Abstract
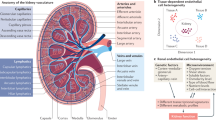
The development and proper functioning of the kidney is dependent on the interaction of kidney cells with the surrounding extracellular matrix (ECM). These interactions are mediated by heterodimeric membrane-bound receptors called integrins, which bind to the ECM via their extracellular domain and via their cytoplasmic tail to intracellular adaptor proteins, to assemble large macromolecular adhesion complexes. These interactions enable integrins to control cellular functions such as intracellular signalling and organization of the actin cytoskeleton and are therefore crucial to organ function. The different nephron segments and the collecting duct system have unique morphologies, functions and ECM environments and are thus equipped with unique sets of integrins with distinct specificities for the ECM with which they interact. These cell-type-specific functions are facilitated by specific intracellular integrin binding proteins, which are critical in determining the integrin activation status, ligand-binding affinity and the type of ECM signals that are relayed to the intracellular structures. The spatiotemporal expression of integrins and their specific interactions with binding partners underlie the proper development, function and repair processes of the kidney. This Review summarizes our current understanding of how integrins, their binding partners and the actin cytoskeleton regulate kidney development, physiology and pathology.
Key points
-
Integrins are heterodimeric transmembrane receptors that consist of an α- and a β-subunit, which are non-covalently bound to each other; in the kidney, integrins control interactions between cells and the extracellular matrix to regulate cellular functions such as intracellular signalling and organization of the actin cytoskeleton.
-
Different parts of the nephron and the collecting system differ in their morphology, function and extracellular matrix environment and are therefore equipped with a unique set of integrins.
-
The laminin-binding integrin, α3β1, which is stabilized by the tetraspanin CD151, is essential for podocyte health; in mesangial cells, the collagen binding integrin α2β1 promotes whereas α1β1 inhibits collagen production following injury.
-
Integrin-binding proteins such as talins, kindlins and the ILK–PINCH–parvin complex differentially regulate cellular functions in various kidney segments.
-
Integrin-controlled Rho GTPase signalling is intricately involved in the complex reorganization of the actin cytoskeleton during kidney development and repair.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Blanc, T. et al. Three-dimensional architecture of nephrons in the normal and cystic kidney. Kidney Int. 99, 632–645 (2021).
Mathew, S., Chen, X., Pozzi, A. & Zent, R. Integrins in renal development. Pediatr. Nephrol. 27, 891–900 (2012).
Naylor, R. W., Morais, M. & Lennon, R. Complexities of the glomerular basement membrane. Nat. Rev. Nephrol. 17, 112–127 (2021).
Borza, C. M., Chen, X., Zent, R. & Pozzi, A. Cell receptor-basement membrane interactions in health and disease: a kidney-centric view. Curr. Top. Membr. 76, 231–253 (2015).
Pozzi, A. & Zent, R. Integrins in kidney disease. J. Am. Soc. Nephrol. 24, 1034–1039 (2013).
Sun, Z., Costell, M. & Fassler, R. Integrin activation by talin, kindlin and mechanical forces. Nat. Cell Biol. 21, 25–31 (2019).
Moser, M., Legate, K. R., Zent, R. & Fassler, R. The tail of integrins, talin, and kindlins. Science 324, 895–899 (2009).
Lelongt, B. & Ronco, P. Role of extracellular matrix in kidney development and repair. Pediatr. Nephrol. 18, 731–742 (2003).
Scarpellini, A. et al. Syndecan-4 knockout leads to reduced extracellular transglutaminase-2 and protects against tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 25, 1013–1027 (2014).
Vogel, W. et al. Discoidin domain receptor 1 is activated independently of β1 integrin. J. Biol. Chem. 275, 5779–5784 (2000).
Dorison, A. & Chantziantoniou, C. DDR1: a major player in renal diseases. Cell Adh. Migr. 12, 299–304 (2018).
Chiusa, M. et al. The extracellular matrix receptor discoidin domain receptor 1 regulates collagen transcription by translocating to the nucleus. J. Am. Soc. Nephrol. 30, 1605–1624 (2019).
Borza, C. M. et al. DDR1 contributes to kidney inflammation and fibrosis by promoting the phosphorylation of BCR and STAT3. JCI Insight 7, e150887 (2022).
