 (2020).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).
Mosteiro, L., Pantoja, C., de Martino, A. & Serrano, M. Senescence promotes in vivo reprogramming through p16INK4a and IL-6. Aging Cell 17, e12711 (2018).
Iannello, A., Thompson, T. W., Ardolino, M., Lowe, S. W. & Raulet, D. H. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J. Exp. Med. 210, 2057–2069 (2013).
Kang, T. W. et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 (2011).
Alimbetov, D. et al. Suppression of the senescence-associated secretory phenotype (SASP) in human fibroblasts using small molecule inhibitors of p38 MAP kinase and MK2. Biogerontology 17, 305–315 (2016).
Rana, T. et al. PAI-1 regulation of TGF-β1-induced alveolar type II cell senescence, SASP secretion, and SASP-mediated activation of alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 62, 319–330 (2020).
Liu, D. & Hornsby, P. J. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 67, 3117–3126 (2007).
Barinda, A. J. et al. Endothelial progeria induces adipose tissue senescence and impairs insulin sensitivity through senescence associated secretory phenotype. Nat. Commun. 11, 481 (2020).
Zuccolo, E. et al. The microRNA-34a-induced senescence-associated secretory phenotype (SASP) favors vascular smooth muscle cells calcification. Int. J. Mol. Sci. 21, 4454 (2020).
Salotti, J. & Johnson, P. F. Regulation of senescence and the SASP by the transcription factor C/EBPβ. Exp. Gerontol. 128, 110752 (2019).
Takahashi, A. et al. Downregulation of cytoplasmic DNases is implicated in cytoplasmic DNA accumulation and SASP in senescent cells. Nat. Commun. 9, 1249 (2018).
En, A., Takauji, Y., Ayusawa, D. & Fujii, M. The role of lamin B receptor in the regulation of senescence-associated secretory phenotype (SASP). Exp. Cell Res. 390, 111927 (2020).
Tripathi, U. et al. SARS-CoV-2 causes senescence in human cells and exacerbates the senescence-associated secretory phenotype through TLR-3. Aging 13, 21838–21854 (2021).
Debacq-Chainiaux, F., Erusalimsky, J. D., Campisi, J. & Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 4, 1798–1806 (2009).
de Mera-Rodríguez, J. A. et al. Is senescence-associated β-galactosidase a reliable in vivo marker of cellular senescence during embryonic development? Front. Cell Dev. Biol. 9, 623175 (2021).
Severino, J., Allen, R. G., Balin, S., Balin, A. & Cristofalo, V. J. Is beta-galactosidase staining a marker of senescence in vitro and in vivo? Exp. Cell Res. 257, 162–171 (2000).
Palmer, A. et al. Expression of p16 within myenteric neurons of the aged colon: a potential marker of declining function. Front. Neurosci. 15, 747067 (2021).
López-Domínguez, J. A. et al. Cdkn1a transcript variant 2 is a marker of aging and cellular senescence. Aging 13, 13380–13392 (2021).
Boichuck, M., Zorea, J., Elkabets, M., Wolfson, M. & Fraifeld, V. E. c-Met as a new marker of cellular senescence. Aging 11, 2889–2897 (2019).
Kwak, I. H., Kim, H. S., Choi, O. R., Ryu, M. S. & Lim, I. K. Nuclear accumulation of globular actin as a cellular senescence marker. Cancer Res. 64, 572–580 (2004).
Bernadotte, A., Mikhelson, V. M. & Spivak, I. M. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging 8, 3–11 (2016).
Turinetto, V. & Giachino, C. Multiple facets of histone variant H2AX: a DNA double-strand-break marker with several biological functions. Nucleic Acids Res. 43, 2489–2498 (2015).
Corpet, A. & Stucki, M. Chromatin maintenance and dynamics in senescence: a spotlight on SAHF formation and the epigenome of senescent cells. Chromosoma 123, 423–436 (2014).
Olivieri, F. et al. MiR-146a as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodelling. Age 35, 1157–1172 (2013).
Zhou, R. et al. Cytosolic dsDNA is a novel senescence marker associated with pyroptosis activation. Tissue Cell 72, 101554 (2021).
Hernandez-Segura, A., Nehme, J. & Demaria, M. Hallmarks of cellular senescence. Trends Cell Biol. 28, 436–453 (2018).
