Abstract
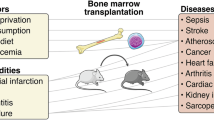
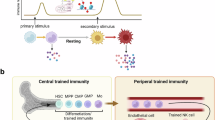
Trained immunity is a functional state of the innate immune response and is characterized by long-term epigenetic reprogramming of innate immune cells. This concept originated in the field of infectious diseases — training of innate immune cells, such as monocytes, macrophages and/or natural killer cells, by infection or vaccination enhances immune responses against microbial pathogens after restimulation. Although initially reported in circulating monocytes and tissue macrophages (termed peripheral trained immunity), subsequent findings indicate that immune progenitor cells in the bone marrow can also be trained (that is, central trained immunity), which explains the long-term innate immunity-mediated protective effects of vaccination against heterologous infections. Although trained immunity is beneficial against infections, its inappropriate induction by endogenous stimuli can also lead to aberrant inflammation. For example, in systemic lupus erythematosus and systemic sclerosis, trained immunity might contribute to inflammatory activity, which promotes disease progression. In organ transplantation, trained immunity has been associated with acute rejection and suppression of trained immunity prolonged allograft survival. This novel concept provides a better understanding of the involvement of the innate immune response in different pathological conditions, and provides a new framework for the development of therapies and treatment strategies that target epigenetic and metabolic pathways of the innate immune system.
Key points
-
Trained immunity is a functional state of the innate immune system that is characterized by long-term epigenetic and metabolic reprogramming of cells associated with potent immune responses.
-
Experimental and clinical studies have demonstrated that exogenous pathogen-associated molecular patterns and endogenous danger-associated molecular patterns induce trained immunity.
-
Trained immunity is a functional adaptation of the innate immune system against secondary infections, but can lead to aberrant inflammatory activity in conditions such as autoimmunity.
-
Sterile inflammation owing to ischaemia–reperfusion injury and organ transplantation induces trained immunity and precipitates allograft rejection.
-
Therapeutic inhibition of trained immunity (for example, in autoimmunity or transplantation) or its induction (for example, in infections or cancer) are promising strategies for treating immunity-related diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Janeway, C. A. Jr. & Medzhitov, R. Introduction: the role of innate immunity in the adaptive immune response. Semin. Immunol. 10, 349–350 (1998).
Murphy, K. & Weaver, C. Janeway’s Immunobiology (Garland Science, 2016).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Bianchi, M. E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5 (2007).
Iwasaki, A. & Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 16, 343–353 (2015).
Guermonprez, P., Valladeau, J., Zitvogel, L., Thery, C. & Amigorena, S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 20, 621–667 (2002).
Netea, M. G., Schlitzer, A., Placek, K., Joosten, L. A. B. & Schultze, J. L. Innate and adaptive immune memory: an evolutionary continuum in the host’s response to pathogens. Cell Host Microbe 25, 13–26 (2019).
Titley, M. A., Snaddon, J. L. & Turner, E. C. Scientific research on animal biodiversity is systematically biased towards vertebrates and temperate regions. PLoS One 12, e0189577 (2017).
Kurtz, J. & Franz, K. Innate defence: evidence for memory in invertebrate immunity. Nature 425, 37–38 (2003).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020).
Netea, M. G., Quintin, J. & van der Meer, J. W. Trained immunity: a memory for innate host defense. Cell Host Microbe 9, 355–361 (2011).
Divangahi, M. et al. Trained immunity, tolerance, priming and differentiation: distinct immunological processes. Nat. Immunol. 22, 2–6 (2021).
Kaufmann, E. et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172, 176–190 e119 (2018).
Bekkering, S., Dominguez-Andres, J., Joosten, L. A. B., Riksen, N. P. & Netea, M. G. Trained immunity: reprogramming innate immunity in health and disease. Annu. Rev. Immunol. 39, 667–693 (2021).
Kleinnijenhuis, J. et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl Acad. Sci. USA 109, 17537–17542 (2012).
Ochando, J., Fayad, Z. A., Madsen, J. C., Netea, M. G. & Mulder, W. J. M. Trained immunity in organ transplantation. Am. J. Transpl. 20, 10–18 (2020).
Zhao, S. et al. H3K4 methylation regulates LPS-induced proinflammatory cytokine expression and release in macrophages. Shock 51, 401–406 (2019).