Borza, C. M., Bolas, G. & Pozzi, A. Genetic and pharmacological tools to study the role of discoidin domain receptors in kidney disease. Front. Pharmacol. 13, 1001122 (2022).
Nordenfelt, P., Elliott, H. L. & Springer, T. A. Coordinated integrin activation by actin-dependent force during T-cell migration. Nat. Commun. 7, 13119 (2016).
Li, J. et al. Ligand binding initiates single-molecule integrin conformational activation. Cell 187, 2990–3005.e17 (2024).
Avraham, S., Korin, B., Chung, J. J., Oxburgh, L. & Shaw, A. S. The mesangial cell — the glomerular stromal cell. Nat. Rev. Nephrol. 17, 855–864 (2021).
Satchell, S. The role of the glomerular endothelium in albumin handling. Nat. Rev. Nephrol. 9, 717–725 (2013).
Kreidberg, J. A. & Symons, J. M. Integrins in kidney development, function, and disease. Am. J. Physiol. Renal Physiol. 279, F233–F242 (2000).
Sachs, N. et al. Kidney failure in mice lacking the tetraspanin CD151. J. Cell Biol. 175, 33–39 (2006).
Pozzi, A. et al. Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev. Biol. 316, 288–301 (2008).
Has, C. et al. Integrin alpha3 mutations with kidney, lung, and skin disease. N. Engl. J. Med. 366, 1508–1514 (2012).
Nicolaou, N. et al. Gain of glycosylation in integrin α3 causes lung disease and nephrotic syndrome. J. Clin. Invest. 122, 4375–4387 (2012).
Sachs, N. et al. Blood pressure influences end-stage renal disease of Cd151 knockout mice. J. Clin. Invest. 122, 348–358 (2012).
Remuzzi, A. et al. Role of ultrastructural determinants of glomerular permeability in ultrafiltration function loss. JCI Insight 5, e137249 (2020).
Naylor, R. W. et al. Basement membrane defects in CD151-associated glomerular disease. Pediatr. Nephrol. 37, 3105–3115 (2022).
Wright, M. D. et al. Characterization of mice lacking the tetraspanin superfamily member CD151. Mol. Cell Biol. 24, 5978–5988 (2004).
Bufi, R. & Korstanje, R. The impact of genetic background on mouse models of kidney disease. Kidney Int. 102, 38–44 (2022).
Gardner, H., Kreidberg, J., Koteliansky, V. & Jaenisch, R. Deletion of integrin α1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev. Biol. 175, 301–313 (1996).
Cosgrove, D. et al. Integrin α1β1 and transforming growth factor-β1 play distinct roles in Alport glomerular pathogenesis and serve as dual targets for metabolic therapy. Am. J. Pathol. 157, 1649–1659 (2000).
Zent, R. et al. Glomerular injury is exacerbated in diabetic integrin α1-null mice. Kidney Int. 70, 460–470 (2006).
Girgert, R. et al. Integrin α2-deficient mice provide insights into specific functions of collagen receptors in the kidney. Fibrogenes. Tissue Repair. 3, 19 (2010).
Wei, C. et al. SuPAR mediates viral response proteinuria by rapidly changing podocyte function. Nat. Commun. 14, 4414 (2023).
Hayek, S. S. et al. A tripartite complex of suPAR, APOL1 risk variants and αvβ3 integrin on podocytes mediates chronic kidney disease. Nat. Med. 23, 945–953 (2017).
Wei, C. et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat. Med. 17, 952–960 (2011).
Gallon, L. & Quaggin, S. E. SuPAR and FSGS: is the jury still out? Nat. Rev. Nephrol. 13, 593 (2017).
Sinha, A. et al. Serum-soluble urokinase receptor levels do not distinguish focal segmental glomerulosclerosis from other causes of nephrotic syndrome in children. Kidney Int. 85, 649–658 (2014).
Sudhini, Y. R., Wei, C. & Reiser, J. suPAR: an inflammatory mediator for kidneys. Kidney Dis. 8, 265–274 (2022).
