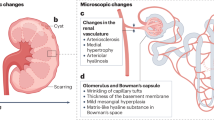
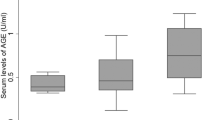
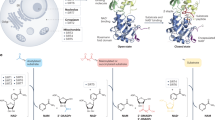
Abstract
The kidney is a metabolically active organ that requires energy to drive processes such as tubular reabsorption and secretion, and shows a decline in function with advancing age. Various molecular mechanisms, including genomic instability, telomere attrition, inflammation, autophagy, mitochondrial function, and changes to the sirtuin and Klotho signalling pathways, are recognized regulators of individual lifespan and pivotal factors that govern kidney ageing. Thus, mechanisms that contribute to ageing not only dictate renal outcomes but also exert a substantial influence over life expectancy. Conversely, kidney dysfunction, in the context of chronic kidney disease (CKD), precipitates an expedited ageing trajectory in individuals, leading to premature ageing and a disconnect between biological and chronological age. As CKD advances, age-related manifestations such as frailty become increasingly conspicuous. Hence, the pursuit of healthy ageing necessitates not only the management of age-related complications but also a comprehensive understanding of the processes and markers that underlie systemic ageing. Here, we examine the hallmarks of ageing, focusing on the mechanisms by which they affect kidney health and contribute to premature organ ageing. We also review diagnostic methodologies and interventions for premature ageing, with special consideration given to the potential of emerging therapeutic avenues to target age-related kidney diseases.
Key points
-
The kidney is a metabolically active organ that requires energy for processes such as tubular reabsorption and secretion; however, kidney function declines with age.
-
Various molecular mechanisms, including cellular senescence, inflammation, mitochondrial function, changes to the sirtuin and Klotho signalling pathways, and the autophagy–lysosome system, are recognized as regulators of individual lifespan and are important factors that govern kidney ageing.
-
Chronic kidney disease (CKD) and premature ageing share several common features and pathophysiological mechanisms; CKD is therefore considered a disease associated with accelerated or premature ageing.
-
The accelerated ageing phenotype of the kidney in the context of CKD results in a disconnect between the biological age of the kidney and the chronological age of the individual, known as the ‘age gap’.
-
Emerging technologies and biomarkers hold promise for improving the early detection, diagnosis and management of age-related kidney diseases and premature ageing.
-
Targeting the pathways associated with inflammation, mitochondrial function, oxidative stress, senescence and the autophagy–lysosome system holds promise for developing therapeutic interventions to prevent, delay or attenuate age-related kidney diseases and promote healthy ageing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ishani, A. et al. Acute kidney injury increases risk of ESRD among elderly. J. Am. Soc. Nephrol. 20, 223–228 (2009).
Ferenbach, D. A. & Bonventre, J. V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat. Rev. Nephrol. 11, 264–276 (2015).
Wang, X., Bonventre, J. V. & Parrish, A. R. The aging kidney: increased susceptibility to nephrotoxicity. Int. J. Mol. Sci. 15, 15358–15376 (2014).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Mizushima, N. & Levine, B. Autophagy in human diseases. N. Engl. J. Med. 383, 1564–1576 (2020).
Takabatake, Y., Kimura, T., Takahashi, A. & Isaka, Y. Autophagy and the kidney: health and disease. Nephrol. Dial. Transpl. 29, 1639–1647 (2014).
Tang, C., Livingston, M. J., Liu, Z. & Dong, Z. Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 16, 489–508 (2020).
Kaushik, S. et al. Autophagy and the hallmarks of aging. Ageing Res. Rev. 72, 101468 (2021).
Aman, Y. et al. Autophagy in healthy aging and disease. Nat. Aging 1, 634–650 (2021).
Kooman, J. P., Kotanko, P., Schols, A. M., Shiels, P. G. & Stenvinkel, P. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 10, 732–742 (2014).
Tian, Y. E. et al. Heterogeneous aging across multiple organ systems and prediction of chronic disease and mortality. Nat. Med. 29, 1221–1231 (2023).
Hommos, M. S., Glassock, R. J. & Rule, A. D. Structural and functional changes in human kidneys with healthy aging. J. Am. Soc. Nephrol. 28, 2838–2844 (2017).
Tan, J. C. et al. Effects of aging on glomerular function and number in living kidney donors. Kidney Int. 78, 686–692 (2010).