Jamieson, A. M. et al. Role of tissue protection in lethal respiratory viral-bacterial coinfection. Science 340, 1230–1234 (2013).
Aegerter, H. et al. Influenza-induced monocyte-derived alveolar macrophages confer prolonged antibacterial protection. Nat. Immunol. 21, 145–157 (2020).
Cheng, S. C. et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat. Immunol. 17, 406–413 (2016).
Roquilly, A. et al. Alveolar macrophages are epigenetically altered after inflammation, leading to long-term lung immunoparalysis. Nat. Immunol. 21, 636–648 (2020).
Novakovic, B. et al. β-Glucan reverses the epigenetic state of LPS-induced immunological tolerance. Cell 167, 1354–1368 e1314 (2016).
Saeed, S. et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345, 1251086 (2014).
Quintin, J. et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12, 223–232 (2012).
Fanucchi, S., Dominguez-Andres, J., Joosten, L. A. B., Netea, M. G. & Mhlanga, M. M. The intersection of epigenetics and metabolism in trained immunity. Immunity 54, 32–43 (2021).
Cheng, S. C. et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345, 1250684 (2014).
Laplante, M. & Sabatini, D. M. mTOR signaling in growth control and disease. Cell 149, 274–293 (2012).
Arts, R. J. W. et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. 17, 2562–2571 (2016).
Abubakar, I. et al. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guerin vaccination against tuberculosis. Health Technol. Assess. 17, 1–372 (2013).
Nguipdop-Djomo, P., Heldal, E., Rodrigues, L. C., Abubakar, I. & Mangtani, P. Duration of BCG protection against tuberculosis and change in effectiveness with time since vaccination in Norway: a retrospective population-based cohort study. Lancet Infect. Dis. 16, 219–226 (2016).
Giamarellos-Bourboulis, E. J. et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell 183, 315–323.e9 (2020).
Tsilika, M. et al. ACTIVATE-2: a double-blind randomized trial of BCG vaccination against COVID-19 in individuals at risk. Front. Immunol. 13, 873067 (2022).
Kleinnijenhuis, J. et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 6, 152–158 (2014).
Cirovic, B. et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe 28, 322–334.e5 (2020).
Mitroulis, I. et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172, 147–161.e12 (2018).
Brown, G. D. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 6, 33–43 (2006).
Kalafati, L. et al. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell 183, 771–785.e12 (2020).
Moorlag, S. et al. BCG vaccination induces long-term functional reprogramming of human neutrophils. Cell Rep. 33, 108387 (2020).
Kleinnijenhuis, J. et al. BCG-induced trained immunity in NK cells: role for non-specific protection to infection. Clin. Immunol. 155, 213–219 (2014).
Placek, K., Schultze, J. L. & Netea, M. G. Immune memory characteristics of innate lymphoid cells. Curr. Opin. Infect. Dis. 32, 196–203 (2019).
Hamada, A., Torre, C., Drancourt, M. & Ghigo, E. Trained immunity carried by non-immune cells. Front. Microbiol. 9, 3225 (2018).
Shao, Y. et al. Vascular endothelial cells and innate immunity. Arterioscler. Thromb. Vasc. Biol. 40, e138–e152 (2020).
Bigot, J. et al. Respiratory epithelial cells can remember infection: a proof-of-concept study. J. Infect. Dis. 221, 1000–1005 (2020).
Babel, N., Hugo, C. & Westhoff, T. H. Vaccination in patients with kidney failure: lessons from COVID-19. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-022-00617-5 (2022).
Yu, F., Haas, M., Glassock, R. & Zhao, M. H. Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat. Rev. Nephrol. 13, 483–495 (2017).
Tsokos, G. C., Lo, M. S., Costa Reis, P. & Sullivan, K. E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 12, 716–730 (2016).
Ma, W. T., Gao, F., Gu, K. & Chen, D. K. The role of monocytes and macrophages in autoimmune diseases: a comprehensive review. Front. Immunol. 10, 1140 (2019).
Baumann, I. et al. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 46, 191–201 (2002).
Frank, M. M., Hamburger, M. I., Lawley, T. J., Kimberly, R. P. & Plotz, P. H. Defective reticuloendothelial system Fc-receptor function in systemic lupus erythematosus. N. Engl. J. Med. 300, 518–523 (1979).
