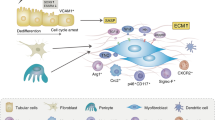
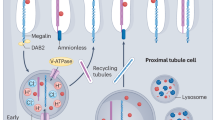
Abstract
Models of kidney injury have classically concentrated on glomeruli as the primary site of injury leading to glomerulosclerosis or on tubules as the primary site of injury leading to tubulointerstitial fibrosis. However, current evidence on the mechanisms of progression of chronic kidney disease indicates that a complex interplay between glomeruli and tubules underlies progressive kidney injury. Primary glomerular injury can clearly lead to subsequent tubule injury. For example, damage to the glomerular filtration barrier can expose tubular cells to serum proteins, including complement and cytokines, that would not be present in physiological conditions and can promote the development of tubulointerstitial fibrosis and progressive decline in kidney function. In addition, although less well-studied, increasing evidence suggests that tubule injury, whether primary or secondary, can also promote glomerular damage. This feedback from the tubule to the glomerulus might be mediated by changes in the reabsorptive capacity of the tubule, which can affect the glomerular filtration rate, or by mediators released by injured proximal tubular cells that can induce damage in both podocytes and parietal epithelial cells. Examining the crosstalk between the various compartments of the kidney is important for understanding the mechanisms underlying kidney pathology and identifying potential therapeutic interventions.
Key points
-
Primary glomerular injury can lead to the development of subsequent tubule injury that promotes the development of tubulointerstitial fibrosis and a progressive decline in kidney function.
-
Serum factors — for example, immunoglobulins, complement proteins and lipids — rather than albumin itself seem to be key mediators of proximal tubule dysfunction associated with high proteinuria.
-
Increasing evidence suggests that injurious factors released from injured tubules, or loss of protective factors, can feedback to the glomerulus, where they promote or exacerbate glomerular injury.
-
In models of acute kidney injury, glomerulosclerosis is attenuated in atubular glomeruli. This effect might be related to reduced filtration in the atubular glomeruli and less oxidative stress in podocytes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Striker, G. E., Schainuck, L. I., Cutler, R. E. & Benditt, E. P. Structural-functional correlations in renal disease. I. A method for assaying and classifying histopathologic changes in renal disease. Hum. Pathol. 1, 615–630 (1970).
Schainuck, L. I., Striker, G. E., Cutler, R. E. & Benditt, E. P. Structural-functional correlations in renal disease. II. The correlations. Hum. Pathol. 1, 631–641 (1970).
Brenner, B. M., Goldszer, R. C. & Hostetter, T. H. Glomerular response to renal injury. Contrib. Nephrol. 33, 48–66 (1982).
Ferenbach, D. A. & Bonventre, J. V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat. Rev. Nephrol. 11, 264–276 (2015).
Basile, D. P. et al. Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J. Am. Soc. Nephrol. 27, 687–697 (2016).
Basile, D. P., Anderson, M. D. & Sutton, T. A. Pathophysiology of acute kidney injury. Compr. Physiol. 2, 1303–1353 (2012).
Harris, R. C., Seifter, J. L. & Brenner, B. M. Adaptation of Na+-H+ exchange in renal microvillus membrane vesicles. Role of dietary protein and uninephrectomy. J. Clin. Invest. 74, 1979–1987 (1984).
Liu, H. et al. Epigenomic and transcriptomic analyses define core cell types, genes and targetable mechanisms for kidney disease. Nat. Genet. 54, 950–962 (2022).
Qiu, C. et al. Renal compartment-specific genetic variation analyses identify new pathways in chronic kidney disease. Nat. Med. 24, 1721–1731 (2018).
Russo, L. M. et al. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J. Am. Soc. Nephrol. 20, 489–494 (2009).
Haymann, J. P. et al. Characterization and localization of the neonatal Fc receptor in adult human kidney. J. Am. Soc. Nephrol. 11, 632–639 (2000).