Glassock, R. J. & Rule, A. D. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int. 82, 270–277 (2012).
Roeder, S. S. et al. Changes in glomerular parietal epithelial cells in mouse kidneys with advanced age. Am. J. Physiol. Ren. Physiol. 309, F164–F178 (2015).
Wiggins, J. E. Aging in the glomerulus. J. Gerontol. A Biol. Sci. Med. Sci. 67, 1358–1364 (2012).
Goligorsky, M. S. Emerging insights into glomerular vascular pole and microcirculation. J. Am. Soc. Nephrol. 33, 1641–1648 (2022).
Nangaku, M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J. Am. Soc. Nephrol. 17, 17–25 (2006).
Tanaka, T. et al. Hypoxia and expression of hypoxia-inducible factor in the aging kidney. J. Gerontol. A Biol. Sci. Med. Sci. 61, 795–805 (2006).
Ryu, D. R. et al. Sirt1-hypoxia-inducible factor-1α interaction is a key mediator of tubulointerstitial damage in the aged kidney. Aging Cell 18, e12904 (2019).
Perico, L., Remuzzi, G. & Benigni, A. Sirtuins in kidney health and disease. Nat. Rev. Nephrol. 20, 313–329 (2024).
Semenza, G. L. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim. Biophys. Acta 1813, 1263–1268 (2011).
Kume, S. et al. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J. Clin. Invest. 120, 1043–1055 (2010).
Mohandes, S. et al. Molecular pathways that drive diabetic kidney disease. J. Clin. Invest. 133, e165654 (2023).
Chuang, P. Y. et al. Reduction in podocyte SIRT1 accelerates kidney injury in aging mice. Am. J. Physiol. Ren. Physiol. 313, F621–F628 (2017).
Randles, M. J. et al. Identification of an altered matrix signature in kidney aging and disease. J. Am. Soc. Nephrol. 32, 1713–1732 (2021).
Isaka, Y. Targeting TGF-β signaling in kidney fibrosis. Int. J. Mol. Sci. 19, 2532 (2018).
Schumacher, B., Pothof, J., Vijg, J. & Hoeijmakers, J. H. J. The central role of DNA damage in the ageing process. Nature 592, 695–703 (2021).
Garaycoechea, J. I., Quinlan, C. & Luijsterburg, M. S. Pathological consequences of DNA damage in the kidney. Nat. Rev. Nephrol. 19, 229–243 (2023).
Wilson, P. C. et al. Mosaic loss of Y chromosome is associated with aging and epithelial injury in chronic kidney disease. Genome Biol. 25, 36 (2024).
Melk, A. et al. Telomere shortening in kidneys with age. J. Am. Soc. Nephrol. 11, 444–453 (2000).
Park, S. et al. A Mendelian randomization study found causal linkage between telomere attrition and chronic kidney disease. Kidney Int. 100, 1063–1070 (2021).
Carrero, J. J. et al. Telomere attrition is associated with inflammation, low fetuin-A levels and high mortality in prevalent haemodialysis patients. J. Intern. Med. 263, 302–312 (2008).
Shiels, P. G., McGuinness, D., Eriksson, M., Kooman, J. P. & Stenvinkel, P. The role of epigenetics in renal ageing. Nat. Rev. Nephrol. 13, 471–482 (2017).
Di Micco, R., Krizhanovsky, V., Baker, D. & d’Adda di Fagagna, F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 22, 75–95 (2021).
Docherty, M. H., O’Sullivan, E. D., Bonventre, J. V. & Ferenbach, D. A. Cellular senescence in the kidney. J. Am. Soc. Nephrol. 30, 726–736 (2019).
Sturmlechner, I., Durik, M., Sieben, C. J., Baker, D. J. & van Deursen, J. M. Cellular senescence in renal ageing and disease. Nat. Rev. Nephrol. 13, 77–89 (2017).
Huang, W., Hickson, L. J., Eirin, A., Kirkland, J. L. & Lerman, L. O. Cellular senescence: the good, the bad and the unknown. Nat. Rev. Nephrol. 18, 611–627 (2022).
Kim, S. R. et al. Progressive cellular senescence mediates renal dysfunction in ischemic nephropathy. J. Am. Soc. Nephrol. 32, 1987–2004 (2021).
