Abstract
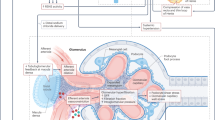
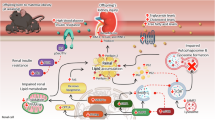
Obesity is associated with severe consequences for the renal system, including chronic kidney disease, kidney failure and increased mortality. Obesity has both direct and indirect effects on kidney health through several mechanisms, including activation of the renin–angiotensin system, mechanical compression, inflammation, fibrosis, increased filtration barrier permeability and renal nerve activity. The expansion of adipose tissue through hypertrophy and hyperplasia can induce haemodynamic changes that promote glomerular hyperfiltration to compensate for the greater metabolic demands of the increased body weight. Adipose expansion is also associated with the release of adipokines and pro-inflammatory cytokines, hyperinsulinaemia and insulin resistance, which exert direct and indirect effects on kidney function via various mechanisms. Increased uptake of fatty acids by the kidney leads to alterations in lipid metabolism and lipotoxicity, also contributing to the pro-inflammatory and pro-fibrotic environment. The role of the adipose tissue–brain–kidney axis in the obesity-associated decline in renal function is sustained by studies showing that stimulation of adipose tissue sensory neurons by locally released factors increases renal sympathetic nerve activity. Conversely, pre-existent kidney disease can contribute to adipose dysfunction through the accumulation of uraemic toxins and hormonal changes. These findings highlight the importance of crosstalk between adipose tissue and the kidneys and provide insights into the mechanisms underlying the associations between obesity and kidney disease.
Key points
-
The mechanisms by which adipose tissue exerts pathogenic effects on the kidney structure and function are multifactorial and include mechanical compression, haemodynamic effects, alterations in fatty acid metabolism, lipotoxicity, oxidative stress, inflammation and altered adipokine release.
-
In the context of obesity and metabolic disease, sensory neurons that innervate adipose tissue are stimulated by numerous locally released factors, sending signals to brain areas that increase the sympathetic outflow to the kidneys.
-
Elevated levels of uraemic toxins in patients with chronic kidney disease (CKD) promote adipose tissue loss and the ectopic accumulation of lipids, which in turn may contribute to worsening kidney function.
-
The exploration of therapeutic targets to down-modulate activity of the adipose afferent reflex could be a potential approach to reducing obesity-associated CKD.
-
Improved understanding of the pathological interactions between adipose tissue and the kidneys in the context of obesity and CKD will aid the identification of new renoprotective targets; drug-based, surgical and lifestyle interventions are under investigation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Centers for Disease Control and Prevention. Chronic kidney disease in the United States, 2023. https://www.cdc.gov/kidneydisease/publications-resources/CKD-national-facts.html (2024).
Martínez-Montoro, J. I., Morales, E., Cornejo-Pareja, I., Tinahones, F. J. & Fernández-García, J. C. Obesity-related glomerulopathy: current approaches and future perspectives. Obes. Rev. 23, e13450 (2022).
Friedman, A. N., Ogden, C. L. & Hales, C. M. Prevalence of obesity and CKD among adults in the United States, 2017–2020. Kidney Med. 5, 100568 (2023).
Jiang, Z. et al. Obesity and chronic kidney disease. Am. J. Physiol. Endocrinol. Metab. 324, E24–E41 (2023).
Virtue, S. & Vidal-Puig, A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome — an allostatic perspective. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1801, 338–349 (2010).
D’Agati, V. D. et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 12, 453–471 (2016).
Hall, J. E., do Carmo, J. M., da Silva, A. A., Wang, Z. & Hall, M. E. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat. Rev. Nephrol. 15, 367–385 (2019).
de Vries, A. P. et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2, 417–426 (2014).
Chen, H. M. et al. Podocyte lesions in patients with obesity-related glomerulopathy. Am. J. Kidney Dis. 48, 772–779 (2006).
Kravets, I. & Mallipattu, S. K. The role of podocytes and podocyte-associated biomarkers in diagnosis and treatment of diabetic kidney disease. J. Endocr. Soc. 4, bvaa029 (2020).
Trimarchi, H. Mechanisms of podocyte detachment, podocyturia, and risk of progression of glomerulopathies. Kidney Dis. 6, 324–329 (2020).
Yang, C. et al. Research progress on multiple cell death pathways of podocytes in diabetic kidney disease. Mol. Med. 29, 135 (2023).
Yau, K., Kuah, R., Cherney, D. Z. I. & Lam, T. K. T. Obesity and the kidney: mechanistic links and therapeutic advances. Nat. Rev. Endocrinol. 20, 321–335 (2024).
Tsuboi, N., Okabayashi, Y., Shimizu, A. & Yokoo, T. The renal pathology of obesity. Kidney Int. Rep. 2, 251–260 (2017).
Zeisberg, M. & Neilson, E. G. Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 21, 1819–1834 (2010).