Labonte, A. C. et al. Identification of alterations in macrophage activation associated with disease activity in systemic lupus erythematosus. PLoS One 13, e0208132 (2018).
Aringer, M. et al. Increased bioactive TNF in human systemic lupus erythematosus: associations with cell death. Lupus 11, 102–108 (2002).
Umare, V. et al. Effect of proinflammatory cytokines (IL-6, TNF-α, and IL-1β) on clinical manifestations in Indian SLE patients. Mediators Inflamm. 2014, 385297 (2014).
Hill, G. S. et al. Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages. Kidney Int. 59, 304–316 (2001).
Jing, C. et al. Macrophage metabolic reprogramming presents a therapeutic target in lupus nephritis. Proc. Natl Acad. Sci. USA 117, 15160–15171 (2020).
Sullivan, K. E. et al. The TNFα locus is altered in monocytes from patients with systemic lupus erythematosus. Clin. Immunol. 123, 74–81 (2007).
Leung, Y. T. et al. Interferon regulatory factor 1 and histone H4 acetylation in systemic lupus erythematosus. Epigenetics 10, 191–199 (2015).
Zhang, Z., Maurer, K., Perin, J. C., Song, L. & Sullivan, K. E. Cytokine-induced monocyte characteristics in SLE. J. Biomed. Biotechnol. 2010, 507475 (2010).
Zhang, Z., Song, L., Maurer, K., Petri, M. A. & Sullivan, K. E. Global H4 acetylation analysis by ChIP-chip in systemic lupus erythematosus monocytes. Genes. Immun. 11, 124–133 (2010).
Zhang, Z. et al. Interferon regulatory factor 1 marks activated genes and can induce target gene expression in systemic lupus erythematosus. Arthritis Rheumatol. 67, 785–796 (2015).
Kamada, R. et al. Interferon stimulation creates chromatin marks and establishes transcriptional memory. Proc. Natl Acad. Sci. USA 115, E9162–E9171 (2018).
Grigoriou, M. et al. Transcriptome reprogramming and myeloid skewing in haematopoietic stem and progenitor cells in systemic lupus erythematosus. Ann. Rheum. Dis. 79, 242–253 (2020).
Kokkinopoulos, I. et al. Patrolling human SLE haematopoietic progenitors demonstrate enhanced extramedullary colonisation; implications for peripheral tissue injury. Sci. Rep. 11, 15759 (2021).
Nakou, M. et al. Gene expression in systemic lupus erythematosus: bone marrow analysis differentiates active from inactive disease and reveals apoptosis and granulopoiesis signatures. Arthritis Rheum. 58, 3541–3549 (2008).
Mishra, N., Reilly, C. M., Brown, D. R., Ruiz, P. & Gilkeson, G. S. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J. Clin. Invest. 111, 539–552 (2003).
Rother, N. et al. Hydroxychloroquine inhibits the trained innate immune response to interferons. Cell Rep. Med. 1, 100146 (2020).
Jeljeli, M. et al. Trained immunity modulates inflammation-induced fibrosis. Nat. Commun. 10, 5670 (2019).
Betjes, M. G. Immune cell dysfunction and inflammation in end-stage renal disease. Nat. Rev. Nephrol. 9, 255–265 (2013).
Kara, I. H., Yilmaz, M. E., Suner, A., Kadiroglu, A. K. & Isikoglu, B. The evaluation of immune responses that occur after HBV infection and HBV vaccination in hemodialysis patients. Vaccine 22, 3963–3967 (2004).
Garcia, P. et al. COVID-19 vaccine type and humoral immune response in patients receiving dialysis. J. Am. Soc. Nephrol. 33, 33–37 (2022).
Council, E.-E. & Group, E. W. Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol. Dial. Transpl. 36, 87–94 (2021).
Hilbrands, L. B. et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol. Dial. Transpl. 35, 1973–1983 (2020).
Akar, H., Akar, G. C., Carrero, J. J., Stenvinkel, P. & Lindholm, B. Systemic consequences of poor oral health in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 6, 218–226 (2011).
Sarnak, M. J. & Jaber, B. L. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 58, 1758–1764 (2000).