Nusshag, C. et al. suPAR links a dysregulated immune response to tissue inflammation and sepsis-induced acute kidney injury. JCI Insight 8, e165740 (2023).
Madhusudhan, T. et al. Podocyte integrin-β3 and activated protein C coordinately restrict RhoA signaling and ameliorate diabetic nephropathy. J. Am. Soc. Nephrol. 31, 1762–1780 (2020).
Elphick, G. F. et al. Recombinant human activated protein C inhibits integrin-mediated neutrophil migration. Blood 113, 4078–4085 (2009).
He, B. et al. Single-cell RNA sequencing reveals the mesangial identity and species diversity of glomerular cell transcriptomes. Nat. Commun. 12, 2141 (2021).
Chen, X. et al. Lack of integrin α1β1 leads to severe glomerulosclerosis after glomerular injury. Am. J. Pathol. 165, 617–630 (2004).
Chen, X. et al. Integrin α1β1 controls reactive oxygen species synthesis by negatively regulating epidermal growth factor receptor-mediated Rac activation. Mol. Cell Biol. 27, 3313–3326 (2007).
Borza, C. M. et al. Integrin α1β1 promotes caveolin-1 dephosphorylation by activating T cell protein-tyrosine phosphatase. J. Biol. Chem. 285, 40114–40124 (2010).
Chen, X. et al. Integrin-mediated type II TGF-β receptor tyrosine dephosphorylation controls SMAD-dependent profibrotic signaling. J. Clin. Invest. 124, 3295–3310 (2014).
Heppner, D. E. et al. The NADPH oxidases DUOX1 and NOX2 play distinct roles in redox regulation of epidermal growth factor receptor signaling. J. Biol. Chem. 291, 23282–23293 (2016).
Chiusa, M. et al. EGF receptor-mediated FUS phosphorylation promotes its nuclear translocation and fibrotic signaling. J. Cell Biol. 219, e202001120 (2020).
Chiusa, M. et al. Cytoplasmic retention of the DNA/RNA-binding protein FUS ameliorates organ fibrosis in mice. J. Clin. Invest. 134, e175158 (2024).
Shi, M. et al. Enhancing integrin alpha1 inserted (I) domain affinity to ligand potentiates integrin α1β1-mediated down-regulation of collagen synthesis. J. Biol. Chem. 287, 35139–35152 (2012).
Gardner, H., Broberg, A., Pozzi, A., Laato, M. & Heino, J. Absence of integrin α1β1 in the mouse causes loss of feedback regulation of collagen synthesis in normal and wounded dermis. J. Cell Sci. 112, 263–272 (1999).
Williams, A. S. et al. Integrin α1-null mice exhibit improved fatty liver when fed a high fat diet despite severe hepatic insulin resistance. J. Biol. Chem. 290, 6546–6557 (2015).
Borza, C. M. et al. Inhibition of integrin α2β1 ameliorates glomerular injury. J. Am. Soc. Nephrol. 23, 1027–1038 (2012).
Lakhe-Reddy, S., Li, V., Arnold, T. D., Khan, S. & Schelling, J. R. Mesangial cell αvβ8-integrin regulates glomerular capillary integrity and repair. Am. J. Physiol. Renal Physiol. 306, F1400–F1409 (2014).
Khan, S. et al. Mesangial cell integrin αvβ8 provides glomerular endothelial cell cytoprotection by sequestering TGF-β and regulating PECAM-1. Am. J. Pathol. 178, 609–620 (2011).
Hartner, A., Schocklmann, H., Prols, F., Muller, U. & Sterzel, R. B. α8 Integrin in glomerular mesangial cells and in experimental glomerulonephritis. Kidney Int. 56, 1468–1480 (1999).
Hartner, A. et al. The α8 integrin chain affords mechanical stability to the glomerular capillary tuft in hypertensive glomerular disease. Am. J. Pathol. 160, 861–867 (2002).
Bieritz, B. et al. Role of α8 integrin in mesangial cell adhesion, migration, and proliferation. Kidney Int. 64, 119–127 (2003).
Zimmerman, S. E. et al. Nephronectin regulates mesangial cell adhesion and behavior in glomeruli. J. Am. Soc. Nephrol. 29, 1128–1140 (2018).