Czaja-Stolc, S., Potrykus, M., Stankiewicz, M., Kaska, Ł. & Małgorzewicz, S. Pro-inflammatory profile of adipokines in obesity contributes to pathogenesis, nutritional disorders, and cardiovascular risk in chronic kidney disease. Nutrients 14, 1457 (2022).
Koenen, M., Hill, M. A., Cohen, P. & Sowers, J. R. Obesity, adipose tissue and vascular dysfunction. Circ. Res. 128, 951–968 (2021).
Couch, C. A., Fowler, L. A., Goss, A. M. & Gower, B. A. Associations of renal sinus fat with blood pressure and ectopic fat in a diverse cohort of adults. Int. J. Cardiol. Cardiovasc. Risk Prev. 16, 200165 (2023).
D’Marco, L. et al. Perirenal fat thickness is associated with metabolic risk factors in patients with chronic kidney disease. Kidney Res. Clin. Pract. 38, 365–372 (2019).
Spit, K. A. et al. Renal sinus fat and renal hemodynamics: a cross-sectional analysis. MAGMA 33, 73–80 (2020).
Qiu, X. et al. The role of perirenal adipose tissue deposition in chronic kidney disease progression: mechanisms and therapeutic implications. Life Sci. 352, 122866 (2024).
Huang, N. et al. Novel insight into perirenal adipose tissue: a neglected adipose depot linking cardiovascular and chronic kidney disease. World J. Diabetes 11, 115–125 (2020).
Hammoud, S. H., AlZaim, I., Al-Dhaheri, Y., Eid, A. H. & El-Yazbi, A. F. Perirenal adipose tissue inflammation: novel insights linking metabolic dysfunction to renal diseases. Front. Endocrinol. 12, 707126 (2021).
Kotsis, V., Martinez, F., Trakatelli, C. & Redon, J. Impact of obesity in kidney diseases. Nutrients 13, 4482 (2021).
Hall, J. E., do Carmo, J. M., da Silva, A. A., Wang, Z. & Hall, M. E. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ. Res. 116, 991–1006 (2015).
Mende, C. & Einhorn, D. Fatty kidney disease: the importance of ectopic fat deposition and the potential value of imaging. J. Diabetes 14, 73–78 (2022).
Castro, B. B. A., Foresto-Neto, O., Saraiva-Camara, N. O. & Sanders-Pinheiro, H. Renal lipotoxicity: insights from experimental models. Clin. Exp. Pharmacol. Physiol. 48, 1579–1588 (2021).
Mitrofanova, A., Merscher, S. & Fornoni, A. Kidney lipid dysmetabolism and lipid droplet accumulation in chronic kidney disease. Nat. Rev. Nephrol. 19, 629–645 (2023).
Mount, P., Davies, M., Choy, S. W., Cook, N. & Power, D. Obesity-related chronic kidney disease — the role of lipid metabolism. Metabolites 5, 720–732 (2015).
Hou, Y. et al. Mitochondrial oxidative damage reprograms lipid metabolism of renal tubular epithelial cells in the diabetic kidney. Cell Mol. Life Sci. 81, 23 (2024).
Yuan, Q., Tang, B. & Zhang, C. Signaling pathways of chronic kidney diseases, implications for therapeutics. Signal. Transduct. Target. Ther. 7, 182 (2022).
Zatterale, F. et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front. Physiol. 10, 1607 (2019).
Samovski, D., Jacome-Sosa, M. & Abumrad, N. A. Fatty acid transport and signaling: mechanisms and physiological implications. Annu. Rev. Physiol. 85, 317–337 (2023).
Hua, W. et al. CD36-mediated podocyte lipotoxicity promotes foot process effacement. Open Med. 19, 20240918 (2024).
Zhang, D. et al. Short-chain fatty acids in diseases. Cell Commun. Signal. 21, 212 (2023).
Yang, X. et al. CD36 in chronic kidney disease: novel insights and therapeutic opportunities. Nat. Rev. Nephrol. 13, 769–781 (2017).
Mori, Y. et al. KIM-1 mediates fatty acid uptake by renal tubular cells to promote progressive diabetic kidney disease. Cell Metab. 33, 1042–1061.e7 (2021).
Zhao, X. et al. Kidney injury molecule-1 is upregulated in renal lipotoxicity and mediates palmitate-induced tubular cell injury and inflammatory response. Int. J. Mol. Sci. 20, 3406 (2019).
Gai, Z. et al. Lipid accumulation and chronic kidney disease. Nutrients 11, 629–645 (2019).
Zhao, J. et al. CD36-mediated lipid accumulation and activation of nlrp3 inflammasome lead to podocyte injury in obesity-related glomerulopathy. Mediators Inflamm. 2019, 3172647 (2019).
Mukhi, D. et al. ACSS2 gene variants determine kidney disease risk by controlling de novo lipogenesis in kidney tubules. J. Clin. Invest. 134, e172963 (2023).
Cassis, L. A., Police, S. B., Yiannikouris, F. & Thatcher, S. E. Local adipose tissue renin-angiotensin system. Curr. Hypertens. Rep. 10, 93–98 (2008).