Thorin-Trescases, N., Labbé, P., Mury, P., Lambert, M. & Thorin, E. Angptl2 is a marker of cellular senescence: the physiological and pathophysiological impact of Angptl2-related senescence. Int. J. Mol. Sci. 22, 12232 (2021).
Walter, R., Murasko, D. M. & Sierra, F. T-kininogen is a biomarker of senescence in rats. Mech. Ageing Dev. 106, 129–144 (1998).
Gan, W. et al. Age-dependent increases in the oxidative damage of DNA, RNA, and their metabolites in normal and senescence-accelerated mice analyzed by LC-MS/MS: urinary 8-oxoguanosine as a novel biomarker of aging. Free Radic. Biol. Med. 52, 1700–1707 (2012).
Bian, X. et al. Senescence marker activin A is increased in human diabetic kidney disease: association with kidney function and potential implications for therapy. BMJ Open. Diabetes Res. Care 7, e000720 (2019).
Santelli, A. et al. Senescent kidney cells in hypertensive patients release urinary extracellular vesicles. J. Am. Heart Assoc. 8, e012584 (2019).
Tresini, M., Pignolo, R. J., Allen, R. G. & Cristofalo, V. J. Effects of donor age on the expression of a marker of replicative senescence (EPC-1) in human dermal fibroblasts. J. Cell Physiol. 179, 11–17 (1999).
Parish, S. T., Wu, J. E. & Effros, R. B. Sustained CD28 expression delays multiple features of replicative senescence in human CD8 T lymphocytes. J. Clin. Immunol. 30, 798–805 (2010).
Henson, S. M. & Akbar, A. N. KLRG1-more than a marker for T cell senescence. Age 31, 285–291 (2009).
Günther, J. et al. Identification of the activating cytotoxicity receptor NKG2D as a senescence marker in zero-hour kidney biopsies is indicative for clinical outcome. Kidney Int. 91, 1447–1463 (2017).
Snyder, L. M. et al. Irreversible spectrin-haemoglobin crosslinking in vivo: a marker for red cell senescence. Br. J. Haematol. 53, 379–384 (1983).
Blanco, F. J. & Bernabéu, C. The splicing factor SRSF1 as a marker for endothelial senescence. Front. Physiol. 3, 54 (2012).
Bascones-Martínez, A. et al. Differences in the expression of five senescence markers in oral cancer, oral leukoplakia and control samples in humans. Oncol. Lett. 3, 1319–1325 (2012).
Bertolo, A., Baur, M., Guerrero, J., Pötzel, T. & Stoyanov, J. Autofluorescence is a reliable in vitro marker of cellular senescence in human mesenchymal stromal cells. Sci. Rep. 9, 2074 (2019).
Yamagishi, S. et al. Upregulation of cannabinoid receptor type 2, but not TSPO, in senescence-accelerated neuroinflammation in mice: a positron emission tomography study. J. Neuroinflammation 16, 208 (2019).
Khandjian, E. W., Salomon, C., Léonard, N., Tremblay, S. & Türler, H. Fibronectin gene expression in proliferating, quiescent, and SV40-infected mouse kidney cells. Exp. Cell Res. 202, 464–470 (1992).
Chen, C. et al. Lipoxin A4 restores septic renal function via blocking crosstalk between inflammation and premature senescence. Front. Immunol. 12, 637753 (2021).
Bae, E. et al. Paricalcitol attenuates contrast-induced acute kidney injury by regulating mitophagy and senescence. Oxid. Med. Cell Longev. 2020, 7627934 (2020).
Khan, S., Loi, V. & Rosner, M. H. Drug-induced kidney injury in the elderly. Drugs Aging 34, 729–741 (2017).
Marquez-Exposito, L. et al. Acute kidney injury is aggravated in aged mice by the exacerbation of proinflammatory processes. Front. Pharmacol. 12, 662020 (2021).
Li, Y. & Lerman, L. O. Cellular senescence: a new player in kidney injury. Hypertension 76, 1069–1075 (2020).
Johnson, A. C. & Zager, R. A. Plasma and urinary p21: potential biomarkers of AKI and renal aging. Am. J. Physiol. Renal Physiol. 315, F1329–F1335 (2018).
Castellano, G. et al. Complement component C5a induces aberrant epigenetic modifications in renal tubular epithelial cells accelerating senescence by Wnt4/βcatenin signaling after ischemia/reperfusion injury. Aging 11, 4382–4406 (2019).