Nielsen, R., Christensen, E. I. & Birn, H. Megalin and cubilin in proximal tubule protein reabsorption: from experimental models to human disease. Kidney Int. 89, 58–67 (2016).
Park, C. H. & Maack, T. Albumin absorption and catabolism by isolated perfused proximal convoluted tubules of the rabbit. J. Clin. Invest. 73, 767–777 (1984).
Gudehithlu, K. P., Pegoraro, A. A., Dunea, G., Arruda, J. A. & Singh, A. K. Degradation of albumin by the renal proximal tubule cells and the subsequent fate of its fragments. Kidney Int. 65, 2113–2122 (2004).
Molitoris, B. A. & Wagner, M. C. Is albumin toxic to the kidney: it depends? Clin. J. Am. Soc. Nephrol. 18, 1222–1224 (2023).
Bedin, M. et al. Human C-terminal CUBN variants associate with chronic proteinuria and normal renal function. J. Clin. Invest. 130, 335–344 (2020).
Molitoris, B. A., Sandoval, R. M., Yadav, S. P. S. & Wagner, M. C. Albumin uptake and processing by the proximal tubule: physiological, pathological, and therapeutic implications. Physiol. Rev. 102, 1625–1667 (2022).
Weyer, K. et al. Abolishment of proximal tubule albumin endocytosis does not affect plasma albumin during nephrotic syndrome in mice. Kidney Int. 93, 335–342 (2018).
Birn, H., Nielsen, R. & Weyer, K. Tubular albumin uptake: is there evidence for a quantitatively important, receptor-independent mechanism? Kidney Int. 104, 1069–1073 (2023).
Kantarci, S. et al. Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes. Nat. Genet. 39, 957–959 (2007).
Charlton, J. R. et al. Beyond the tubule: pathological variants of LRP2, encoding the megalin receptor, result in glomerular loss and early progressive chronic kidney disease. Am. J. Physiol. Renal Physiol. 319, F988–F999 (2020).
Faivre, A. et al. Spatiotemporal landscape of kidney tubular responses to glomerular proteinuria. J. Am. Soc. Nephrol. 35, 854–869 (2024).
Langelueddecke, C. et al. Lipocalin-2 (24p3/neutrophil gelatinase-associated lipocalin (NGAL)) receptor is expressed in distal nephron and mediates protein endocytosis. J. Biol. Chem. 287, 159–169 (2012).
Dizin, E. et al. Albuminuria induces a proinflammatory and profibrotic response in cortical collecting ducts via the 24p3 receptor. Am. J. Physiol. Renal Physiol. 305, F1053–F1063 (2013).
Hinrichs, G. R. et al. Urokinase-type plasminogen activator contributes to amiloride-sensitive sodium retention in nephrotic range glomerular proteinuria in mice. Acta Physiol. 227, e13362 (2019).
Svenningsen, P. et al. Plasmin in nephrotic urine activates the epithelial sodium channel. J. Am. Soc. Nephrol. 20, 299–310 (2009).
Bohnert, B. N. et al. Urokinase-type plasminogen activator (uPA) is not essential for epithelial sodium channel (ENaC)-mediated sodium retention in experimental nephrotic syndrome. Acta Physiol. 227, e13286 (2019).
Staehr, M. et al. Aberrant glomerular filtration of urokinase-type plasminogen activator in nephrotic syndrome leads to amiloride-sensitive plasminogen activation in urine. Am. J. Physiol. Renal Physiol. 309, F235–F241 (2015).
Xiao, M. et al. Plasminogen deficiency does not prevent sodium retention in a genetic mouse model of experimental nephrotic syndrome. Acta Physiol. 231, e13512 (2021).
Zoja, C., Benigni, A. & Remuzzi, G. Cellular responses to protein overload: key event in renal disease progression. Curr. Opin. Nephrol. Hypertens. 13, 31–37 (2004).