Schutten, M. T., Houben, A. J., de Leeuw, P. W. & Stehouwer, C. D. The link between adipose tissue renin-angiotensin-aldosterone system signaling and obesity-associated hypertension. Physiology 32, 197–209 (2017).
Thethi, T., Kamiyama, M. & Kobori, H. The link between the renin-angiotensin-aldosterone system and renal injury in obesity and the metabolic syndrome. Curr. Hypertens. Rep. 14, 160–169 (2012).
Siragy, H. M. & Carey, R. M. Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am. J. Nephrol. 31, 541–550 (2010).
Biancardi, V. C., Son, S. J., Ahmadi, S., Filosa, J. A. & Stern, J. E. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension 63, 572–579 (2014).
Kim, S. et al. The adipose renin-angiotensin system modulates systemic markers of insulin sensitivity and activates the intrarenal renin-angiotensin system. J. Biomed. Biotechnol. 2006, 027012 (2006).
Lin, H. et al. Kidney angiotensin in cardiovascular disease: formation and drug targeting. Pharmacol. Rev. 74, 462–505 (2022).
Briones, A. M. et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension 59, 1069–1078 (2012).
Hosoya, K. et al. Insulin resistance in chronic kidney disease is ameliorated by spironolactone in rats and humans. Kidney Int. 87, 749–760 (2015).
Remuzzi, G., Cattaneo, D. & Perico, N. The aggravating mechanisms of aldosterone on kidney fibrosis. J. Am. Soc. Nephrol. 19, 1459–1462 (2008).
Davel, A. P., Jaffe, I. Z., Tostes, R. C., Jaisser, F. & Belin de Chantemele, E. J. New roles of aldosterone and mineralocorticoid receptors in cardiovascular disease: translational and sex-specific effects. Am. J. Physiol. Heart Circ. Physiol 315, H989–H999 (2018).
Ichihara, A. & Yatabe, M. S. The (pro)renin receptor in health and disease. Nat. Rev. Nephrol. 15, 693–712 (2019).
Shamansurova, Z. et al. Adipose tissue (P)RR regulates insulin sensitivity, fat mass and body weight. Mol. Metab. 5, 959–969 (2016).
Fukushima, A. et al. Increased plasma soluble (pro)renin receptor levels are correlated with renal dysfunction in patients with heart failure. Int. J. Cardiol. 168, 4313–4314 (2013).
Visniauskas, B. et al. Sex differences in soluble prorenin receptor in patients with type 2 diabetes. Biol. Sex. Differ. 12, 33 (2021).
Gatineau, E., Gong, M. C. & Yiannikouris, F. Soluble prorenin receptor increases blood pressure in high fat-fed male mice. Hypertension 74, 1014–1020 (2019).
Fu, Z. et al. Soluble (pro)renin receptor induces endothelial dysfunction and hypertension in mice with diet-induced obesity via activation of angiotensin II type 1 receptor. Clin. Sci. 135, 793–810 (2021).
Quadri, S. & Siragy, H. M.(Pro)renin receptor contributes to regulation of renal epithelial sodium channel. J. Hypertens. 34, 486–494 (2016).
Zhu, Q. & Scherer, P. E. Immunologic and endocrine functions of adipose tissue: implications for kidney disease. Nat. Rev. Nephrol. 14, 105–120 (2018).
Mascali, A. et al. Obesity and kidney disease: beyond the hyperfiltration. Int. J. Immunopathol. Pharmacol. 29, 354–363 (2016).
Haynes, W. G., Morgan, D. A., Walsh, S. A., Mark, A. L. & Sivitz, W. I. Receptor-mediated regional sympathetic nerve activation by leptin. J. Clin. Invest. 100, 270–278 (1997).
Rahmouni, K. Leptin-induced sympathetic nerve activation: signaling mechanisms and cardiovascular consequences in obesity. Curr. Hypertens. Rev. 6, 104–209 (2010).
Hall, J. E. et al. Obesity, kidney dysfunction, and inflammation: interactions in hypertension. Cardiovasc. Res. 117, 1859–1876 (2021).
Lago, R., Gomez, R., Lago, F., Gomez-Reino, J. & Gualillo, O. Leptin beyond body weight regulation-current concepts concerning its role in immune function and inflammation. Cell Immunol. 252, 139–145 (2008).
Pedone, C. et al. Longitudinal association between serum leptin concentration and glomerular filtration rate in humans. PLoS ONE 10, e0117828 (2015).
Korczynska, J., Czumaj, A., Chmielewski, M., Swierczynski, J. & Sledzinski, T. The causes and potential injurious effects of elevated serum leptin levels in chronic kidney disease patients. Int. J. Mol. Sci. 22, 4685 (2021).
Briffa, J. F., Grinfeld, E., Poronnik, P., McAinch, A. J. & Hryciw, D. H. Uptake of leptin and albumin via separate pathways in proximal tubule cells. Int. J. Biochem. Cell Biol. 79, 194–198 (2016).