Zhang, L. et al. C/EBPα deficiency in podocytes aggravates podocyte senescence and kidney injury in aging mice. Cell Death Dis. 10, 684 (2019).
Fang, Y. et al. Age-related GSK3β overexpression drives podocyte senescence and glomerular aging. J. Clin. Invest. 132, e141848 (2022).
Pippin, J. W. et al. Upregulated PD-1 signaling antagonizes glomerular health in aged kidneys and disease. J. Clin. Invest. 132, e156250 (2022).
Mylonas, K. J. et al. Cellular senescence inhibits renal regeneration after injury in mice, with senolytic treatment promoting repair. Sci. Transl. Med. 13, eabb0203 (2021).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Jin, H. et al. Epithelial innate immunity mediates tubular cell senescence after kidney injury. JCI Insight 4, e125490 (2019).
Paez-Ribes, M., Gonzalez-Gualda, E., Doherty, G. J. & Munoz-Espin, D. Targeting senescent cells in translational medicine. EMBO Mol. Med. 11, e10234 (2019).
Chaib, S., Tchkonia, T. & Kirkland, J. L. Cellular senescence and senolytics: the path to the clinic. Nat. Med. 28, 1556–1568 (2022).
Johmura, Y. et al. Senolysis by glutaminolysis inhibition ameliorates various age-associated disorders. Science 371, 265–270 (2021).
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C. & Santoro, A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14, 576–590 (2018).
Evenepoel, P., Stenvinkel, P., Shanahan, C. & Pacifici, R. Inflammation and gut dysbiosis as drivers of CKD-MBD. Nat. Rev. Nephrol. 19, 646–657 (2023).
Sato, Y., Silina, K., van den Broek, M., Hirahara, K. & Yanagita, M. The roles of tertiary lymphoid structures in chronic diseases. Nat. Rev. Nephrol. 19, 525–537 (2023).
Stenvinkel, P. et al. Chronic inflammation in chronic kidney disease progression: role of Nrf2. Kidney Int. Rep. 6, 1775–1787 (2021).
Shelton, L. M., Park, B. K. & Copple, I. M. Role of Nrf2 in protection against acute kidney injury. Kidney Int. 84, 1090–1095 (2013).
Nezu, M. et al. Transcription factor Nrf2 hyperactivation in early-phase renal ischemia-reperfusion injury prevents tubular damage progression. Kidney Int. 91, 387–401 (2017).
Jo, M. J. et al. Impaired NRF2 inhibits recovery from ischemic reperfusion injury in the aging kidney. Antioxidants 12, 1440 (2023).
Martini, S. et al. Integrative biology identifies shared transcriptional networks in CKD. J. Am. Soc. Nephrol. 25, 2559–2572 (2014).
Salminen, A., Kaarniranta, K. & Kauppinen, A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging 4, 166–175 (2012).
Komada, T. & Muruve, D. A. The role of inflammasomes in kidney disease. Nat. Rev. Nephrol. 15, 501–520 (2019).
Kaverina, N. et al. Inhibiting NLRP3 signaling in aging podocytes improves their life- and health-span. Aging 15, 6658–6689 (2023).
Sato, Y. et al. Heterogeneous fibroblasts underlie age-dependent tertiary lymphoid tissues in the kidney. JCI Insight 1, e87680 (2016).
Ligon, M. M. et al. Single cell and tissue-transcriptomic analysis of murine bladders reveals age- and TNFalpha-dependent but microbiota-independent tertiary lymphoid tissue formation. Mucosal Immunol. 13, 908–918 (2020).
Singh, P. et al. Lymphoid neogenesis and immune infiltration in aged liver. Hepatology 47, 1680–1690 (2008).
Sato, Y. & Yanagita, M. Immunology of the ageing kidney. Nat. Rev. Nephrol. 15, 625–640 (2019).
Sato, Y. et al. Developmental stages of tertiary lymphoid tissue reflect local injury and inflammation in mouse and human kidneys. Kidney Int. 98, 448–463 (2020).
Sato, Y. et al. CD153-CD30 signaling promotes age-dependent tertiary lymphoid tissue expansion and kidney injury. J. Clin. Invest. 132, e146071 (2021).
Lee, Y. H. et al. Advanced tertiary lymphoid tissues in protocol biopsies are associated with progressive graft dysfunction in kidney transplant recipients. J. Am. Soc. Nephrol. 33, 186–200 (2021).