Lidberg, K. A. et al. Serum protein exposure activates a core regulatory program driving human proximal tubule injury. J. Am. Soc. Nephrol. 33, 949–965 (2022).
Morais, C., Westhuyzen, J., Metharom, P. & Healy, H. High molecular weight plasma proteins induce apoptosis and Fas/FasL expression in human proximal tubular cells. Nephrol. Dial. Transpl. 20, 50–58 (2005).
Mackinnon, B. et al. Urinary transferrin, high molecular weight proteinuria and the progression of renal disease. Clin. Nephrol. 59, 252–258 (2003).
Kalim, S. et al. Protein carbamylation and chronic kidney disease progression in the Chronic Renal Insufficiency Cohort Study. Nephrol. Dial. Transpl. 37, 139–147 (2021).
Noels, H. et al. Post-translational modifications in kidney diseases and associated cardiovascular risk. Nat. Rev. Nephrol. 20, 495–512 (2024).
Yadav, S. P. S. et al. Mechanism of how carbamylation reduces albumin binding to FcRn contributing to increased vascular clearance. Am. J. Physiol. Renal Physiol. 320, F114–F129 (2021).
Figueroa, S. M. et al. Oxidized albumin as a mediator of kidney disease. Antioxidants 10, 404 (2021).
Mishra, V. & Heath, R. J. Structural and biochemical features of human serum albumin essential for eukaryotic cell culture. Int. J. Mol. Sci. 22, 8411 (2021).
Afshinnia, F. et al. Increased lipogenesis and impaired β-oxidation predict type 2 diabetic kidney disease progression in American Indians. JCI Insight 4, e130317 (2019).
Arici, M., Chana, R., Lewington, A., Brown, J. & Brunskill, N. J. Stimulation of proximal tubular cell apoptosis by albumin-bound fatty acids mediated by peroxisome proliferator activated receptor-γ. J. Am. Soc. Nephrol. 14, 17–27 (2003).
Kamijo, A. et al. Urinary free fatty acids bound to albumin aggravate tubulointerstitial damage. Kidney Int. 62, 1628–1637 (2002).
Khan, S. et al. Kidney proximal tubule lipoapoptosis is regulated by fatty acid transporter-2 (FATP2). J. Am. Soc. Nephrol. 29, 81–91 (2018).
Khan, S. et al. Lipotoxic disruption of NHE1 interaction with PI(4,5)P2 expedites proximal tubule apoptosis. J. Clin. Invest. 124, 1057–1068 (2014).
Mitrofanova, A., Merscher, S. & Fornoni, A. Kidney lipid dysmetabolism and lipid droplet accumulation in chronic kidney disease. Nat. Rev. Nephrol. 19, 629–645 (2023).
Long, K. R., Rbaibi, Y., Gliozzi, M. L., Ren, Q. & Weisz, O. A. Differential kidney proximal tubule cell responses to protein overload by albumin and its ligands. Am. J. Physiol. Renal Physiol. 318, F851–F859 (2020).
Sun, H. et al. Nonesterified free fatty acids enhance the inflammatory response in renal tubules by inducing extracellular ATP release. Am. J. Physiol. Renal Physiol. 319, F292–F303 (2020).
Khan, S. et al. Fatty acid transport protein-2 regulates glycemic control and diabetic kidney disease progression. JCI Insight 5, e136845 (2020).
Schelling, J. R. The contribution of lipotoxicity to diabetic kidney disease. Cells 11, 114465 (2022).
Chen, Y. et al. Involvement of FATP2-mediated tubular lipid metabolic reprogramming in renal fibrogenesis. Cell Death Dis. 11, 994 (2020).
Mori, Y. et al. KIM-1 mediates fatty acid uptake by renal tubular cells to promote progressive diabetic kidney disease. Cell Metab. 33, 1042–1061.e7 (2021).
Kon, V., Yang, H. C., Smith, L. E., Vickers, K. C. & Linton, M. F. High-density lipoproteins in kidney disease. Int. J. Mol. Sci. 22, 8201 (2021).