Briffa, J. F. et al. Acute leptin exposure reduces megalin expression and upregulates TGFβ1 in cultured renal proximal tubule cells. Mol. Cell Endocrinol. 401, 25–34 (2015).
Young, C. N., Morgan, D. A., Butler, S. D., Mark, A. L. & Davisson, R. L. The brain subfornical organ mediates leptin-induced increases in renal sympathetic activity but not its metabolic effects. Hypertension 61, 737–744 (2013).
Patel, M. et al. Central mechanisms in sympathetic nervous dysregulation in obesity. J. Neurophysiol. 130, 1414–1424 (2023).
Zhao, J., Miyamoto, S., You, Y. H. & Sharma, K. AMP-activated protein kinase (AMPK) activation inhibits nuclear translocation of Smad4 in mesangial cells and diabetic kidneys. Am. J. Physiol. Renal Physiol 308, F1167–F1177 (2015).
Nasrallah, M. P. & Ziyadeh, F. N. Overview of the physiology and pathophysiology of leptin with special emphasis on its role in the kidney. Semin. Nephrol. 33, 54–65 (2013).
Dalmasso, C., Leachman, J. R., Osborn, J. L. & Loria, A. S. Sensory signals mediating high blood pressure via sympathetic activation: role of adipose afferent reflex. Am. J. Physiol. Regul. Integr. Comp. Physiol 318, R379–R389 (2020).
Xiong, X. Q., Chen, W. W. & Zhu, G. Q. Adipose afferent reflex: sympathetic activation and obesity hypertension. Acta Physiol. 210, 468–478 (2014).
Mao, S. et al. Leptin and chronic kidney diseases. J. Recept. Signal. Transduct. Res. 38, 89–94 (2018).
Podkowińska, A. & Formanowicz, D. Chronic kidney disease as oxidative stress- and inflammatory-mediated cardiovascular disease. Antioxidants 9, 752 (2020).
Han, D. C. et al. Leptin stimulates type I collagen production in db/db mesangial cells: glucose uptake and TGF-β type II receptor expression. Kidney Int. 59, 1315–1323 (2001).
Thieme, K. & Oliveira-Souza, M. Renal hemodynamic and morphological changes after 7 and 28 days of leptin treatment: the participation of angiotensin II via the AT1 receptor. PLoS ONE 10, e0122265 (2015).
Chen, H.-H. et al. Chronic kidney disease: interaction of adiponectin gene polymorphisms and diabetes. Int. J. Mol. Sci. 24, 8128 (2023).
Vahdat, S. The complex effects of adipokines in the patients with kidney disease. J. Res. Med. Sci. 23, 60 (2018).
Przybyciński, J., Dziedziejko, V., Puchałowicz, K., Domański, L. & Pawlik, A. Adiponectin in chronic kidney disease. Int. J. Mol. Sci. 21, 9375 (2020).
Khoramipour, K. et al. Adiponectin: structure, physiological functions, role in diseases, and effects of nutrition. Nutrients 13, 1180 (2021).
Ahima, R. S. Linking adiponectin to proteinuria. J. Clin. Invest. 118, 1619–1622 (2008).
Ohashi, K. et al. Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Arterioscler. Thromb. Vasc. Biol. 27, 1910–1917 (2007).
Zha, D., Wu, X. & Gao, P. Adiponectin and its receptors in diabetic kidney disease: molecular mechanisms and clinical potential. Endocrinology 158, 2022–2034 (2017).
Sharma, K. et al. Adiponectin regulates albuminuria and podocyte function in mice. J. Clin. Invest. 118, 1645–1656 (2008).
Miyamoto, S. & Sharma, K. Adipokines protecting CKD. Nephrol. Dial. Transpl. 28 (Suppl. 4) iv15–iv22 (2013).
Kim, Y. & Park, C. W. Mechanisms of adiponectin action: implication of adiponectin receptor agonism in diabetic kidney disease. Int. J. Mol. Sci. 20, 1782 (2019).
Baldelli, S. et al. The role of adipose tissue and nutrition in the regulation of adiponectin. Nutrients 16, 2436 (2024).
Slyvka, Y. et al. Antioxidant diet and sex interact to regulate NOS isoform expression and glomerular mesangium proliferation in Zucker diabetic rat kidney. Acta Histochem. 118, 183–193 (2016).
Martinez Cantarin, M. P., Keith, S. W., Waldman, S. A. & Falkner, B. Adiponectin receptor and adiponectin signaling in human tissue among patients with end-stage renal disease. Nephrol. Dial. Transpl. 29, 2268–2277 (2014).
Park, C. H. & Yoo, T. H. TGF-β inhibitors for therapeutic management of kidney fibrosis. Pharmaceuticals 15, 1485 (2022).
Choi, S. R. et al. Adiponectin receptor agonist AdipoRon decreased ceramide, and lipotoxicity, and ameliorated diabetic nephropathy. Metabolism 85, 348–360 (2018).