Yoshikawa, T. et al. Tertiary lymphoid tissues are microenvironments with intensive interactions between immune cells and proinflammatory parenchymal cells in aged kidneys. J. Am. Soc. Nephrol. 34, 1687–1708 (2023).
Doke, T. & Susztak, K. The multifaceted role of kidney tubule mitochondrial dysfunction in kidney disease development. Trends Cell Biol. 32, 841–853 (2022).
Guan, Y. et al. Nicotinamide mononucleotide, an NAD+ precursor, rescues age-associated susceptibility to AKI in a sirtuin 1-dependent manner. J. Am. Soc. Nephrol. 28, 2337–2352 (2017).
Yamamoto, T. et al. Time-dependent dysregulation of autophagy: implications in aging and mitochondrial homeostasis in the kidney proximal tubule. Autophagy 12, 801–813 (2016).
Tang, C. et al. Mitochondrial quality control in kidney injury and repair. Nat. Rev. Nephrol. 17, 299–318 (2021).
Miwa, S., Kashyap, S., Chini, E. & von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Invest. 132, e158447 (2022).
Kishi, S., Nagasu, H., Kidokoro, K. & Kashihara, N. Oxidative stress and the role of redox signalling in chronic kidney disease. Nat. Rev. Nephrol. 20, 101–119 (2024).
Salminen, A., Kaarniranta, K. & Kauppinen, A. Regulation of longevity by FGF21: interaction between energy metabolism and stress responses. Ageing Res. Rev. 37, 79–93 (2017).
Minami, S. et al. FGF21 and autophagy coordinately counteract kidney disease progression during aging and obesity. Autophagy 20, 489–504 (2023).
Yamamoto, T. et al. High-fat diet-induced lysosomal dysfunction and impaired autophagic flux contribute to lipotoxicity in the kidney. J. Am. Soc. Nephrol. 28, 1534–1551 (2017).
Yamamoto, T. et al. Eicosapentaenoic acid attenuates renal lipotoxicity by restoring autophagic flux. Autophagy 17, 1700–1713 (2021).
Minami, S. et al. Lipophagy maintains energy homeostasis in the kidney proximal tubule during prolonged starvation. Autophagy 13, 1629–1647 (2017).
Matsumoto, A. et al. Spatiotemporally quantitative in vivo imaging of mitochondrial fatty acid β-oxidation at cellular-level resolution in mice. Am. J. Physiol. Endocrinol. Metab. 325, E552–E561 (2023).
Noels, H., Lehrke, M., Vanholder, R. & Jankowski, J. Lipoproteins and fatty acids in chronic kidney disease: molecular and metabolic alterations. Nat. Rev. Nephrol. 17, 528–542 (2021).
Mitrofanova, A., Merscher, S. & Fornoni, A. Kidney lipid dysmetabolism and lipid droplet accumulation in chronic kidney disease. Nat. Rev. Nephrol. 19, 629–645 (2023).
Mutlu, A. S., Duffy, J. & Wang, M. C. Lipid metabolism and lipid signals in aging and longevity. Dev. Cell 56, 1394–1407 (2021).
Braun, F. et al. Altered lipid metabolism in the aging kidney identified by three layered omic analysis. Aging 8, 441–457 (2016).
Kang, H. M. et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 21, 37–46 (2015).
Dhillon, P. et al. The nuclear receptor ESRRA protects from kidney disease by coupling metabolism and differentiation. Cell Metab. 33, 379–394.e8 (2021).
Chung, K. W. et al. Impairment of PPARα and the fatty acid oxidation pathway aggravates renal fibrosis during aging. J. Am. Soc. Nephrol. 29, 1223–1237 (2018).
Lee, G. et al. PGC-1α, a potential therapeutic target against kidney aging. Aging Cell 18, e12994 (2019).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997).
Kurosu, H. et al. Suppression of aging in mice by the hormone Klotho. Science 309, 1829–1833 (2005).
Shi, M. et al. αKlotho mitigates progression of AKI to CKD through activation of autophagy. J. Am. Soc. Nephrol. 27, 2331–2345 (2016).
Wang, Y. et al. Imbalanced lipid homeostasis caused by membrane αKlotho deficiency contributes to the acute kidney injury to chronic kidney disease transition. Kidney Int. 104, 956–974 (2023).