Rodrigues, C. E. et al. Human umbilical cord-derived mesenchymal stromal cells protect against premature renal senescence resulting from oxidative stress in rats with acute kidney injury. Stem Cell Res. Ther. 8, 19 (2017).
Westhoff, J. H. et al. Telomere shortening reduces regenerative capacity after acute kidney injury. J. Am. Soc. Nephrol. 21, 327–336 (2010).
Cheng, H., Fan, X., Lawson, W. E., Paueksakon, P. & Harris, R. C. Telomerase deficiency delays renal recovery in mice after ischemia-reperfusion injury by impairing autophagy. Kidney Int. 88, 85–94 (2015).
Sari, F. T., Sari, F. T., Sari, F. T., Arfian, N. & Sari, D. C. R. Effect of kidney ischemia/reperfusion injury on proliferation, apoptosis, and cellular senescence in acute kidney injury in mice. Med. J. Malays. 75, 20–23 (2020).
Jia, Y. et al. Nicotinamide mononucleotide attenuates renal interstitial fibrosis after AKI by suppressing tubular DNA damage and senescence. Front. Physiol. 12, 649547 (2021).
DiRocco, D. P. et al. CDK4/6 inhibition induces epithelial cell cycle arrest and ameliorates acute kidney injury. Am. J. Physiol. Renal Physiol. 306, F379–F388 (2014).
Kim, S. R. et al. Progressive cellular senescence mediates renal dysfunction in ischemic nephropathy. J. Am. Soc. Nephrol. 32, 1987–2004 (2021).
Schroth, J., Thiemermann, C. & Henson, S. M. Senescence and the aging immune system as major drivers of chronic kidney disease. Front. Cell Dev. Biol. 8, 564461 (2020).
Carracedo, J. et al. Mechanisms of cardiovascular disorders in patients with chronic kidney disease: a process related to accelerated senescence. Front. Cell Dev. Biol. 8, 185 (2020).
Crépin, T. et al. Uraemia-induced immune senescence and clinical outcomes in chronic kidney disease patients. Nephrol. Dial. Transpl. 35, 624–632 (2020).
Sosa, P. et al. Hyperphosphatemia promotes senescence of myoblasts by impairing autophagy through Ilk overexpression, a possible mechanism involved in sarcopenia. Aging Dis. 9, 769–784 (2018).
Olmos, G. et al. Hyperphosphatemia induces senescence in human endothelial cells by increasing endothelin-1 production. Aging Cell 16, 1300–1312 (2017).
Troyano, N. et al. Hyperphosphatemia induces cellular senescence in human aorta smooth muscle cells through integrin linked kinase (ILK) up-regulation. Mech. Ageing Dev. 152, 43–55 (2015).
Okada, A. et al. D-serine, a novel uremic toxin, induces senescence in human renal tubular cells via GCN2 activation. Sci. Rep. 7, 11168 (2017).
Dong, D. et al. Alleviation of senescence and epithelial-mesenchymal transition in aging kidney by short-term caloric restriction and caloric restriction mimetics via modulation of AMPK/mTOR signaling. Oncotarget 8, 16109–16121 (2017).
Gong, W. et al. Brahma-related gene-1 promotes tubular senescence and renal fibrosis through Wnt/β-catenin/autophagy axis. Clin. Sci. 135, 1873–1895 (2021).
Luo, C. et al. Wnt9a promotes renal fibrosis by accelerating cellular senescence in tubular epithelial cells. J. Am. Soc. Nephrol. 29, 1238–1256 (2018).
Zhou, L., Li, Y., Zhou, D., Tan, R. J. & Liu, Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J. Am. Soc. Nephrol. 24, 771–785 (2013).
Li, C., Shen, Y., Huang, L., Liu, C. & Wang, J. Senolytic therapy ameliorates renal fibrosis postacute kidney injury by alleviating renal senescence. FASEB J. 35, e21229 (2021).
Zhang, L. et al. C/EBPα deficiency in podocytes aggravates podocyte senescence and kidney injury in aging mice. Cell Death Dis. 10, 684 (2019).
Juvet, C. et al. Renal programming by transient postnatal overfeeding: the role of senescence pathways. Front. Physiol. 11, 511 (2020).