Muller, U. et al. Integrin α8β1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell 88, 603–613 (1997).
Humbert, C. et al. Integrin α8 recessive mutations are responsible for bilateral renal agenesis in humans. Am. J. Hum. Genet. 94, 288–294 (2014).
Elias, B. C. et al. The integrin β1 subunit regulates paracellular permeability of kidney proximal tubule cells. J. Biol. Chem. 289, 8532–8544 (2014).
Zheng, G. et al. α3 Integrin of cell-cell contact mediates kidney fibrosis by integrin-linked kinase in proximal tubular e-cadherin deficient mice. Am. J. Pathol. 186, 1847–1860 (2016).
Sucre, J. M. et al. Alveolar repair following LPS-induced injury requires cell-ECM interactions. JCI Insight 8, e167211 (2023).
Haake, S. M. et al. Ligand-independent integrin β1 signaling supports lung adenocarcinoma development. JCI Insight 7, e154098 (2022).
Plosa, E. J. et al. β1 Integrin regulates adult lung alveolar epithelial cell inflammation. JCI Insight 5, e129259 (2020).
Ramovs, V., Krotenberg Garcia, A., Kreft, M. & Sonnenberg, A. Integrin α3β1 is a key regulator of several protumorigenic pathways during skin carcinogenesis. J. Invest. Dermatol. 141, 732–741.e6 (2021).
Mia, M. S. et al. Integrin β1 is a key determinant of the expression of angiotensin-converting enzyme 2 (ACE2) in the kidney epithelial cells. Eur. J. Cell Biol. 102, 151316 (2023).
Hahm, K. et al. αvβ6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am. J. Pathol. 170, 110–125 (2007).
Ma, L. J. et al. Transforming growth factor-β-dependent and -independent pathways of induction of tubulointerstitial fibrosis in β6−/− mice. Am. J. Pathol. 163, 1261–1273 (2003).
Chang, Y. et al. Pharmacologic blockade of alphavbeta1 integrin ameliorates renal failure and fibrosis in vivo. J. Am. Soc. Nephrol. 28, 1998–2005 (2017).
Munger, J. S. et al. The integrin αvβ6 binds and activates latent TGF β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96, 319–328 (1999).
Wu, W. et al. β1-Integrin is required for kidney collecting duct morphogenesis and maintenance of renal function. Am. J. Physiol. Renal Physiol. 297, F210–F217 (2009).
Zhang, X. et al. β1 Integrin is necessary for ureteric bud branching morphogenesis and maintenance of collecting duct structural integrity. Development 136, 3357–3366 (2009).
Lee, K., Boctor, S., Barisoni, L. M. & Gusella, G. L. Inactivation of integrin-β1 prevents the development of polycystic kidney disease after the loss of polycystin-1. J. Am. Soc. Nephrol. 26, 888–895 (2015).
Morais, M. et al. Kidney organoids recapitulate human basement membrane assembly in health and disease. Elife 11, e73486 (2022).
Kreidberg, J. A. et al. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development 122, 3537–3547 (1996).
Yazlovitskaya, E. M. et al. Integrin α3β1 regulates kidney collecting duct development via TRAF6-dependent K63-linked polyubiquitination of Akt. Mol. Biol. Cell 26, 1857–1874 (2015).
Viquez, O. M. et al. Integrin α6 maintains the structural integrity of the kidney collecting system. Matrix Biol. 57-58, 244–257 (2017).
Yazlovitskaya, E. M. et al. The laminin-binding integrins regulate nuclear factor κB-dependent epithelial cell polarity and inflammation. J. Cell Sci. 134, jcs259161 (2021).
Yazlovitskaya, E. M. et al. The laminin binding α3 and α6 integrins cooperate to promote epithelial cell adhesion and growth. Matrix Biol. 77, 101–116 (2019).
Yang, D. H. et al. Renal collecting system growth and function depend upon embryonic γ1 laminin expression. Development 138, 4535–4544 (2011).
Georges-Labouesse, E. et al. Absence of integrin α6 leads to epidermolysis bullosa and neonatal death in mice. Nat. Genet. 13, 370–373 (1996).