Morita, Y. et al. The role of complement in the pathogenesis of tubulointerstitial lesions in rat mesangial proliferative glomerulonephritis. J. Am. Soc. Nephrol. 8, 1363–1372 (1997).
Nomura, A. et al. Role of complement in acute tubulointerstitial injury of rats with aminonucleoside nephrosis. Am. J. Pathol. 151, 539–547 (1997).
Nangaku, M., Pippin, J. & Couser, W. G. Complement membrane attack complex (C5b-9) mediates interstitial disease in experimental nephrotic syndrome. J. Am. Soc. Nephrol. 10, 2323–2331 (1999).
Rangan, G. K., Pippin, J. W., Coombes, J. D. & Couser, W. G. C5b-9 does not mediate chronic tubulointerstitial disease in the absence of proteinuria. Kidney Int. 67, 492–503 (2005).
Rangan, G. K. C5b-9 does not mediate tubulointerstitial injury in experimental acute glomerular disease characterized by selective proteinuria. World J. Nephrol. 5, 288–299 (2016).
Clark, E. C., Nath, K. A., Hostetter, M. K. & Hostetter, T. H. Role of ammonia in tubulointerstitial injury. Min. Electrolyte Metab. 16, 315–321 (1990).
Worn, M. et al. Proteasuria in nephrotic syndrome-quantification and proteomic profiling. J. Proteom. 230, 103981 (2021).
Branten, A. J., Kock-Jansen, M., Klasen, I. S. & Wetzels, J. F. Urinary excretion of complement C3d in patients with renal diseases. Eur. J. Clin. Invest. 33, 449–456 (2003).
Timmerman, J. J. et al. Extrahepatic C6 is as effective as hepatic C6 in the generation of renal C5b-9 complexes. Kidney Int. 51, 1788–1796 (1997).
Isaksson, G. L. et al. Urine excretion of C3dg and sC5b-9 coincide with proteinuria and development of preeclampsia in pregnant women with type-1 diabetes. J. Hypertens. 41, 223–232 (2023).
Isaksson, G. L. et al. Proteinuria is accompanied by intratubular complement activation and apical membrane deposition of C3dg and C5b-9 in kidney transplant recipients. Am. J. Physiol. Renal Physiol. 322, F150–F163 (2022).
Chen, S. J., Lv, L. L., Liu, B. C. & Tang, R. N. Crosstalk between tubular epithelial cells and glomerular endothelial cells in diabetic kidney disease. Cell Prolif. 53, e12763 (2020).
Munkonda, M. N. et al. Podocyte-derived microparticles promote proximal tubule fibrotic signaling via p38 MAPK and CD36. J. Extracell. Vesicles 7, 1432206 (2018).
Leung, J. C. K., Lai, K. N. & Tang, S. C. W. Role of mesangial-podocytic-tubular cross-talk in IgA nephropathy. Semin. Nephrol. 38, 485–495 (2018).
Zhang, J. et al. Role of human mesangial-tubular crosstalk in secretory IgA-induced IgA nephropathy. Kidney Blood Press. Res. 46, 286–297 (2021).
Jeon, J. S. et al. microRNA in extracellular vesicles released by damaged podocytes promote apoptosis of renal tubular epithelial cells. Cells 9, 1409 (2020).
Rana, R. et al. Glomerular-tubular crosstalk via cold shock Y-box binding protein-1 in the kidney. Kidney Int. 105, 65–83 (2024).
Miao, Z. et al. Single cell regulatory landscape of the mouse kidney highlights cellular differentiation programs and disease targets. Nat. Commun. 12, 2277 (2021).
Muto, Y. et al. Single cell transcriptional and chromatin accessibility profiling redefine cellular heterogeneity in the adult human kidney. Nat. Commun. 12, 2190 (2021).
Nolin, A. C. et al. Proteinuria causes dysfunctional autophagy in the proximal tubule. Am. J. Physiol. Renal Physiol. 311, F1271–F1279 (2016).