Stewart, J. H. et al. The pattern of excess cancer in dialysis and transplantation. Nephrol. Dial. Transpl. 24, 3225–3231 (2009).
Yeun, J. Y., Levine, R. A., Mantadilok, V. & Kaysen, G. A. C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am. J. Kidney Dis. 35, 469–476 (2000).
Himmelfarb, J., Stenvinkel, P., Ikizler, T. A. & Hakim, R. M. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 62, 1524–1538 (2002).
Samouilidou, E. C. et al. Lipid abnormalities and oxidized LDL in chronic kidney disease patients on hemodialysis and peritoneal dialysis. Ren. Fail. 34, 160–164 (2012).
Diepeveen, S. H. et al. Oxidative stress in patients with end-stage renal disease prior to the start of renal replacement therapy. Nephron Clin. Pract. 98, c3–c7 (2004).
Bentzon, J. F., Otsuka, F., Virmani, R. & Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 114, 1852–1866 (2014).
Swirski, F. K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117, 195–205 (2007).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Rogacev, K. S. et al. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur. Heart J. 32, 84–92 (2011).
Pertosa, G., Grandaliano, G., Gesualdo, L. & Schena, F. P. Clinical relevance of cytokine production in hemodialysis. Kidney Int. Suppl. 76, S104–S111 (2000).
de Cal, M. et al. Oxidative stress and ‘monocyte reprogramming’ after kidney transplant: a longitudinal study. Blood Purif. 26, 105–110 (2008).
Sela, S. et al. Primed peripheral polymorphonuclear leukocyte: a culprit underlying chronic low-grade inflammation and systemic oxidative stress in chronic kidney disease. J. Am. Soc. Nephrol. 16, 2431–2438 (2005).
Zawada, A. M. et al. SuperTAG methylation-specific digital karyotyping reveals uremia-induced epigenetic dysregulation of atherosclerosis-related genes. Circ. Cardiovasc. Genet. 5, 611–620 (2012).
Li, X. et al. Maladaptive innate immune training of myelopoiesis links inflammatory comorbidities. Cell 185, 1709–1727 e1718 (2022).
Bekkering, S. et al. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 34, 1731–1738 (2014).
Crisan, T. O. et al. Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann. Rheum. Dis. 75, 755–762 (2016).
Cabau, G., Crisan, T. O., Kluck, V., Popp, R. A. & Joosten, L. A. B. Urate-induced immune programming: consequences for gouty arthritis and hyperuricemia. Immunol. Rev. 294, 92–105 (2020).
Joosten, L. A. B., Crisan, T. O., Bjornstad, P. & Johnson, R. J. Asymptomatic hyperuricaemia: a silent activator of the innate immune system. Nat. Rev. Rheumatol. 16, 75–86 (2020).
Zawada, A. M. et al. Serum uric acid and mortality risk among hemodialysis patients. Kidney Int. Rep. 5, 1196–1206 (2020).
Eltzschig, H. K. & Eckle, T. Ischemia and reperfusion–from mechanism to translation. Nat. Med. 17, 1391–1401 (2011).
Fernandez, A. R., Sanchez-Tarjuelo, R., Cravedi, P., Ochando, J. & Lopez-Hoyos, M. Review: ischemia reperfusion injury-a translational perspective in organ transplantation. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21228549 (2020).
Iyer, S. S. et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl Acad. Sci. USA 106, 20388–20393 (2009).
McDonald, B. et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330, 362–366 (2010).
Amores-Iniesta, J. et al. Extracellular ATP activates the NLRP3 inflammasome and is an early danger signal of skin allograft rejection. Cell Rep. 21, 3414–3426 (2017).
Barbera-Cremades, M. et al. P2X7 receptor induces tumor necrosis factor-alpha converting enzyme activation and release to boost TNF-α production. Front. Immunol. 8, 862 (2017).
Vergani, A. et al. Long-term heart transplant survival by targeting the ionotropic purinergic receptor P2X7. Circulation 127, 463–475 (2013).
Isenberg, J. S., Frazier, W. A. & Roberts, D. D. Thrombospondin-1: a physiological regulator of nitric oxide signaling. Cell Mol. Life Sci. 65, 728–742 (2008).
Thakar, C. V. et al. Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia. J. Clin. Invest. 115, 3451–3459 (2005).