He, Y., Esser, P., Heinemann, A., Bruckner-Tuderman, L. & Has, C. Kindlin-1 and -2 have overlapping functions in epithelial cells implications for phenotype modification. Am. J. Pathol. 178, 975–982 (2011).
Gough, R. E. & Goult, B. T. The tale of two talins — two isoforms to fine-tune integrin signalling. FEBS Lett. 592, 2108–2125 (2018).
Bandyopadhyay, A., Rothschild, G., Kim, S., Calderwood, D. A. & Raghavan, S. Functional differences between kindlin-1 and kindlin-2 in keratinocytes. J. Cell Sci. 125, 2172–2184 (2012).
Ciobanasu, C., Faivre, B. & Le Clainche, C. Actomyosin-dependent formation of the mechanosensitive talin-vinculin complex reinforces actin anchoring. Nat. Commun. 5, 3095 (2014).
Mathew, S. et al. Talin regulates integrin β1-dependent and -independent cell functions in ureteric bud development. Development 144, 4148–4158 (2017).
Monkley, S. J. et al. Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev. Dyn. 219, 560–574 (2000).
Theodosiou, M. et al. Kindlin-2 cooperates with talin to activate integrins and induces cell spreading by directly binding paxillin. Elife 5, e10130 (2016).
Rognoni, E., Ruppert, R. & Fassler, R. The kindlin family: functions, signaling properties and implications for human disease. J. Cell Sci. 129, 17–27 (2016).
Bottcher, R. T. et al. Kindlin-2 recruits paxillin and Arp2/3 to promote membrane protrusions during initial cell spreading. J. Cell Biol. 216, 3785–3798 (2017).
Bouaouina, M. & Calderwood, D. A. Kindlins. Curr. Biol. 21, R99–R101 (2011).
Yates, L. A. et al. Structural and functional characterization of the kindlin-1 pleckstrin homology domain. J. Biol. Chem. 287, 43246–43261 (2012).
Montanez, E. et al. Kindlin-2 controls bidirectional signaling of integrins. Genes. Dev. 22, 1325–1330 (2008).
Schell, C. & Huber, T. B. The evolving complexity of the podocyte cytoskeleton. J. Am. Soc. Nephrol. 28, 3166–3174 (2017).
Tian, X. et al. Podocyte-associated talin1 is critical for glomerular filtration barrier maintenance. J. Clin. Invest. 124, 1098–1113 (2014).
Lausecker, F. et al. Vinculin is required to maintain glomerular barrier integrity. Kidney Int. 93, 643–655 (2018).
Atherton, P. et al. Relief of talin autoinhibition triggers a force-independent association with vinculin. J. Cell Biol. 219, e201903134 (2020).
Sun, Y. et al. Kindlin-2 association with Rho GDP-dissociation inhibitor α suppresses Rac1 activation and podocyte injury. J. Am. Soc. Nephrol. 28, 3545–3562 (2017).
Qu, H. et al. Kindlin-2 regulates podocyte adhesion and fibronectin matrix deposition through interactions with phosphoinositides and integrins. J. Cell Sci. 124, 879–891 (2011).
Mosaddeghzadeh, N. & Ahmadian, M. R. The RHO family GTPases: mechanisms of regulation and signaling. Cells 10, 1831 (2021).
Bosco, E. E., Mulloy, J. C. & Zheng, Y. Rac1 GTPase: a “Rac” of all trades. Cell Mol. Life Sci. 66, 370–374 (2009).
Wei, X. et al. Kindlin-2 mediates activation of TGF-β/Smad signaling and renal fibrosis. J. Am. Soc. Nephrol. 24, 1387–1398 (2013).
Guo, B., Gao, J., Zhan, J. & Zhang, H. Kindlin-2 interacts with and stabilizes EGFR and is required for EGF-induced breast cancer cell migration. Cancer Lett. 361, 271–281 (2015).
Yu, Y. et al. Kindlin 2 regulates myogenic related factor myogenin via a canonical Wnt signaling in myogenic differentiation. PLoS ONE 8, e63490 (2013).
Godbout, E. et al. Kindlin-2 mediates mechanical activation of cardiac myofibroblasts. Cells 9, 2702 (2020).