Zhuang, Y. et al. Mitochondrial dysfunction confers albumin-induced NLRP3 inflammasome activation and renal tubular injury. Am. J. Physiol. Renal Physiol. 308, F857–F866 (2015).
Doke, T. & Susztak, K. The multifaceted role of kidney tubule mitochondrial dysfunction in kidney disease development. Trends Cell Biol. 32, 841–853 (2022).
Abbate, M., Zoja, C. & Remuzzi, G. How does proteinuria cause progressive renal damage? J. Am. Soc. Nephrol. 17, 2974–2984 (2006).
de Seigneux, S. et al. Proteinuria increases plasma phosphate by altering its tubular handling. J. Am. Soc. Nephrol. 26, 1608–1618 (2015).
Hu, J. et al. Hypoxia inducible factor-1α mediates the profibrotic effect of albumin in renal tubular cells. Sci. Rep. 7, 15878 (2017).
Wilson, P. C. et al. Multimodal single cell sequencing implicates chromatin accessibility and genetic background in diabetic kidney disease progression. Nat. Commun. 13, 5253 (2022).
Kirita, Y., Wu, H., Uchimura, K., Wilson, P. C. & Humphreys, B. D. Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc. Natl Acad. Sci. USA 117, 15874–15883 (2020).
Dhillon, P. et al. The nuclear receptor ESRRA protects from kidney disease by coupling metabolism and differentiation. Cell Metab. 33, 379–394.e8 (2021).
Gerhardt, L. M. S., Liu, J., Koppitch, K., Cippa, P. E. & McMahon, A. P. Single-nuclear transcriptomics reveals diversity of proximal tubule cell states in a dynamic response to acute kidney injury. Proc. Natl Acad. Sci. USA 118, e2026684118 (2021).
Aggarwal, S. et al. SOX9 switch links regeneration to fibrosis at the single-cell level in mammalian kidneys. Science 383, eadd6371 (2024).
Yang, L., Besschetnova, T. Y., Brooks, C. R., Shah, J. V. & Bonventre, J. V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 16, 535–543 (2010).
Taguchi, K. et al. Cyclin G1 induces maladaptive proximal tubule cell dedifferentiation and renal fibrosis through CDK5 activation. J. Clin. Invest 132, e158096 (2022).
Coca, S. G., Singanamala, S. & Parikh, C. R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 81, 442–448 (2012).
Chawla, L. S., Eggers, P. W., Star, R. A. & Kimmel, P. L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 371, 58–66 (2014).
Palant, C. E., Amdur, R. L. & Chawla, L. S. Long-term consequences of acute kidney injury in the perioperative setting. Curr. Opin. Anaesthesiol. 30, 100–104 (2017).
Wang, J., Zhong, J., Yang, H. C. & Fogo, A. B. Cross talk from tubules to glomeruli. Toxicol. Pathol. 46, 944–948 (2018).
Jones, J. et al. Association of complete recovery from acute kidney injury with incident CKD stage 3 and all-cause mortality. Am. J. Kidney Dis. 60, 402–408 (2012).
Harris, R. C. & Zhang, M. Z. Cyclooxygenase metabolites in the kidney. Compr. Physiol. 1, 1729–1758 (2011).
Capasso, G. A new cross-talk pathway between the renal tubule and its own glomerulus. Kidney Int. 71, 1087–1089 (2007).
Sen, T. & Heerspink, H. J. L. A kidney perspective on the mechanism of action of sodium glucose co-transporter 2 inhibitors. Cell Metab. 33, 732–739 (2021).
Tuttle, K. R. Digging deep into cells to find mechanisms of kidney protection by SGLT2 inhibitors. J. Clin. Invest. 133, e167700 (2023).
Nishiyama, A. & Kitada, K. Possible renoprotective mechanisms of SGLT2 inhibitors. Front. Med. 10, 1115413 (2023).