Edmonston, D. et al. Klotho and clinical outcomes in CKD. Am. J. Kidney Dis. https://doi.org/10.1053/j.ajkd.2024.02.008 (2024).
Fujimura, R. et al. Autophagy protects kidney from phosphate-induced mitochondrial injury. Biochem. Biophys. Res. Commun. 524, 636–642 (2020).
Kuro, O. M. Phosphate as a pathogen of arteriosclerosis and aging. J. Atheroscler. Thromb. 28, 203–213 (2021).
Kawai, M., Kinoshita, S., Ozono, K. & Michigami, T. Inorganic phosphate activates the AKT/mTORC1 pathway and shortens the life span of an α-klotho-deficient model. J. Am. Soc. Nephrol. 27, 2810–2824 (2016).
Kuro, O. M. Klotho and calciprotein particles as therapeutic targets against accelerated ageing. Clin. Sci. 135, 1915–1927 (2021).
Shiizaki, K. et al. Calcium phosphate microcrystals in the renal tubular fluid accelerate chronic kidney disease progression. J. Clin. Invest. 131, e145693 (2021).
Mafra, D. et al. Food as medicine: targeting the uraemic phenotype in chronic kidney disease. Nat. Rev. Nephrol. 17, 153–171 (2021).
Avesani, C. M., Cuppari, L., Nerbass, F. B., Lindholm, B. & Stenvinkel, P. Ultraprocessed foods and chronic kidney disease – double trouble. Clin. Kidney J. 16, 1723–1736 (2023).
Dominguez, L. J., Veronese, N. & Barbagallo, M. Magnesium and the hallmarks of aging. Nutrients 16, 496 (2024).
Sakaguchi, Y. et al. Hypomagnesemia in type 2 diabetic nephropathy: a novel predictor of end-stage renal disease. Diabetes Care 35, 1591–1597 (2012).
Oka, T. et al. Proteinuria-associated renal magnesium wasting leads to hypomagnesemia: a common electrolyte abnormality in chronic kidney disease. Nephrol. Dial. Transpl. 34, 1154–1162 (2019).
Du, S., Kim, H., Crews, D. C., White, K. & Rebholz, C. M. Association between ultraprocessed food consumption and risk of incident CKD: a prospective cohort study. Am. J. Kidney Dis. 80, 589–598.e1 (2022).
Su, D. et al. Metabolomic markers of ultra-processed food and incident CKD. Clin. J. Am. Soc. Nephrol. 18, 327–336 (2023).
Rebholz, C. M. et al. Dietary magnesium and kidney function decline: the healthy aging in neighborhoods of diversity across the life span study. Am. J. Nephrol. 44, 381–387 (2016).
Sakaguchi, Y. et al. Low magnesium diet aggravates phosphate-induced kidney injury. Nephrol. Dial. Transpl. 34, 1310–1319 (2019).
Diaz-Tocados, J. M. et al. Dietary magnesium supplementation prevents and reverses vascular and soft tissue calcifications in uremic rats. Kidney Int. 92, 1084–1099 (2017).
Sakaguchi, Y. et al. Magnesium modifies the association between serum phosphate and the risk of progression to end-stage kidney disease in patients with non-diabetic chronic kidney disease. Kidney Int. 88, 833–842 (2015).
Jahan, N. et al. Possible contribution of phosphate to the pathogenesis of chronic kidney disease in dolphins. Sci. Rep. 13, 5161 (2023).
Gupta, N. et al. Targeted inhibition of gut microbial trimethylamine N-oxide production reduces renal tubulointerstitial fibrosis and functional impairment in a murine model of chronic kidney disease. Arterioscler. Thromb. Vasc. Biol. 40, 1239–1255 (2020).
Wang, M. et al. The gut microbial metabolite trimethylamine N-oxide, incident CKD, and kidney function decline. J. Am. Soc. Nephrol. 9, 934113 (2024).
Sakai, S. et al. Proximal tubule autophagy differs in type 1 and 2 diabetes. J. Am. Soc. Nephrol. 30, 929–945 (2019).
Takahashi, A. et al. Autophagy inhibits the accumulation of advanced glycation end products by promoting lysosomal biogenesis and function in the kidney proximal tubules. Diabetes 66, 1359–1372 (2017).