Liu, J. et al. Impact of ER stress-regulated ATF4/p16 signaling on the premature senescence of renal tubular epithelial cells in diabetic nephropathy. Am. J. Physiol. Cell Physiol. 308, C621–C630 (2015).
Yu, S. et al. M1 macrophages accelerate renal glomerular endothelial cell senescence through reactive oxygen species accumulation in streptozotocin-induced diabetic mice. Int. Immunopharmacol. 81, 106294 (2020).
Shi, M. et al. The RAGE/STAT5/autophagy axis regulates senescence in mesangial cells. Cell Signal. 62, 109334 (2019).
Kim, S. R., Eirin, A., Zhang, X., Lerman, A. & Lerman, L. O. Mitochondrial protection partly mitigates kidney cellular senescence in swine atherosclerotic renal artery stenosis. Cell Physiol. Biochem. 52, 617–632 (2019).
Chen, X. J. et al. Renovascular disease induces senescence in renal scattered tubular-like cells and impairs their reparative potency. Hypertension 77, 507–518 (2021).
Liu, J. et al. Accelerated senescence of renal tubular epithelial cells is associated with disease progression of patients with immunoglobulin A (IgA) nephropathy. Transl. Res. 159, 454–463 (2012).
Tilman, G. et al. High p16INK4a, a marker of cellular senescence, is associated with renal injury, impairment and outcome in lupus nephritis. RMD Open 7, e001844 (2021).
Kim, S. R. et al. Increased renal cellular senescence in murine high-fat diet: effect of the senolytic drug quercetin. Transl Res. 213, 112–123 (2019).
Sis, B. et al. Accelerated expression of senescence associated cell cycle inhibitor p16INK4A in kidneys with glomerular disease. Kidney Int. 71, 218–226 (2007).
Kim, D. Y., Lee, M. & Kim, E. J. Involvement of Klotho, TNF‑α; and ADAMs in radiation‑induced senescence of renal epithelial cells. Mol. Med. Rep. 23, 22 (2021).
Eleftheriadis, T., Pissas, G., Filippidis, G., Liakopoulos, V. & Stefanidis, I. The role of indoleamine 2,3-dioxygenase in renal tubular epithelial cells senescence under anoxia or reoxygenation. Biomolecules 11, 1522 (2021).
Troyano-Suárez, N. et al. Glucose oxidase induces cellular senescence in immortal renal cells through ILK by downregulating Klotho gene expression. Oxid. Med. Cell Longev. 2015, 416738 (2015).
Ferlicot, S. et al. The role of replicative senescence in chronic allograft nephropathy. Hum. Pathol. 34, 924–928 (2003).
Pesce, F. et al. DelCFHR3-1 influences graft survival in transplant patients with IgA nephropathy via complement-mediated cellular senescence. Am. J. Transpl. 21, 838–845 (2021).
Lee, D. H., Wolstein, J. M., Pudasaini, B. & Plotkin, M. INK4a deletion results in improved kidney regeneration and decreased capillary rarefaction after ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 302, F183–F191 (2012).
Baar, M. P. et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 169, 132–147 (2017).
Kim, S. G., Sung, J. Y., Kim, J. R. & Choi, H. C. Quercetin-induced apoptosis ameliorates vascular smooth muscle cell senescence through AMP-activated protein kinase signaling pathway. Korean J. Physiol. Pharmacol. 24, 69–79 (2020).
Jiang, Y. H., Jiang, L. Y., Wang, Y. C., Ma, D. F. & Li, X. Quercetin attenuates atherosclerosis via modulating oxidized LDL-induced endothelial cellular senescence. Front. Pharmacol. 11, 512 (2020).
Liu, T. et al. Quercetin alleviates kidney fibrosis by reducing renal tubular epithelial cell senescence through the SIRT1/PINK1/mitophagy axis. Life Sci. 257, 118116 (2020).
Shao, Z. et al. Senolytic agent quercetin ameliorates intervertebral disc degeneration via the Nrf2/NF-κB axis. Osteoarthritis Cartilage 29, 413–422 (2021).
Yu, S. et al. Quercetin reverses cardiac systolic dysfunction in mice fed with a high-fat diet: role of angiogenesis. Oxid. Med. Cell Longev. 2021, 8875729 (2021).
Yousefzadeh, M. J. et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36, 18–28 (2018).
Zhu, Y. et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging 9, 955–963 (2017).