Billing, A. M. et al. Metabolic communication by SGLT2 inhibition. Circulation 149, 860–884 (2024).
Albalawy, W. N. et al. SGLT2-independent effects of canagliflozin on NHE3 and mitochondrial complex I activity inhibit proximal tubule fluid transport and albumin uptake. Am. J. Physiol. Renal Physiol. 326, F1041–F1053 (2024).
Zhang, Y., Thai, K., Kepecs, D. M. & Gilbert, R. E. Sodium-glucose linked cotransporter-2 inhibition does not attenuate disease progression in the rat remnant kidney model of chronic kidney disease. PLoS ONE 11, e0144640 (2016).
Zhu, Z. et al. Finerenone added to RAS/SGLT2 blockade for CKD in Alport syndrome. results of a randomized controlled trial with Col4a3-/- Mice. J. Am. Soc. Nephrol. 34, 1513–1520 (2023).
Schaub, J. A. et al. SGLT2 inhibitors mitigate kidney tubular metabolic and mTORC1 perturbations in youth-onset type 2 diabetes. J. Clin. Invest. 133, e164486 (2023).
Inoki, K. et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J. Clin. Invest. 121, 2181–2196 (2011).
Godel, M. et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J. Clin. Invest. 121, 2197–2209 (2011).
Packer, M. Critical reanalysis of the mechanisms underlying the cardiorenal benefits of SGLT2 inhibitors and reaffirmation of the nutrient deprivation signaling/autophagy hypothesis. Circulation 146, 1383–1405 (2022).
Haase, V. H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 27, 41–53 (2013).
Grgic, I. et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 82, 172–183 (2012).
Gao, L. et al. Restoration of E-cadherin by PPBICA protects against cisplatin-induced acute kidney injury by attenuating inflammation and programmed cell death. Lab. Invest. 98, 911–923 (2018).
Xiong, C. et al. Pharmacological inhibition of Src kinase protects against acute kidney injury in a murine model of renal ischemia/reperfusion. Oncotarget 8, 31238–31253 (2017).
Tan, R. J. et al. Tubular injury triggers podocyte dysfunction by β-catenin-driven release of MMP-7. JCI Insight 4, e122399 (2019).
Ma, Y. et al. Paracrine effects of renal proximal tubular epithelial cells on podocyte injury under hypoxic conditions are mediated by arginase-II and TGF-β1. Int J. Mol. Sci. 24, 3587 (2023).
Lim, B. J. et al. Tubulointerstitial fibrosis can sensitize the kidney to subsequent glomerular injury. Kidney Int. 92, 1395–1403 (2017).
Zou, J. et al. Stabilization of hypoxia-inducible factor ameliorates glomerular injury sensitization after tubulointerstitial injury. Kidney Int. 99, 620–631 (2021).
Xu, C. et al. Renal tubular Bim mediates the tubule-podocyte crosstalk via NFAT2 to induce podocyte cytoskeletal dysfunction. Theranostics 10, 6806–6824 (2020).
Han, X. et al. Targeting Sirtuin1 to treat aging-related tissue fibrosis: from prevention to therapy. Pharmacol. Ther. 229, 107983 (2022).
Hasegawa, K. et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat. Med. 19, 1496–1504 (2013).
Hasegawa, K. et al. Communication from tubular epithelial cells to podocytes through sirt1 and nicotinic acid metabolism. Curr. Hypertens. Rev. 12, 95–104 (2016).
Yasuda, I. et al. Pre-emptive short-term nicotinamide mononucleotide treatment in a mouse model of diabetic nephropathy. J. Am. Soc. Nephrol. 32, 1355–1370 (2021).
Jones, B. A. et al. Nicotinamide riboside activates renal metabolism and protects the kidney in a model of Alport syndrome. Preprint at bioRxiv https://doi.org/10.1101/2024.02.26.580911 (2024).