Li, Y., Qi, X., Tong, X. & Wang, S. Thrombospondin 1 activates the macrophage Toll-like receptor 4 pathway. Cell Mol. Immunol. 10, 506–512 (2013).
Stein, E. V., Miller, T. W., Ivins-O’Keefe, K., Kaur, S. & Roberts, D. D. Secreted thrombospondin-1 regulates macrophage interleukin-1β production and activation through CD47. Sci. Rep. 6, 19684 (2016).
Yamauchi, Y. et al. Thrombospondin-1 differentially regulates release of IL-6 and IL-10 by human monocytic cell line U937. Biochem. Biophys. Res. Commun. 290, 1551–1557 (2002).
Batal, I. et al. The mechanisms of up-regulation of dendritic cell activity by oxidative stress. J. Leukoc. Biol. 96, 283–293 (2014).
Jong, W. M. et al. Reduced acute myocardial ischemia-reperfusion injury in IL-6-deficient mice employing a closed-chest model. Inflamm. Res. 65, 489–499 (2016).
Uehara, M. et al. Ischemia augments alloimmune injury through IL-6-driven CD4+ alloreactivity. Sci. Rep. 8, 2461 (2018).
Solhjou, Z. et al. Novel application of localized nanodelivery of anti-interleukin-6 protects organ transplant from ischemia-reperfusion injuries. Am. J. Transpl. 17, 2326–2337 (2017).
Zhao, H., Alam, A., Soo, A. P., George, A. J. T. & Ma, D. Ischemia-reperfusion injury reduces long term renal graft survival: mechanism and beyond. EBioMedicine 28, 31–42 (2018).
Fagenson, A. M. et al. Liver ischemia reperfusion injury, enhanced by trained immunity, is attenuated in caspase 1/caspase 11 double gene knockout mice. Pathogens https://doi.org/10.3390/pathogens9110879 (2020).
Tammaro, A., Kers, J., Scantlebery, A. M. L. & Florquin, S. Metabolic flexibility and innate immunity in renal ischemia reperfusion injury: the fine balance between adaptive repair and tissue degeneration. Front. Immunol. 11, 1346 (2020).
Nankivell, B. J. & Alexander, S. I. Rejection of the kidney allograft. N. Engl. J. Med. 363, 1451–1462 (2010).
Ochando, J., Ordikhani, F., Boros, P. & Jordan, S. The innate immune response to allotransplants: mechanisms and therapeutic potentials. Cell Mol. Immunol. 16, 350–356 (2019).
Poggio, E. D., Augustine, J. J., Arrigain, S., Brennan, D. C. & Schold, J. D. Long-term kidney transplant graft survival-making progress when most needed. Am. J. Transpl. 21, 2824–2832 (2021).
Garcia, M. R. et al. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J. Clin. Invest. 120, 2486–2496 (2010).
Zecher, D., van Rooijen, N., Rothstein, D. M., Shlomchik, W. D. & Lakkis, F. G. An innate response to allogeneic nonself mediated by monocytes. J. Immunol. 183, 7810–7816 (2009).
Dai, H. et al. Donor SIRPα polymorphism modulates the innate immune response to allogeneic grafts. Sci. Immunol. https://doi.org/10.1126/sciimmunol.aam6202 (2017).
Dai, H. et al. PIRs mediate innate myeloid cell memory to nonself MHC molecules. Science 368, 1122–1127 (2020).
Braza, M. S. et al. Inhibiting inflammation with myeloid cell-specific nanobiologics promotes organ transplant acceptance. Immunity 49, 819–828 e816 (2018).
Thiagarajan, P. S. et al. Vimentin is an endogenous ligand for the pattern recognition receptor Dectin-1. Cardiovasc. Res. 99, 494–504 (2013).
Yang, H. et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc. Natl Acad. Sci. USA 107, 11942–11947 (2010).
Azimzadeh, A. M. et al. Humoral immunity to vimentin is associated with cardiac allograft injury in nonhuman primates. Am. J. Transpl. 5, 2349–2359 (2005).
Huang, Y. et al. Extracellular hmgb1 functions as an innate immune-mediator implicated in murine cardiac allograft acute rejection. Am. J. Transpl. 7, 799–808 (2007).
Fishman, J. A. Infection in organ transplantation. Am. J. Transpl. 17, 856–879 (2017).