Ballabio, A. & Bonifacino, J. S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 21, 101–118 (2020).
Yamamoto, T., Nakamura, J., Takabatake, Y. & Isaka, Y. Obesity-related proximal tubulopathy: an emerging threat to kidney health. Autophagy Rep. 2, https://doi.org/10.1080/27694127.2023.2200341 (2023).
Minami, S., Yamamoto, T., Yamamoto-Imoto, H., Isaka, Y. & Hamasaki, M. Autophagy and kidney aging. Prog. Biophys. Mol. Biol. 179, 10–15 (2023).
Cui, M. et al. HKDC1, a target of TFEB, is essential to maintain both mitochondrial and lysosomal homeostasis, preventing cellular senescence. Proc. Natl Acad. Sci. USA 121, e2306454120 (2024).
Gros, F. & Muller, S. The role of lysosomes in metabolic and autoimmune diseases. Nat. Rev. Nephrol. 19, 366–383 (2023).
Takabatake, Y., Yamamoto, T. & Isaka, Y. Stagnation of autophagy: a novel mechanism of renal lipotoxicity. Autophagy 13, 775–776 (2017).
Nakamura, J. et al. TFEB-mediated lysosomal exocytosis alleviates high-fat diet-induced lipotoxicity in the kidney. JCI Insight 8, e162498 (2023).
Matsunaga, K. et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat. Cell Biol. 11, 385–396 (2009).
Nakamura, S. et al. Suppression of autophagic activity by Rubicon is a signature of aging. Nat. Commun. 10, 847 (2019).
Yamamoto-Imoto, H. et al. Age-associated decline of MondoA drives cellular senescence through impaired autophagy and mitochondrial homeostasis. Cell Rep. 38, 110444 (2022).
Maeda, S. et al. MondoA and AKI and AKI-to-CKD transition. J. Am. Soc. Nephrol. https://doi.org/10.1681/ASN.0000000000000414 (2024).
Kim, J., Kundu, M., Viollet, B. & Guan, K. L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141 (2011).
Huynh, C., Ryu, J., Lee, J., Inoki, A. & Inoki, K. Nutrient-sensing mTORC1 and AMPK pathways in chronic kidney diseases. Nat. Rev. Nephrol. 19, 102–122 (2023).
Zhang, W., Feng, C. & Jiang, H. Novel target for treating Alzheimer’s diseases: crosstalk between the Nrf2 pathway and autophagy. Ageing Res. Rev. 65, 101207 (2021).
Oh, H. S. et al. Organ aging signatures in the plasma proteome track health and disease. Nature 624, 164–172 (2023).
Cohen, N. M. et al. Longitudinal machine learning uncouples healthy aging factors from chronic disease risks. Nat. Aging 4, 129–144 (2024).
Rutledge, J., Oh, H. & Wyss-Coray, T. Measuring biological age using omics data. Nat. Rev. Genet. 23, 715–727 (2022).
Shiels, P. G. et al. Manipulating the exposome to enable better ageing. Biochem. J. 478, 2889–2898 (2021).
Stenvinkel, P., Avesani, C. M., Gordon, L. J., Schalling, M. & Shiels, P. G. Biomimetics provides lessons from nature for contemporary ways to improve human health. J. Clin. Transl. Sci. 5, e128 (2021).
Ebert, T. et al. Inflammation and premature ageing in chronic kidney disease. Toxins 12, 227 (2020).
Singh, M., Stewart, R. & White, H. Importance of frailty in patients with cardiovascular disease. Eur. Heart J. 35, 1726–1731 (2014).
Otobe, Y., Rhee, C. M., Nguyen, M., Kalantar-Zadeh, K. & Kopple, J. D. Current status of the assessment of sarcopenia, frailty, physical performance and functional status in chronic kidney disease patients. Curr. Opin. Nephrol. Hypertens. 31, 109–128 (2022).
Fried, L. P. et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 56, M146–M156 (2001).
Nixon, A. C. et al. Frailty and chronic kidney disease: current evidence and continuing uncertainties. Clin. Kidney J. 11, 236–245 (2018).
Miao, Z., Humphreys, B. D., McMahon, A. P. & Kim, J. Multi-omics integration in the age of million single-cell data. Nat. Rev. Nephrol. 17, 710–724 (2021).
Klinkhammer, B. M. et al. Non-invasive molecular imaging of kidney diseases. Nat. Rev. Nephrol. 17, 688–703 (2021).