Bonventre, J. V. Primary proximal tubule injury leads to epithelial cell cycle arrest, fibrosis, vascular rarefaction, and glomerulosclerosis. Kidney Int. Suppl. 4, 39–44 (2014).
Lasagni, L. & Romagnani, P. Glomerular epithelial stem cells: the good, the bad, and the ugly. J. Am. Soc. Nephrol. 21, 1612–1619 (2010).
Smeets, B. et al. Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J. Am. Soc. Nephrol. 22, 1262–1274 (2011).
Kaverina, N. V. et al. Dual lineage tracing shows that glomerular parietal epithelial cells can transdifferentiate toward the adult podocyte fate. Kidney Int. 96, 597–611 (2019).
Shankland, S. J., Pippin, J. W. & Duffield, J. S. Progenitor cells and podocyte regeneration. Semin. Nephrol. 34, 418–428 (2014).
Kuppe, C. et al. Novel parietal epithelial cell subpopulations contribute to focal segmental glomerulosclerosis and glomerular tip lesions. Kidney Int. 96, 80–93 (2019).
Appel, D. et al. Recruitment of podocytes from glomerular parietal epithelial cells. J. Am. Soc. Nephrol. 20, 333–343 (2009).
Berger, K. & Moeller, M. J. Mechanisms of epithelial repair and regeneration after acute kidney injury. Semin. Nephrol. 34, 394–403 (2014).
Angelotti, M. L. et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cell 30, 1714–1725 (2012).
Ronconi, E. et al. Regeneration of glomerular podocytes by human renal progenitors. J. Am. Soc. Nephrol. 20, 322–332 (2009).
Schulte, K. et al. Origin of parietal podocytes in atubular glomeruli mapped by lineage tracing. J. Am. Soc. Nephrol. 25, 129–141 (2014).
Pippin, J. W. et al. Cells of renin lineage are progenitors of podocytes and parietal epithelial cells in experimental glomerular disease. Am. J. Pathol. 183, 542–557 (2013).
Kaverina, N. V. et al. Tracking the stochastic fate of cells of the renin lineage after podocyte depletion using multicolor reporters and intravital imaging. PLoS ONE 12, e0173891 (2017).
Martinez, M. F. et al. Super-enhancers maintain renin-expressing cell identity and memory to preserve multi-system homeostasis. J. Clin. Invest. 128, 4787–4803 (2018).
Risdon, R. A., Sloper, J. C. & De Wardener, H. E. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 2, 363–366 (1968).
Welch, W. J., Baumgartl, H., Lubbers, D. & Wilcox, C. S. Nephron pO2 and renal oxygen usage in the hypertensive rat kidney. Kidney Int. 59, 230–237 (2001).
Rosenberger, C., Rosen, S., Paliege, A. & Heyman, S. N. Pimonidazole adduct immunohistochemistry in the rat kidney: detection of tissue hypoxia. Methods Mol. Biol. 466, 161–174 (2009).
Palm, F. & Nordquist, L. Renal oxidative stress, oxygenation, and hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R1229–R1241 (2011).
Fine, L. G., Orphanides, C. & Norman, J. T. Progressive renal disease: the chronic hypoxia hypothesis. Kidney Int. Suppl. 65, S74–S78 (1998).
Manotham, K. et al. Transdifferentiation of cultured tubular cells induced by hypoxia. Kidney Int. 65, 871–880 (2004).
Mimura, I. & Nangaku, M. The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nat. Rev. Nephrol. 6, 667–678 (2010).
Haase, V. H. Hypoxia-inducible factors in the kidney. Am. J. Physiol. Renal Physiol. 291, F271–F281 (2006).
Haase, V. H. Pathophysiological consequences of HIF activation: HIF as a modulator of fibrosis. Ann. N. Y. Acad. Sci. 1177, 57–65 (2009).
Sugahara, M., Tanaka, T. & Nangaku, M. Hypoxia-inducible factor and oxygen biology in the kidney. Kidney360 1, 1021–1031 (2020).