Xu, Q. et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat. Metab. 3, 1706–1726 (2021).
Wang, Z. et al. Ginsenoside Rg1 prevents bone marrow mesenchymal stem cell senescence via NRF2 and PI3K/Akt signaling. Free Radic. Biol. Med. 174, 182–194 (2021).
Yokozawa, T., Satoh, A. & Cho, E. J. Ginsenoside-Rd attenuates oxidative damage related to aging in senescence-accelerated mice. J. Pharm. Pharmacol. 56, 107–113 (2004).
Hou, J., Cui, C., Kim, S., Sung, C. & Choi, C. Ginsenoside F1 suppresses astrocytic senescence-associated secretory phenotype. Chem. Biol. Interact. 283, 75–83 (2018).
Ogrodnik, M. et al. Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell 20, e13296 (2021).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2016).
Zhu, Y. et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435 (2016).
Adams, J. M. & Cory, S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 25, 27–36 (2018).
Mylonas, K. J. et al. Cellular senescence inhibits renal regeneration after injury in mice, with senolytic treatment promoting repair. Sci. Transl Med. 13, eabb0203 (2021).
Fuhrmann-Stroissnigg, H. et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun. 8, 422 (2017).
Rinaldi, S. et al. Stem cell senescence. Effects of REAC technology on telomerase-independent and telomerase-dependent pathways. Sci. Rep. 4, 6373 (2014).
Poblocka, M. et al. Targeted clearance of senescent cells using an antibody-drug conjugate against a specific membrane marker. Sci. Rep. 11, 20358 (2021).
Arora, S. et al. Invariant natural killer T cells coordinate removal of senescent cells. Med 2, 938–950 (2021).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132 (2020).
Mendelsohn, A. R. & Larrick, J. W. Antiaging vaccines targeting senescent cells. Rejuvenation Res. 25, 39–45 (2022).
Soukas, A. A., Hao, H. & Wu, L. Metformin as anti-aging therapy: is it for everyone? Trends Endocrinol. Metab. 30, 745–755 (2019).
Moiseeva, O. et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell 12, 489–498 (2013).
Zhang, E. et al. Metformin and resveratrol inhibited high glucose-induced metabolic memory of endothelial senescence through SIRT1/p300/p53/p21 pathway. PLoS ONE 10, e0143814 (2015).
Menendez, J. A. et al. Metformin and the ATM DNA damage response (DDR): accelerating the onset of stress-induced senescence to boost protection against cancer. Aging 3, 1063–1077 (2011).
Xu, M. et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl Acad. Sci. USA 112, E6301–E6310 (2015).
Sasaki, N., Itakura, Y. & Toyoda, M. Rapamycin promotes endothelial-mesenchymal transition during stress-induced premature senescence through the activation of autophagy. Cell Commun. Signal. 18, 43 (2020).
Wang, R. et al. Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell 16, 564–574 (2017).
Correia-Melo, C. et al. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 35, 724–742 (2016).
Menicacci, B. et al. Chronic resveratrol treatment inhibits MRC5 fibroblast SASP-related protumoral effects on melanoma cells. J. Gerontol. A Biol. Sci. Med. Sci. 72, 1187–1195 (2017).
Zhang, B. et al. KDM4 orchestrates epigenomic remodeling of senescent cells and potentiates the senescence-associated secretory phenotype. Nat. Aging 1, 454–472 (2021).
Pazolli, E. et al. Chromatin remodeling underlies the senescence-associated secretory phenotype of tumor stromal fibroblasts that supports cancer progression. Cancer Res. 72, 2251–2261 (2012).
Yu, S. et al. Melatonin regulates PARP1 to control the senescence-associated secretory phenotype (SASP) in human fetal lung fibroblast cells. J. Pineal Res. 63, 12405 (2017).
Yoo, Y. M., Jang, S. K., Kim, G. H., Park, J. Y. & Joo, S. S. Pharmacological advantages of melatonin in immunosenescence by improving activity of T lymphocytes. J. Biomed. Res. 30, 314–321 (2016).
Rodríguez, M. I. et al. Chronic melatonin treatment prevents age-dependent cardiac mitochondrial dysfunction in senescence-accelerated mice. Free Radic. Res. 41, 15–24 (2007).