Jang, H. S., Kim, J. & Padanilam, B. J. Renal sympathetic nerve activation via α2-adrenergic receptors in chronic kidney disease progression. Kidney Res. Clin. Pract. 38, 6–14 (2019).
Chen, L. et al. Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment. Biomed. Pharmacother. 101, 670–681 (2018).
Declèves, A. E., Mathew, A. V., Cunard, R. & Sharma, K. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J. Am. Soc. Nephrol. 22, 1846–1855 (2011).
Du, N. et al. Combination of ginsenoside Rg1 and Astragaloside IV reduces oxidative stress and inhibits TGF-β1/Smads signaling cascade on renal fibrosis in rats with diabetic nephropathy. Drug. Des. Devel. Ther. 12, 3517–3524 (2018).
Li, J. et al. Blockade of endothelial-mesenchymal transition by a smad3 inhibitor delays the early development of streptozotocin-induced diabetic nephropathy. Diabetes 59, 2612–2624 (2010).
Isakova, T., Yanucil, C. & Faul, C. A klotho-derived peptide as a possible novel drug to prevent kidney fibrosis. Am. J. Kidney Dis. 80, 285–288 (2022).
Yuan, Q. et al. A Klotho-derived peptide protects against kidney fibrosis by targeting TGF-β signaling. Nat. Commun. 13, 438 (2022).
Zhang, X. et al. Klotho-derived peptide 1 inhibits cellular senescence in the fibrotic kidney by restoring Klotho expression via posttranscriptional regulation. Theranostics 14, 420–435 (2024).
Pai, R., Ha, H., Kirschenbaum, M. A. & Kamanna, V. S. Role of tumor necrosis factor-alpha on mesangial cell MCP-1 expression and monocyte migration: mechanisms mediated by signal transduction. J. Am. Soc. Nephrol. 7, 914–923 (1996).
Sethi, J. K. & Hotamisligil, G. S. Metabolic messengers: tumour necrosis factor. Nat. Metab. 3, 1302–1312 (2021).
Denhez, B. et al. Saturated fatty acids induce insulin resistance in podocytes through inhibition of IRS1 via activation of both IKKβ and mTORC1. Sci. Rep. 10, 21628 (2020).
Sieber, J. et al. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am. J. Physiol. Renal Physiol 299, F821–829 (2010).
Therrien, F. J., Agharazii, M., Lebel, M. & Larivière, R. Neutralization of tumor necrosis factor-alpha reduces renal fibrosis and hypertension in rats with renal failure. Am. J. Nephrol. 36, 151–161 (2012).
Timoshanko, J. R. & Tipping, P. G. Resident kidney cells and their involvement in glomerulonephritis. Curr. Drug. Targets Inflamm. Allergy 4, 353–362 (2005).
Tirichen, H. et al. Mitochondrial reactive oxygen species and their contribution in chronic kidney disease progression through oxidative stress. Front. Physiol. 12, 627837 (2021).
Su, H., Lei, C. T. & Zhang, C. Interleukin-6 signaling pathway and its role in kidney disease: an update. Front. Immunol. 8, 405 (2017).
Barreto, D. V. et al. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 77, 550–556 (2010).
Mohamed-Ali, V. et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in vivo. J. Clin. Endocrinol. Metab. 82, 4196–4200 (1997).
Trujillo, M. E. et al. Interleukin-6 regulates human adipose tissue lipid metabolism and leptin production in vitro. J. Clin. Endocrinol. Metab. 89, 5577–5582 (2004).
Coletta, I. et al. Selective induction of MCP-1 in human mesangial cells by the IL-6/sIL-6R complex. Exp. Nephrol. 8, 37–43 (2000).
Li, K. et al. Interleukin-6 stimulates epithelial sodium channels in mouse cortical collecting duct cells. Am. J. Physiol. Regul. Integr. Comp. Physiol 299, R590–595 (2010).
Lu, T. C. et al. Knockdown of Stat3 activity in vivo prevents diabetic glomerulopathy. Kidney Int. 76, 63–71 (2009).
Paquissi, F. C. & Abensur, H. The Th17/IL-17 axis and kidney diseases, with focus on lupus nephritis. Front. Med. 8, 654912 (2021).
Yun, H. et al. The chemerin-CMKLR1 axis is functionally important for central regulation of energy homeostasis. Front. Physiol. 13, 897105 (2022).
Behnoush, A. H. et al. Chemerin levels in chronic kidney disease: a systematic review and meta-analysis. Front. Endocrinol. 14, 1120774 (2023).
Leiherer, A. et al. High plasma chemerin is associated with renal dysfunction and predictive for cardiovascular events — insights from phenotype and genotype characterization. Vasc. Pharmacol. 77, 60–68 (2016).
Habas, K. & Shang, L. Alterations in intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in human endothelial cells. Tissue Cell 54, 139–143 (2018).