Kaelin, W. G. Jr. & Ratcliffe, P. J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 (2008).
Nordquist, L. et al. Activation of hypoxia-inducible factors prevents diabetic nephropathy. J. Am. Soc. Nephrol. 26, 328–338 (2015).
Bernhardt, W. M. et al. Involvement of hypoxia-inducible transcription factors in polycystic kidney disease. Am. J. Pathol. 170, 830–842 (2007).
Higgins, D. F. et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Invest. 117, 3810–3820 (2007).
Higgins, D. F., Kimura, K., Iwano, M. & Haase, V. H. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle 7, 1128–1132 (2008).
Rosenberger, C. et al. Immunohistochemical detection of hypoxia-inducible factor-1α in human renal allograft biopsies. J. Am. Soc. Nephrol. 18, 343–351 (2007).
Li, Z. et al. Chromatin-accessibility estimation from single-cell ATAC-seq data with scOpen. Nat. Commun. 12, 6386 (2021).
Zhang, Y. et al. Endothelial progenitor cells-derived exosomal microRNA-21-5p alleviates sepsis-induced acute kidney injury by inhibiting RUNX1 expression. Cell Death Dis. 12, 335 (2021).
Chen, Q., Guan, X., Zuo, X., Wang, J. & Yin, W. The role of high mobility group box 1 (HMGB1) in the pathogenesis of kidney diseases. Acta Pharm. Sin. B 6, 183–188 (2016).
Grigoryev, D. N. et al. The local and systemic inflammatory transcriptome after acute kidney injury. J. Am. Soc. Nephrol. 19, 547–558 (2008).
Lie, M. L. et al. Lung T lymphocyte trafficking and activation during ischemic acute kidney injury. J. Immunol. 189, 2843–2851 (2012).
Ba Aqeel, S. H., Sanchez, A. & Batlle, D. Angiotensinogen as a biomarker of acute kidney injury. Clin. Kidney J. 10, 759–768 (2017).
Marcussen, N. Atubular glomeruli in cisplatin-induced chronic interstitial nephropathy. An experimental stereological investigation. APMIS 98, 1087–1097 (1990).
Marcussen, N. & Olsen, T. S. Atubular glomeruli in patients with chronic pyelonephritis. Lab. Invest. 62, 467–473 (1990).
Najafian, B., Kim, Y., Crosson, J. T. & Mauer, M. Atubular glomeruli and glomerulotubular junction abnormalities in diabetic nephropathy. J. Am. Soc. Nephrol. 14, 908–917 (2003).
Forbes, M. S., Thornhill, B. A. & Chevalier, R. L. Proximal tubular injury and rapid formation of atubular glomeruli in mice with unilateral ureteral obstruction: a new look at an old model. Am. J. Physiol. Renal Physiol. 301, F110–F117 (2011).
Gandhi, M., Olson, J. L. & Meyer, T. W. Contribution of tubular injury to loss of remnant kidney function. Kidney Int. 54, 1157–1165 (1998).
Tanner, G. A., Gretz, N., Connors, B. A., Evan, A. P. & Steinhausen, M. Role of obstruction in autosomal dominant polycystic kidney disease in rats. Kidney Int. 50, 873–886 (1996).
Yang, H. C. et al. Spatial transcriptomic analysis reveals altered gene expression in glomerular parietal epithelial cells following tubular injury. ASN Kidney Week 34, 315 (2023).
Acknowledgements
These studies were supported by NIH grants DK51265, DK95785, DK62794 (R.C.H.), the VA Merit Award 00507969 (R.C.H.), the NIH grant DK56942 (A.F.) and The Vanderbilt Center for Kidney Disease.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Zheng Dong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fogo, A.B., Harris, R.C. Crosstalk between glomeruli and tubules. Nat Rev Nephrol 21, 189–199 (2025). https://doi.org/10.1038/s41581-024-00907-0
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41581-024-00907-0