Han, Y. S., Kim, S. M. & Lee, J. H. Melatonin protects chronic kidney disease mesenchymal stem cells against senescence via PrPC-dependent enhancement of the mitochondrial function. J. Pineal Res. 66, e12535 (2019).
Rais, M., Wilson, R. M., Urbanski, H. F. & Messaoudi, I. Androgen supplementation improves some but not all aspects of immune senescence in aged male macaques. Geroscience 39, 373–384 (2017).
Toillon, R. A. et al. Estrogens decrease gamma-ray-induced senescence and maintain cell cycle progression in breast cancer cells independently of p53. Int. J. Radiat. Oncol. Biol. Phys. 67, 1187–1200 (2007).
Engelmann, F. et al. Accelerated immune senescence and reduced response to vaccination in ovariectomized female rhesus macaques. Age 33, 275–289 (2011).
Poulsen, R. C. et al. Glucocorticoids induce senescence in primary human tenocytes by inhibition of sirtuin 1 and activation of the p53/p21 pathway: in vivo and in vitro evidence. Ann. Rheum. Dis. 73, 1405–1413 (2014).
Zhang, M. et al. Bone marrow mesenchymal stem cell transplantation retards the natural senescence of rat hearts. Stem Cell Transl Med. 4, 494–502 (2015).
Zhang, Y. et al. Rat induced pluripotent stem cells protect H9C2 cells from cellular senescence via a paracrine mechanism. Cardiology 128, 43–50 (2014).
Kim, S. R. et al. Increased cellular senescence in the murine and human stenotic kidney: effect of mesenchymal stem cells. J. Cell Physiol. 236, 1332–1344 (2021).
Cheng, T., Ding, S., Liu, S., Li, Y. & Sun, L. Human umbilical cord-derived mesenchymal stem cell therapy ameliorates lupus through increasing CD4+ T cell senescence via MiR-199a-5p/Sirt1/p53 axis. Theranostics 11, 893–905 (2021).
Xiao, X. et al. Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src. Signal. Transduct. Target. Ther. 6, 354 (2021).
Zhang, L. et al. Selective intrarenal delivery of mesenchymal stem cell-derived extracellular vesicles attenuates myocardial injury in experimental metabolic renovascular disease. Basic. Res. Cardiol. 115, 16 (2020).
Dong, J. et al. Dental pulp stem cell-derived small extracellular vesicle in irradiation-induced senescence. Biochem. Biophys. Res. Commun. 575, 28–35 (2021).
Lei, J. et al. Exosomes from antler stem cells alleviate mesenchymal stem cell senescence and osteoarthritis. Protein Cell 13, 220–226 (2021).
Carroll, J. E. et al. Partial sleep deprivation activates the DNA damage response (DDR) and the senescence-associated secretory phenotype (SASP) in aged adult humans. Brain Behav. Immun. 51, 223–229 (2016).
Sun, L. et al. Alterations in mitochondrial biogenesis and respiratory activity, inflammation of the senescence-associated secretory phenotype, and lipolysis in the perirenal fat and liver of rats following lifelong exercise and detraining. FASEB J. 35, e21890 (2021).
Tylutka, A., Morawin, B., Gramacki, A. & Zembron-Lacny, A. Lifestyle exercise attenuates immunosenescence; flow cytometry analysis. BMC Geriatr. 21, 200 (2021).
Bianchi, A. et al. Moderate exercise inhibits age-related inflammation, liver steatosis, senescence, and tumorigenesis. J. Immunol. 206, 904–916 (2021).
Yzydorczyk, C. et al. Transient postnatal overfeeding causes liver stress-induced premature senescence in adult mice. Sci. Rep. 7, 12911 (2017).
Greeley, E. H., Spitznagel, E., Lawler, D. F., Kealy, R. D. & Segre, M. Modulation of canine immunosenescence by life-long caloric restriction. Vet. Immunol. Immunopathol. 111, 287–299 (2006).
List, E. O. et al. Diet-induced weight loss is sufficient to reduce senescent cell number in white adipose tissue of weight-cycled mice. Nutr. Healthy Aging 4, 95–99 (2016).
Mafra, D. et al. Food as medicine: targeting the uraemic phenotype in chronic kidney disease. Nat. Rev. Nephrol. 17, 153–171 (2021).
Shiels, P. G. et al. Manipulating the exposome to enable better ageing. Biochem. J. 478, 2889–2898 (2021).