Song, J. et al. Kindlin-2 Inhibits the hippo signaling pathway by promoting degradation of MOB1. Cell Rep. 29, 3664–3677.e5 (2019).
Wickstrom, S. A., Lange, A., Montanez, E. & Fassler, R. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J. 29, 281–291 (2010).
Sepulveda, J. L. & Wu, C. The parvins. Cell Mol. Life Sci. 63, 25–35 (2006).
Liang, X. et al. PINCH1 plays an essential role in early murine embryonic development but is dispensable in ventricular cardiomyocytes. Mol. Cell Biol. 25, 3056–3062 (2005).
Montanez, E., Wickstrom, S. A., Altstatter, J., Chu, H. & Fassler, R. α-parvin controls vascular mural cell recruitment to vessel wall by regulating RhoA/ROCK signalling. EMBO J. 28, 3132–3144 (2009).
Lange, A. et al. Integrin-linked kinase is an adaptor with essential functions during mouse development. Nature 461, 1002–1006 (2009).
Rogg, M. et al. α-Parvin defines a specific integrin adhesome to maintain the glomerular filtration barrier. J. Am. Soc. Nephrol. 33, 786–808 (2022).
El-Aouni, C. et al. Podocyte-specific deletion of integrin-linked kinase results in severe glomerular basement membrane alterations and progressive glomerulosclerosis. J. Am. Soc. Nephrol. 17, 1334–1344 (2006).
Schordan, S., Schordan, E., Endlich, K. & Endlich, N. αV-Integrins mediate the mechanoprotective action of osteopontin in podocytes. Am. J. Physiol. Renal Physiol. 300, F119–F132 (2011).
Li, Y., Yang, J., Dai, C., Wu, C. & Liu, Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J. Clin. Invest. 112, 503–516 (2003).
Li, Y., Dai, C., Wu, C. & Liu, Y. PINCH-1 promotes tubular epithelial-to-mesenchymal transition by interacting with integrin-linked kinase. J. Am. Soc. Nephrol. 18, 2534–2543 (2007).
Kriz, W., Kaissling, B. & Le Hir, M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J. Clin. Invest. 121, 468–474 (2011).
Li, Y. et al. Inhibition of integrin-linked kinase attenuates renal interstitial fibrosis. J. Am. Soc. Nephrol. 20, 1907–1918 (2009).
Kuppe, C. et al. Decoding myofibroblast origins in human kidney fibrosis. Nature 589, 281–286 (2021).
Sheng, L. & Zhuang, S. New insights into the role and mechanism of partial epithelial-mesenchymal transition in kidney fibrosis. Front. Physiol. 11, 569322 (2020).
Smeeton, J. et al. Integrin-linked kinase regulates p38 MAPK-dependent cell cycle arrest in ureteric bud development. Development 137, 3233–3243 (2010).
Bulus, N. et al. Disruption of the integrin-linked kinase (ILK) pseudokinase domain affects kidney development in mice. J. Biol. Chem. 296, 100361 (2021).
Eke, I., Leonhardt, F., Storch, K., Hehlgans, S. & Cordes, N. The small molecule inhibitor QLT0267 radiosensitizes squamous cell carcinoma cells of the head and neck. PLoS ONE 4, e6434 (2009).
Huang, M. et al. Integrin-linked kinase deficiency in collecting duct principal cell promotes necroptosis of principal cell and contributes to kidney inflammation and fibrosis. J. Am. Soc. Nephrol. 30, 2073–2090 (2019).
Fujiu, K., Manabe, I. & Nagai, R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J. Clin. Invest. 121, 3425–3441 (2011).
DeMali, K. A., Wennerberg, K. & Burridge, K. Integrin signaling to the actin cytoskeleton. Curr. Opin. Cell Biol. 15, 572–582 (2003).
Delon, I. & Brown, N. H. Integrins and the actin cytoskeleton. Curr. Opin. Cell Biol. 19, 43–50 (2007).
Svitkina, T. The actin cytoskeleton and actin-based motility. Cold Spring Harb. Perspect. Biol. 10, a018267 (2018).
Mukherjee, K. et al. Simultaneous stabilization of actin cytoskeleton in multiple nephron-specific cells protects the kidney from diverse injury. Nat. Commun. 13, 2422 (2022).