Hsu, C. Y. et al. Increased circulating visfatin is associated with progression of kidney disease in non-diabetic hypertensive patients. Am. J. Hypertens. 29, 528–536 (2016).
Axelsson, J. et al. Circulating levels of visfatin/pre-B-cell colony-enhancing factor 1 in relation to genotype, GFR, body composition, and survival in patients with CKD. Am. J. Kidney Dis. 49, 237–244 (2007).
Song, H. K. et al. Visfatin: a new player in mesangial cell physiology and diabetic nephropathy. Am. J. Physiol. Renal Physiol. 295, F1485–1494 (2008).
Huang, Q. et al. Visfatin stimulates a cellular renin-angiotensin system in cultured rat mesangial cells. Endocr. Res. 36, 93–100 (2011).
Boini, K. M. et al. Visfatin-induced lipid raft redox signaling platforms and dysfunction in glomerular endothelial cells. Biochim. Biophys. Acta 1801, 1294–1304 (2010).
Huang, D. et al. Contribution of podocyte inflammatory exosome release to glomerular inflammation and sclerosis during hyperhomocysteinemia. Biochim. Biophys. Acta Mol. Basis Dis. 1867, 166146 (2021).
Fan, X. et al. Interleukin-10 attenuates renal injury after myocardial infarction in diabetes. J. Investig. Med. 70, 1233–1242 (2022).
Fang, M., Jeon, Y., Echouffo-Tcheugui, J. B. & Selvin, E. Prevalence and management of obesity in U.S. adults with type 1 diabetes. Ann. Intern. Med. 176, 427–429 (2023).
Bonner, R., Albajrami, O., Hudspeth, J. & Upadhyay, A. Diabetic kidney disease. Prim. Care 47, 645–659 (2020).
Cherney, D. Z. et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129, 587–597 (2014).
Nordheim, E. & Geir Jenssen, T. Chronic kidney disease in patients with diabetes mellitus. Endocr. Connect. 10, R151–R159 (2021).
Pyram, R., Kansara, A., Banerji, M. A. & Loney-Hutchinson, L. Chronic kidney disease and diabetes. Maturitas 71, 94–103 (2012).
Katsoulieris, E. et al. Lipotoxicity in renal proximal tubular cells: relationship between endoplasmic reticulum stress and oxidative stress pathways. Free. Radic. Biol. Med. 48, 1654–1662 (2010).
García-Carro, C. et al. A nephrologist perspective on obesity: from kidney injury to clinical management. Front. Med. 8, 655871 (2021).
Zhang, Y. et al. Hyperinsulinemia can cause kidney disease in the IGT stage of OLETF rats via the INS/IRS-1/PI3-K/Akt signaling pathway. J. Diabetes Res. 2019, 4709715 (2019).
Whaley-Connell, A. & Sowers, J. R. Insulin resistance in kidney disease: is there a distinct role separate from that of diabetes or obesity. Cardiorenal Med. 8, 41–49 (2017).
Daza-Arnedo, R. et al. Insulin and the kidneys: a contemporary view on the molecular basis. Front. Nephrol. 3, 1133352 (2023).
Brosolo, G. et al. Insulin resistance and high blood pressure: mechanistic insight on the role of the kidney. Biomedicines 10, 2374 (2022).
Zeni, L., Norden, A. G. W., Cancarini, G. & Unwin, R. J. A more tubulocentric view of diabetic kidney disease. J. Nephrol. 30, 701–717 (2017).
Gilbert, R. E. Proximal tubulopathy: prime mover and key therapeutic target in diabetic kidney disease. Diabetes 66, 791–800 (2017).
Pagtalunan, M. E. et al. Podocyte loss and progressive glomerular injury in type II diabetes. J. Clin. Invest. 99, 342–348 (1997).
Vallon, V. & Thomson, S. C. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat. Rev. Nephrol. 16, 317–336 (2020).
Serrano, E., Shenoy, P. & Martinez Cantarin, M. P. Adipose tissue metabolic changes in chronic kidney disease. Immunometabolism 5, e00023 (2023).
Tanaka, S. et al. Indoxyl sulfate contributes to adipose tissue inflammation through the activation of NADPH oxidase. Toxins 12, 502 (2020).
Vanholder, R. et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 63, 1934–1943 (2003).
Glassock, R. J. & Massry, S. G. in Nutritional Management of Renal Disease 4th edn. (eds Kopple, J. D., Massry, S. G., Kalantar-Zadeh, K., & Fouque, D.) 77–89 (Academic Press, 2022).
Nishi, H., Takemura, K., Higashihara, T. & Inagi, R. Uremic sarcopenia: clinical evidence and basic experimental approach. Nutrients 12, 1814 (2020).
Zhao, H. L. et al. Fat redistribution and adipocyte transformation in uninephrectomized rats. Kidney Int. 74, 467–477 (2008).