Schreibing, F. & Kramann, R. Mapping the human kidney using single-cell genomics. Nat. Rev. Nephrol. 18, 347–360 (2022).
He, X., Memczak, S., Qu, J., Belmonte, J. C. I. & Liu, G. H. Single-cell omics in ageing: a young and growing field. Nat. Metab. 2, 293–302 (2020).
Kirita, Y., Wu, H., Uchimura, K., Wilson, P. C. & Humphreys, B. D. Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc. Natl Acad. Sci. USA 117, 15874–15883 (2020).
Ahadi, S. et al. Personal aging markers and ageotypes revealed by deep longitudinal profiling. Nat. Med. 26, 83–90 (2020).
Baek, J., He, C., Afshinnia, F., Michailidis, G. & Pennathur, S. Lipidomic approaches to dissect dysregulated lipid metabolism in kidney disease. Nat. Rev. Nephrol. 18, 38–55 (2022).
Tsugawa, H. et al. A lipidome landscape of aging in mice. Nat. Aging 4, 709–726 (2024).
Burtscher, J. et al. Mitochondrial stress and mitokines in aging. Aging Cell 22, e13770 (2023).
Kim, K. H. et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat. Med. 19, 83–92 (2013).
Hamano, T. et al. Fetuin-mineral complex reflects extraosseous calcification stress in CKD. J. Am. Soc. Nephrol. 21, 1998–2007 (2010).
Miura, Y., Kurosu, H. & Kuro, O. M. Quantification of calciprotein particles (CPPs) in serum/plasma samples using a fluorescent bisphosphonate. Methods Mol. Biol. 2664, 333–341 (2023).
Mukai, H. et al. Skin autofluorescence, arterial stiffness and Framingham risk score as predictors of clinical outcome in chronic kidney disease patients: a cohort study. Nephrol. Dial. Transpl. 34, 442–448 (2019).
Reurean-Pintilei, D. et al. Skin autofluorescence as a potential adjunctive marker for cardiovascular risk assessment in type 2 diabetes: a systematic review. Int. J. Mol. Sci. 25, 3889 (2024).
Neytchev, O. et al. Epigenetic clocks indicate that kidney transplantation and not dialysis mitigate the effects of renal ageing. J. Intern. Med. 295, 79–90 (2024).
Suzuki, T. et al. Mitochonic acid 5 binds mitochondria and ameliorates renal tubular and cardiac myocyte damage. J. Am. Soc. Nephrol. 27, 1925–1932 (2016).
Matsuhashi, T. et al. Mitochonic acid 5 (MA-5) facilitates ATP synthase oligomerization and cell survival in various mitochondrial diseases. EBioMedicine 20, 27–38 (2017).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Zhang, L. et al. Cellular senescence: a key therapeutic target in aging and diseases. J. Clin. Invest. 132, e158450 (2022).
Hickson, L. J. et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456 (2019).
US National Library of Medicine. ClinicalTrials.gov www.clinicaltrials.gov/study/NCT02848131?term=NCT02848131&rank=1 (2024).
US National Library of Medicine. ClinicalTrials.gov www.clinicaltrials.gov/study/NCT04313634?term=NCT04313634&rank=1 (2024).
US National Library of Medicine. ClinicalTrials.gov www.clinicaltrials.gov/study/NCT04733534?term=NCT04733534&rank=1 (2023).
Katsuumi, G. et al. SGLT2 inhibition eliminates senescent cells and alleviates pathological aging. Nat. Aging 10.1038/s43587-024-00642-y (2024).
Grosse, L. et al. Defined p16High senescent cell types are indispensable for mouse healthspan. Cell Metab. 32, 87–99.e6 (2020).
Namba, T. et al. Autophagic clearance of mitochondria in the kidney copes with metabolic acidosis. J. Am. Soc. Nephrol. 25, 2254–2266 (2014).
Zhang, Y. et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife 1, e00065 (2012).
Bhatt, D. L. et al. The FGF21 analog pegozafermin in severe hypertriglyceridemia: a randomized phase 2 trial. Nat. Med. 29, 1782–1792 (2023).
Loomba, R. et al. Randomized, controlled trial of the FGF21 analogue pegozafermin in NASH. N. Engl. J. Med. 389, 998–1008 (2023).