Molitoris, B. A. Actin cytoskeleton in ischemic acute renal failure. Kidney Int. 66, 871–883 (2004).
Lawson, C. D. & Burridge, K. The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration. Small GTPases 5, e27958 (2014).
Spiering, D. & Hodgson, L. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adh Migr. 5, 170–180 (2011).
Suleiman, H. Y. et al. Injury-induced actin cytoskeleton reorganization in podocytes revealed by super-resolution microscopy. JCI Insight 2, e94137 (2017).
Qu, C. et al. Three-dimensional visualization of the podocyte actin network using integrated membrane extraction, electron microscopy, and machine learning. J. Am. Soc. Nephrol. 33, 155–173 (2022).
Haydak, J. & Azeloglu, E. U. Role of biophysics and mechanobiology in podocyte physiology. Nat. Rev. Nephrol. 20, 371–385 (2024).
Zhu, L., Jiang, R., Aoudjit, L., Jones, N. & Takano, T. Activation of RhoA in podocytes induces focal segmental glomerulosclerosis. J. Am. Soc. Nephrol. 22, 1621–1630 (2011).
Yu, H. et al. Rac1 activation in podocytes induces rapid foot process effacement and proteinuria. Mol. Cell Biol. 33, 4755–4764 (2013).
Robins, R. et al. Rac1 activation in podocytes induces the spectrum of nephrotic syndrome. Kidney Int. 92, 349–364 (2017).
Zamboni, V. et al. Hyperactivity of Rac1-GTPase pathway impairs neuritogenesis of cortical neurons by altering actin dynamics. Sci. Rep. 8, 7254 (2018).
Scott, R. P. et al. Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J. Am. Soc. Nephrol. 23, 1149–1154 (2012).
Etienne-Manneville, S. Cdc42 — the centre of polarity. J. Cell Sci. 117, 1291–1300 (2004).
Blattner, S. M. et al. Divergent functions of the Rho GTPases Rac1 and Cdc42 in podocyte injury. Kidney Int. 84, 920–930 (2013).
Rogg, M. et al. SRGAP1 controls small rho GTPases to regulate podocyte foot process maintenance. J. Am. Soc. Nephrol. 32, 563–579 (2021).
Bieling, P. & Rottner, K. From WRC to Arp2/3: collective molecular mechanisms of branched actin network assembly. Curr. Opin. Cell Biol. 80, 102156 (2023).
Schell, C. et al. ARP3 controls the podocyte architecture at the kidney filtration barrier. Dev. Cell 47, 741–757.e8 (2018).
Schell, C. et al. N-wasp is required for stabilization of podocyte foot processes. J. Am. Soc. Nephrol. 24, 713–721 (2013).
Pernier, J., Shekhar, S., Jegou, A., Guichard, B. & Carlier, M. F. Profilin interaction with actin filament barbed end controls dynamic instability, capping, branching, and motility. Dev. Cell 36, 201–214 (2016).
Tian, X. et al. Profilin1 is required for prevention of mitotic catastrophe in murine and human glomerular diseases. J. Clin. Invest. 133, e171237 (2023).
Bock, F. et al. Rac1 promotes kidney collecting duct repair by mechanically coupling cell morphology to mitotic entry. Sci. Adv. 10, eadi7840 (2024).
Wioland, H. et al. ADF/cofilin accelerates actin dynamics by severing filaments and promoting their depolymerization at both ends. Curr. Biol. 27, 1956–1967.e7 (2017).
Garg, P. et al. Actin-depolymerizing factor cofilin-1 is necessary in maintaining mature podocyte architecture. J. Biol. Chem. 285, 22676–22688 (2010).
Kuure, S. et al. Actin depolymerizing factors cofilin1 and destrin are required for ureteric bud branching morphogenesis. PLoS Genet. 6, e1001176 (2010).
Elias, B. C. et al. Cdc42 regulates epithelial cell polarity and cytoskeletal function during kidney tubule development. J. Cell Sci. 128, 4293–4305 (2015).
Bock, F. et al. Rac1 promotes kidney collecting duct integrity by limiting actomyosin activity. J. Cell Biol. 220, e202103080 (2021).