Stocker, S. D., Kinsman, B. J. & Sved, A. F. Recent advances in neurogenic hypertension: dietary salt, obesity, and inflammation. Hypertension https://doi.org/10.1161/HYPERTENSIONAHA.117.08936 (2017).
do Carmo, J. M. et al. Obesity-induced hypertension: brain signaling pathways. Curr. Hypertens. Rep. 18, 58 (2016).
DiBona, G. F. Sympathetic nervous system and hypertension. Hypertension 61, 556–560 (2013).
Asirvatham-Jeyaraj, N. et al. Renal denervation normalizes arterial pressure with no effect on glucose metabolism or renal inflammation in obese hypertensive mice. Hypertension 68, 929–936 (2016).
Ott, C. et al. Renal denervation preserves renal function in patients with chronic kidney disease and resistant hypertension. J. Hypertens. 33, 1261–1266 (2015).
Id, D. et al. Predictors of blood pressure response: obesity is associated with a less pronounced treatment response after renal denervation. Catheter. Cardiovasc. Interv. 87, E30–E38 (2016).
Kopp, U. C. Role of renal sensory nerves in physiological and pathophysiological conditions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R79–R95 (2015).
Tyshynsky, R., Vulchanova, L. & Osborn, J. in Renal Denervation: Treatment and Device-Based Neuromodulation. (eds Heuser, R. R., Schlaich, M. P., Hering, D., & Bertog, S. C.) 3–9 (Springer International Publishing, 2023).
Lambert, E. A. et al. Obesity-associated organ damage and sympathetic nervous activity. Hypertension 73, 1150–1159 (2019).
Yu, S. Q., Ma, S. & Wang, D. H. Activation of TRPV1-expressing renal sensory nerves of rats with N-oleoyldopamine attenuates high-fat-diet-induced impairment of renal function. Int. J. Mol. Sci. 24, 6207 (2023).
Banek, C. T. et al. Targeted afferent renal denervation reduces arterial pressure but not renal inflammation in established DOCA-salt hypertension in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 314, R883–R891 (2018).
Foss, J. D., Fink, G. D. & Osborn, J. W. Differential role of afferent and efferent renal nerves in the maintenance of early- and late-phase Dahl S hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R262–R267 (2016).
Cao, W. et al. Adipocytes initiate an adipose-cerebral-peripheral sympathetic reflex to induce insulin resistance during high-fat feeding. Clin. Sci. 133, 1883–1899 (2019).
Ding, L. et al. Adipose afferent reflex response to insulin is mediated by melanocortin 4 type receptors in the paraventricular nucleus in insulin resistance rats. Acta Physiol. 214, 450–466 (2015).
Xiong, X. Q. et al. Enhanced adipose afferent reflex contributes to sympathetic activation in diet-induced obesity hypertension. Hypertension 60, 1280–1286 (2012).
Niijima, A. Afferent signals from leptin sensors in the white adipose tissue of the epididymis, and their reflex effect in the rat. J. Autonomic Nerv. Syst. 73, 19–25 (1998).
Niijima, A. Reflex effects from leptin sensors in the white adipose tissue of the epididymis to the efferent activity of the sympathetic and vagus nerve in the rat. Neurosci. Lett. 262, 125–128 (1999).
Murphy, K. T. et al. Leptin-sensitive sensory nerves innervate white fat. Am. J. Physiol. Endocrinol. Metab. 304, E1338–E1347 (2013).
Tanida, M., Iwashita, S., Ootsuka, Y., Terui, N. & Suzuki, M. Leptin injection into white adipose tissue elevates renal sympathetic nerve activity dose-dependently through the afferent nerves pathway in rats. Neurosci. Lett. 293, 107–110 (2000).
Song, C. K., Schwartz, G. J. & Bartness, T. J. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R501–R511 (2009).
Shi, Z. et al. Sympathetic activation by chemical stimulation of white adipose tissues in rats. J. Appl. Physiol. 112, 1008–1014 (2012).
Bartness, T. J. & Song, C. K. Brain-adipose tissue neural crosstalk. Physiol. Behav. 91, 343–351 (2007).
Bartness, T. J., Liu, Y., Shrestha, Y. B. & Ryu, V. Neural innervation of white adipose tissue and the control of lipolysis. Front. Neuroendocrinol. 35, 473–493 (2014).
Stern, J. E. Neuroendocrine-autonomic integration in the paraventricular nucleus: novel roles for dendritically released neuropeptides. J. Neuroendocrinol. 27, 487–497 (2015).
Seravalle, G. & Grassi, G. Sympathetic nervous system, hypertension, obesity and metabolic syndrome. High. Blood Press. Cardiovasc. Prev. 23, 175–179 (2016).
de Kloet, A. D. & Herman, J. P. Fat-brain connections: adipocyte glucocorticoid control of stress and metabolism. Front. Neuroendocrinol. 48, 50–57 (2018).
Dalmasso, C. et al. Epididymal fat-derived sympathoexcitatory signals exacerbate neurogenic hypertension in obese male mice exposed to early life stress. Hypertension 78, 1434–1449 (2021).