Lee, Y. I. & Kim, E. Synergistic effect of 300 μm needle-depth fractional microneedling radiofrequency on the treatment of senescence-induced aging hyperpigmentation of the skin. Int. J. Mol. Sci. 22, 7480 (2021).
Hickson, L. J. et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456 (2019).
Torres, B. et al. Impact of switching to raltegravir and/or adding losartan in lymphoid tissue fibrosis and inflammation in people living with HIV. A randomized clinical trial. HIV Med. 22, 674–681 (2021).
Wong, G. C. L. et al. Horticultural therapy reduces biomarkers of immunosenescence and inflammaging in community-dwelling older adults: a feasibility pilot randomized controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 76, 307–317 (2021).
Sharma, A. K. et al. The senolytic drug navitoclax (ABT-263) causes trabecular bone loss and impaired osteoprogenitor function in aged mice. Front. Cell Dev. Biol. 8, 354 (2020).
Puglisi, M. et al. A phase I study of the safety, pharmacokinetics and efficacy of navitoclax plus docetaxel in patients with advanced solid tumors. Future Oncol. 17, 2747–2758 (2021).
Rodríguez, J. A. et al. Antagonistic pleiotropy and mutation accumulation influence human senescence and disease. Nat. Ecol. Evol. 1, 55 (2017).
Lei, J., Jiang, X., Li, W. & Ren, J. Exosomes from antler stem cells alleviate mesenchymal stem cell senescence and osteoarthritis. Protein Cell 13, 220–226 (2022).
Acknowledgements
The authors’ work was supported by National Institutes of Health grant numbers DK120292, DK122734, AG062104, AG013925 and AG61456, the Connor Fund, Robert P. and Arlene R. Kogod, Robert J. and Theresa W. Ryan, and the Noaber Foundation.
Author information
Authors and Affiliations
Contributions
W.H. researched the data for the article and wrote the text. W.H. and L.O.L contributed substantially to discussion of the content. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
L.O.L. is an adviser to AstraZeneca, CureSpec, Beren, Ribocure Pharmaceuticals and Butterfly Biosciences. Patents on senolytic drugs and their uses are held by the Mayo Clinic. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies. The other authors declare no conflicts of interest.
Peer review
Peer review information
Nature Reviews Nephrology thanks Giuseppe Castellano and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- DREAM complex
-
A multisubunit complex formed by the assembly of p130 and p107 with their dimerization partner, E2F4/5, and a multi-vulva class-B core complex.
- Somatic hypermutation
-
A programmed process of adaptation to new foreign elements (such as microbes) whereby changes are introduced to the nucleotide sequences of immunoglobulin genes during B cell development.
- Efferocytosis
-
The process by which apoptotic cells are removed by phagocytic cells.
- Ramified
-
Used to describe cells that have long branch-like processes.
- Geroprotective protein
-
A protein that has anti-ageing effects.
- Radio-electric asymmetric conveyer technology
-
A technology delivering very low-intensity radio-electric emission to generate microcurrents in tissues or culture media and thereby produce biological effects.
- Metabolic memory
-
The durable effect of prior hyperglycaemia on the initiation and progression of diabetic complications.
- Tenocytes
-
Elongated fibroblasts and fibrocytes that reside between collagen fibres.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, W., Hickson, L.J., Eirin, A. et al. Cellular senescence: the good, the bad and the unknown. Nat Rev Nephrol 18, 611–627 (2022). https://doi.org/10.1038/s41581-022-00601-z
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41581-022-00601-z
This article is cited by
-
Mitochondria–endoplasmic reticulum contact sites in hepatocytic senescence
Cellular & Molecular Biology Letters (2026)
-
Matricellular protein SMOC2 safeguards tubular integrity in acute kidney injury via integrin β3-dependent inhibition of CCND1-CDK4/6 axis
Molecular Biomedicine (2026)
-
Evidence gaps in the effects of exercise on SASP-Related biomarkers in older adults: a systematic review and meta-analysis of randomized controlled trials
BMC Geriatrics (2026)
-
Validation of a novel genomic biomarker of mesenchymal stem cell scalability and implications of genotype status on cellular senescence phenotypes
Scientific Reports (2026)
-
A machine learning-defined cellular senescence signature systematically enhances prognostication and guides immunotherapy strategies for the treatment of gliomas
npj Precision Oncology (2026)