Salehi, S. & Reed, E. F. The divergent roles of macrophages in solid organ transplantation. Curr. Opin. Organ. Transpl. 20, 446–453 (2015).
Jiang, X., Tian, W., Sung, Y. K., Qian, J. & Nicolls, M. R. Macrophages in solid organ transplantation. Vasc. Cell 6, 5 (2014).
Ahmed, E. B., Wang, T., Daniels, M., Alegre, M. L. & Chong, A. S. IL-6 induced by Staphylococcus aureus infection prevents the induction of skin allograft acceptance in mice. Am. J. Transpl. 11, 936–946 (2011).
Kapetanovic, R. et al. Contribution of phagocytosis and intracellular sensing for cytokine production by Staphylococcus aureus-activated macrophages. Infect. Immun. 75, 830–837 (2007).
Wang, T. et al. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am. J. Transpl. 10, 1524–1533 (2010).
Wang, T. et al. Prevention of allograft tolerance by bacterial infection with Listeria monocytogenes. J. Immunol. 180, 5991–5999 (2008).
Shen, H. & Goldstein, D. R. IL-6 and TNF-α synergistically inhibit allograft acceptance. J. Am. Soc. Nephrol. 20, 1032–1040 (2009).
Kaminski, H. & Fishman, J. A. The cell biology of cytomegalovirus: implications for transplantation. Am. J. Transpl. 16, 2254–2269 (2016).
Kapoor, A., Forman, M. & Arav-Boger, R. Activation of nucleotide oligomerization domain 2 (NOD2) by human cytomegalovirus initiates innate immune responses and restricts virus replication. PLoS One 9, e92704 (2014).
Mitchell, B. M., Leung, A. & Stevens, J. G. Murine cytomegalovirus DNA in peripheral blood of latently infected mice is detectable only in monocytes and polymorphonuclear leukocytes. Virology 223, 198–207 (1996).
Pulliam, L., Moore, D. & West, D. C. Human cytomegalovirus induces IL-6 and TNF α from macrophages and microglial cells: possible role in neurotoxicity. J. Neurovirol. 1, 219–227 (1995).
Hargett, D. & Shenk, T. E. Experimental human cytomegalovirus latency in CD14+ monocytes. Proc. Natl Acad. Sci. USA 107, 20039–20044 (2010).
Smith, P. D. et al. Cytomegalovirus enhances macrophage TLR expression and MyD88-mediated signal transduction to potentiate inducible inflammatory responses. J. Immunol. 193, 5604–5612 (2014).
Sen, P. et al. Linking indirect effects of cytomegalovirus in transplantation to modulation of monocyte innate immune function. Sci. Adv. 6, eaax9856 (2020).
Cook, C. H. et al. Disruption of murine cardiac allograft acceptance by latent cytomegalovirus. Am. J. Transpl. 9, 42–53 (2009).
Zuhair, M. et al. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev. Med. Virol. 29, e2034 (2019).
Brown, G. D. et al. Dectin-1 mediates the biological effects of β-glucans. J. Exp. Med. 197, 1119–1124 (2003).
Tang, C. et al. Inhibition of Dectin-1 signaling ameliorates colitis by inducing lactobacillus-mediated regulatory T cell expansion in the intestine. Cell Host Microbe 18, 183–197 (2015).
Xie, J. et al. Laminarin-mediated targeting to Dectin-1 enhances antigen-specific immune responses. Biochem. Biophys. Res. Commun. 391, 958–962 (2010).
Wang, J. E. et al. Peptidoglycan primes for LPS-induced release of proinflammatory cytokines in whole human blood. Shock 16, 178–182 (2001).
Wray, G. M., Foster, S. J., Hinds, C. J. & Thiemermann, C. A cell wall component from pathogenic and non-pathogenic gram-positive bacteria (peptidoglycan) synergises with endotoxin to cause the release of tumour necrosis factor-α, nitric oxide production, shock, and multiple organ injury/dysfunction in the rat. Shock 15, 135–142 (2001).
Juarez-Verdayes, M. A., Rodriguez-Martinez, S., Cancino-Diaz, M. E. & Cancino-Diaz, J. C. Peptidoglycan and muramyl dipeptide from Staphylococcus aureus induce the expression of VEGF-A in human limbal fibroblasts with the participation of TLR2-NFB and NOD2-EGFR. Graefes Arch. Clin. Exp. Ophthalmol. 251, 53–62 (2013).