Sorce, B. et al. Mitotic cells contract actomyosin cortex and generate pressure to round against or escape epithelial confinement. Nat. Commun. 6, 8872 (2015).
Ramkumar, N. & Baum, B. Coupling changes in cell shape to chromosome segregation. Nat. Rev. Mol. Cell Biol. 17, 511–521 (2016).
Pham, T. D. et al. Angiotensin II acts through Rac1 to upregulate pendrin: role of NADPH oxidase. Am. J. Physiol. Renal Physiol. 326, F202–F218 (2024).
Ayuzawa, N. et al. Rac1 deficiency impairs postnatal development of the renal papilla. Sci. Rep. 12, 20310 (2022).
Pang, X. et al. Targeting integrin pathways: mechanisms and advances in therapy. Signal. Transduct. Target. Ther. 8, 1 (2023).
Shen, A. R. et al. Integrin, exosome and kidney disease. Front. Physiol. 11, 627800 (2020).
Tang, T. T. et al. Employing macrophage-derived microvesicle for kidney-targeted delivery of dexamethasone: an efficient therapeutic strategy against renal inflammation and fibrosis. Theranostics 9, 4740–4755 (2019).
Kumar, S. Cellular and molecular pathways of renal repair after acute kidney injury. Kidney Int. 93, 27–40 (2018).
Bonventre, J. V. & Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Invest. 121, 4210–4221 (2011).
Little, M. H. & Kairath, P. Does renal repair recapitulate kidney development? J. Am. Soc. Nephrol. 28, 34–46 (2017).
Okamura, D. M. et al. Spiny mice activate unique transcriptional programs after severe kidney injury regenerating organ function without fibrosis. iScience 24, 103269 (2021).
Korhonen, M., Ylanne, J., Laitinen, L. & Virtanen, I. The α1-α6 subunits of integrins are characteristically expressed in distinct segments of developing and adult human nephron. J. Cell Biol. 111, 1245–1254 (1990).
Voigt, S. et al. Distribution and quantification of α1-integrin subunit in rat organs. Histochem. J. 27, 123–132 (1995).
Patey, N., Halbwachs-Mecarelli, L., Droz, D., Lesavre, P. & Noel, L. H. Distribution of integrin subunits in normal human kidney. Cell Adhes. Commun. 2, 159–167 (1994).
Kuhara, T., Kagami, S. & Kuroda, Y. Expression of β1-integrins on activated mesangial cells in human glomerulonephritis. J. Am. Soc. Nephrol. 8, 1679–1687 (1997).
Rahilly, M. A. & Fleming, S. Differential expression of integrin α chains by renal epithelial cells. J. Pathol. 167, 327–334 (1992).
Acknowledgements
This work was supported in part by NIH grants K08 DK134879 (to F.B.), DK069921 (to R.Z.), DK088327 (to R.Z.), DK127589 (to R.Z.), R01 DK119212 (to A.P.), P30 DK114809 (to A.P.) and VA Merit awards I01-BX002196 (to R.Z.) and 1I01BX002025 (to A.P.). A.P. is the recipient of a Department of Veterans Affairs Senior Research Career Scientist Award IK6 BX005240. F.B. is supported by the Research Scholar Award by the Southern Society for Clinical Investigation. R.Z. is supported by a Keck Foundation Grant.
Author information
Authors and Affiliations
Contributions
F.B., S.L. and R.Z. researched data for the article. F.B., A.P. and R.Z. wrote the article. All authors contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors do not declare any conflicts of interest.
Peer review
Peer review information
Nature Reviews Nephrology thanks Shuta Ishibe and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bock, F., Li, S., Pozzi, A. et al. Integrins in the kidney — beyond the matrix. Nat Rev Nephrol 21, 157–174 (2025). https://doi.org/10.1038/s41581-024-00906-1
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41581-024-00906-1
This article is cited by
-
ncRNA-mediated ITGB1 upregulation correlates with poor prognosis and tumor-immune infiltration in gastric cancer
European Journal of Medical Research (2025)
-
Integrative analysis of Helicobacter pylori-driven stomach adenocarcinoma reveals epigenetic deregulation, immune evasion, and therapeutic resistance
Hereditas (2025)