Yang, T., Richards, E. M., Pepine, C. J. & Raizada, M. K. The gut microbiota and the brain–gut–kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 14, 442–456 (2018).
Buffa, J. A. et al. The microbial gbu gene cluster links cardiovascular disease risk associated with red meat consumption to microbiota l-carnitine catabolism. Nat. Microbiol. 7, 73–86 (2022).
Lew, Q. J. et al. Red meat intake and risk of ESRD. J. Am. Soc. Nephrol. 28, 304–312 (2017).
Taguchi, K., Fukami, K., Elias, B. C. & Brooks, C. R. Dysbiosis-related advanced glycation endproducts and trimethylamine N-oxide in chronic kidney disease. Toxins 13, 361 (2021).
Dai, L. et al. The association between TMAO, CMPF, and clinical outcomes in advanced chronic kidney disease: results from the European QUALity (EQUAL) study. Am. J. Clin. Nutr. 116, 1842–1851 (2022).
Fontana, L. & Partridge, L. Promoting health and longevity through diet: from model organisms to humans. Cell 161, 106–118 (2015).
Windahl, K. et al. The safety of a low protein diet in older adults with advanced chronic kidney disease. Nephrol. Dial. Transpl. https://doi.org/10.1093/ndt/gfae077 (2024).
Carrero, J. J. et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 16, 525–542 (2020).
Sakaguchi, Y., Kaimori, J. Y. & Isaka, Y. Plant-dominant low protein diet: a potential alternative dietary practice for patients with chronic kidney disease. Nutrients 15, 1002 (2023).
Sakaguchi, Y., Hamano, T. & Isaka, Y. Effects of magnesium on the phosphate toxicity in chronic kidney disease: time for intervention studies. Nutrients 9, 112 (2017).
Lu, Y., Zhang, Y., Lou, Y., Cui, W. & Miao, L. Sulforaphane suppresses obesity-related glomerulopathy-induced damage by enhancing autophagy via Nrf2. Life Sci. 258, 118153 (2020).
Mohammad, R. S., Lokhandwala, M. F. & Banday, A. A. Age-related mitochondrial impairment and renal injury is ameliorated by sulforaphane via activation of transcription factor NRF2. Antioxidants 11, 156 (2022).
Luis, C. et al. Nutritional senolytics and senomorphics: implications to immune cells metabolism and aging – from theory to practice. Front. Nutr. 9, 958563 (2022).
Jylhava, J., Pedersen, N. L. & Hagg, S. Biological age predictors. EBioMedicine 21, 29–36 (2017).
Acknowledgements
The authors’ work is supported by Japan Society for the Promotion of Science (21K16163, 23K07671 to T.Y. and 21H02935 to Y.I.), Japan Agency for Medical Research and Development (JP23ek0310022 to T.Y. and JP22gm1410014 to Y.I.), the Astellas Foundation for Research on Metabolic Disorders, Naito Foundation, Kato Memorial Bioscience Foundation, Salt Science Research Foundation, Sumitomo Insurance Welfare Foundation, G-7 Scholarship Foundation, and Takeda Medical Research Foundation (to T. Y.).
Author information
Authors and Affiliations
Contributions
Both authors made substantial contributions to discussions of the content and wrote, reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Jeroen Kooman, Motoko Yanagita, Oliver Wessely and Peter Stenvinkel for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yamamoto, T., Isaka, Y. Pathological mechanisms of kidney disease in ageing. Nat Rev Nephrol 20, 603–615 (2024). https://doi.org/10.1038/s41581-024-00868-4
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41581-024-00868-4
This article is cited by
-
Allogeneic bone marrow-derived mesenchymal stem cells in the aging kidney: secondary results of a Parkinson’s disease clinical trial
Stem Cell Research & Therapy (2025)
-
Spicy food consumption and biological aging across multiple organ systems: a longitudinal analysis from the China Multi-Ethnic cohort
Nutrition Journal (2025)
-
Klotho antiaging protein: molecular mechanisms and therapeutic potential in diseases
Molecular Biomedicine (2025)
-
Mendelian randomization study revealed a gut microbiota-immune system-kidney junction axis in chronic kidney disease
Scientific Reports (2025)
-
Identification of aging-related biomarkers and immune infiltration analysis in renal stones by integrated bioinformatics analysis
Scientific Reports (2025)