Ogawa, C., Liu, Y. J. & Kobayashi, K. S. Muramyl dipeptide and its derivatives: peptide adjuvant in immunological disorders and cancer therapy. Curr. Bioact. Compd. 7, 180–197 (2011).
Rickard, D. J. et al. Identification of benzimidazole diamides as selective inhibitors of the nucleotide-binding oligomerization domain 2 (NOD2) signaling pathway. PLoS One 8, e69619 (2013).
Lenis, A. T., Lec, P. M., Chamie, K. & Mshs, M. D. Bladder cancer: a review. JAMA 324, 1980–1991 (2020).
Lobo, N. et al. 100 years of Bacillus Calmette-Guerin immunotherapy: from cattle to COVID-19. Nat. Rev. Urol. 18, 611–622 (2021).
Bisiaux, A. et al. Molecular analyte profiling of the early events and tissue conditioning following intravesical bacillus Calmette-Guerin therapy in patients with superficial bladder cancer. J. Urol. 181, 1571–1580 (2009).
Priem, B. et al. Trained immunity-promoting nanobiologic therapy suppresses tumor growth and potentiates checkpoint inhibition. Cell 183, 786–801 e719 (2020).
Morales, A., Eidinger, D. & Bruce, A. W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 116, 180–183 (1976).
Patard, J. J. et al. Intravesical Bacillus Calmette-Guerin treatment improves patient survival in T1G3 bladder tumours. Eur. Urol. 41, 635–641 (2002).
Moorlag, S., Arts, R. J. W., van Crevel, R. & Netea, M. G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 25, 1473–1478 (2019).
Teufel, L. U., Arts, R. J. W., Netea, M. G., Dinarello, C. A. & Joosten, L. A. B. IL-1 family cytokines as drivers and inhibitors of trained immunity. Cytokine 150, 155773 (2022).
Dinarello, C. A., Simon, A. & van der Meer, J. W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11, 633–652 (2012).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Dinarello, C. A., Koch, K. M. & Shaldon, S. Interleukin-1 and its relevance in patients treated with hemodialysis. Kidney Int. Suppl. 24, S21–S26 (1988).
Hung, A. M., Ellis, C. D., Shintani, A., Booker, C. & Ikizler, T. A. IL-1β receptor antagonist reduces inflammation in hemodialysis patients. J. Am. Soc. Nephrol. 22, 437–442 (2011).
Nowak, K. L. et al. IL-1 inhibition and vascular function in CKD. J. Am. Soc. Nephrol. 28, 971–980 (2017).
Mulder, W. J. M., Ochando, J., Joosten, L. A. B., Fayad, Z. A. & Netea, M. G. Therapeutic targeting of trained immunity. Nat. Rev. Drug. Discov. 18, 553–566 (2019).
Lawrence, R. E. & Zoncu, R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 21, 133–142 (2019).
Bekkering, S. et al. Metabolic induction of trained immunity through the mevalonate pathway. Cell 172, 135–146 e139 (2018).
Sohrabi, Y. et al. LXR activation induces a proinflammatory trained innate immunity-phenotype in human monocytes. Front. Immunol. 11, 353 (2020).
van der Heijden, C. et al. Aldosterone induces trained immunity: the role of fatty acid synthesis. Cardiovasc. Res. 116, 317–328 (2020).
Kiefer, J. et al. An unbiased flow cytometry-based approach to assess subset-specific circulating monocyte activation and cytokine profile in whole blood. Front. Immunol. 12, 641224 (2021).
Arts, R. J. W. et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 23, 89–100 e105 (2018).
Dos Santos, J. C. et al. β-Glucan-induced trained immunity protects against Leishmania braziliensis infection: a crucial role for IL-32. Cell Rep. 28, 2659–2672 e2656 (2019).
Arts, R. J. et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 24, 807–819 (2016).
Bulua, A. C. et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 208, 519–533 (2011).
Chacko, B. K. et al. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab. Invest. 93, 690–700 (2013).
Nicholls, D. G. et al. Bioenergetic profile experiment using C2C12 myoblast cells. J. Vis. Exp. https://doi.org/10.3791/2511 (2010).
Mourits, V. P. et al. Lysine methyltransferase G9a is an important modulator of trained immunity. Clin. Transl. Immunol. 10, e1253 (2021).
van der Heijden, C. et al. Catecholamines induce trained immunity in monocytes in vitro and in vivo. Circ. Res. 127, 269–283 (2020).
Rodriguez-Ubreva, J. & Ballestar, E. Chromatin immunoprecipitation. Methods Mol. Biol. 1094, 309–318 (2014).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Kaya-Okur, H. S. et al. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun. 10, 1930 (2019).
Skene, P. J., Henikoff, J. G. & Henikoff, S. Targeted in situ genome-wide profiling with high efficiency for low cell numbers. Nat. Protoc. 13, 1006–1019 (2018).
de Almeida, M. C., Silva, A. C., Barral, A. & Barral Netto, M. A simple method for human peripheral blood monocyte isolation. Mem. Inst. Oswaldo Cruz 95, 221–223 (2000).
Meital, L. T. et al. A simple and effective method for the isolation and culture of human monocytes from small volumes of peripheral blood. J. Immunol. Methods 472, 75–78 (2019).
Ziegler-Heitbrock, L. et al. Nomenclature of monocytes and dendritic cells in blood. Blood 116, e74–e80 (2010).
Llitjos, J. F. et al. Assessing the functional heterogeneity of monocytes in human septic shock: a proof-of-concept microfluidic assay of TNFα secretion. Front. Immunol. 12, 686111 (2021).
Marimuthu, R. et al. Characterization of human monocyte subsets by whole blood flow cytometry analysis. J. Vis. Exp. https://doi.org/10.3791/57941 (2018).
Ong, S. M. et al. A novel, five-marker alternative to CD16-CD14 gating to identify the three human monocyte subsets. Front. Immunol. 10, 1761 (2019).
Garaude, J. et al. Mitochondrial respiratory-chain adaptations in macrophages contribute to antibacterial host defense. Nat. Immunol. 17, 1037–1045 (2016).
Monteiro, L. B., Davanzo, G. G., de Aguiar, C. F. & Moraes-Vieira, P. M. M. Using flow cytometry for mitochondrial assays. MethodsX 7, 100938 (2020).
Geng, J. et al. Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nat. Immunol. 16, 1142–1152 (2015).
Kauffman, M. E. et al. MitoSOX-based flow cytometry for detecting mitochondrial ROS. React. Oxyg. Species 2, 361–370 (2016).
Acknowledgements
The authors’ work is supported by National Institutes of Health grants R01 AI139623AI (J.O.); Vici grant from the Dutch Research Council (NWO) and European Research Council Advanced Grant (TOLERANCE) (W.J.M.M.); European Research Council Consolidator Grant (310372) and Spinoza Grant of the Netherlands Organization for Scientific Research (M.G.N.); P01 HL158504 (J.C.M.); and Hypatia grant by the Radboud University Medical Center and Senior Kolff grant by the Dutch Kidney Foundation (R.D.).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussions of the content and wrote, reviewed or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
J.O., W.J.M.M., and M.G.N. declare that they are scientific founders of Trained Therapeutics Discovery. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks C. Kurts, X. Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ochando, J., Mulder, W.J.M., Madsen, J.C. et al. Trained immunity — basic concepts and contributions to immunopathology. Nat Rev Nephrol 19, 23–37 (2023). https://doi.org/10.1038/s41581-022-00633-5
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41581-022-00633-5
This article is cited by
-
Evaluation of trained immunity as a potential strategy for a universal Streptococcus suis vaccine using immunogenic proteins in a murine model
BMC Veterinary Research (2026)
-
Recent perspectives on macrophage memory: types, mechanisms, and characteristics in pulmonary diseases
Cell Communication and Signaling (2026)
-
Paclitaxel induces trained immunity via the GPR183–STING axis to enhance host defense against MRSA infection
Veterinary Research (2026)
-
Injectable hydrogels for osteomyelitis treatment induce metabolic reprogramming for protection against reinfection
Nature Communications (2026)
-
Sepsis induces long-term reprogramming of human HSPCs and drives myeloid dysregulation in sepsis survivors
Journal of Inflammation (2026)