Guilherme, A., Henriques, F., Bedard, A. H. & Czech, M. P. Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus. Nat. Rev. Endocrinol. 15, 207–225 (2019).
Szolcsanyi, J., Szallasi, A., Szallasi, Z., Joo, F. & Blumberg, P. M. Resiniferatoxin: an ultrapotent selective modulator of capsaicin-sensitive primary afferent neurons. J. Pharmacol. Exp. Ther. 255, 923–928 (1990).
Ding, L. et al. Superoxide anions in paraventricular nucleus modulate adipose afferent reflex and sympathetic activity in rats. PLoS ONE 8, e83771 (2013).
Cao, W. et al. A renal-cerebral-peripheral sympathetic reflex mediates insulin resistance in chronic kidney disease. EBioMedicine 37, 281–293 (2018).
Cui, B. P. et al. Ionotropic glutamate receptors in paraventricular nucleus mediate adipose afferent reflex and regulate sympathetic outflow in rats. Acta Physiol. 209, 45–54 (2013).
Wang, Y. et al. The role of somatosensory innervation of adipose tissues. Nature 609, 569–574 (2022).
Jiang, H., Ding, X., Cao, Y., Wang, H. & Zeng, W. Dense intra-adipose sympathetic arborizations are essential for cold-induced beiging of mouse white adipose tissue. Cell Metab. 26, 686–692.e683 (2017).
Qiu, F. et al. Potentiation of acid-sensing ion channel activity by the activation of 5-HT(2) receptors in rat dorsal root ganglion neurons. Neuropharmacology 63, 494–500 (2012).
Van Dyken, P. & Lacoste, B. Impact of metabolic syndrome on neuroinflammation and the blood-brain barrier. Front. Neurosci. 12, 930 (2018).
Feng, Z., Fang, C., Ma, Y. & Chang, J. Obesity-induced blood-brain barrier dysfunction: phenotypes and mechanisms. J. Neuroinflammation 21, 110 (2024).
Le Thuc, O. & Garcia-Caceres, C. Obesity-induced inflammation: connecting the periphery to the brain. Nat. Metab. 6, 1237–1252 (2024).
Thaler, J. P., Guyenet, S. J., Dorfman, M. D., Wisse, B. E. & Schwartz, M. W. Hypothalamic inflammation: marker or mechanism of obesity pathogenesis? Diabetes 62, 2629–2634 (2013).
Malkiewicz, M. A. et al. Blood-brain barrier permeability and physical exercise. J. Neuroinflammation 16, 15 (2019).
Salas-Venegas, V. et al. The obese brain: mechanisms of systemic and local inflammation, and interventions to reverse the cognitive deficit. Front. Integr. Neurosci. 16, 798995 (2022).
Garofalo, C. et al. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 91, 1224–1235 (2017).
Ghatage, T., Goyal, S. G., Dhar, A. & Bhat, A. Novel therapeutics for the treatment of hypertension and its associated complications: peptide- and nonpeptide-based strategies. Hypertension Res. 44, 740–755 (2021).
Pergola, P. E. et al. Effect of ziltivekimab on determinants of hemoglobin in patients with CKD stage 3–5: an analysis of a randomized trial (RESCUE). J. Am. Soc. Nephrol. 35, 74–84 (2024).
Adamstein, N. H. et al. Association of interleukin 6 inhibition with ziltivekimab and the neutrophil-lymphocyte ratio: a secondary analysis of the RESCUE clinical trial. JAMA Cardiol. 8, 177–181, (2023).
Ivković, V. & Bruchfeld, A. Endothelin receptor antagonists in diabetic and non-diabetic chronic kidney disease. Clin. Kidney J. 17, sfae072 (2024).
Schiffrin, E. L. & Pollock, D. M. Endothelin system in hypertension and chronic kidney disease. Hypertension 81, 691–701 (2024).
Cheong, A. J. Y. et al. SGLT inhibitors on weight and body mass: a meta-analysis of 116 randomized-controlled trials. Obesity 30, 117–128 (2022).
Zingerman, B. et al. Effect of acetazolamide on obesity-induced glomerular hyperfiltration: a randomized controlled trial. PLoS ONE 10, e0137163 (2015).
Suh, S. H. & Kim, S. W. Dyslipidemia in patients with chronic kidney disease: an updated overview. Diabetes Metab. J. 47, 612–629 (2023).
Perkovic, V. et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N. Engl. J. Med. 391, 109–121 (2024).
Acknowledgements
The authors’ work was supported by National Institutes of Health grants (R01-HL-142969 and R01-HL-1647 to A.S.L.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Toshiro Fujita, Sandra Merscher, Joseph Tam who co-reviewed with Liad Hinden, Raymond Townsend and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmed, N., Dalmasso, C., Turner, M.B. et al. From fat to filter: the effect of adipose tissue-derived signals on kidney function. Nat Rev Nephrol 21, 417–434 (2025). https://doi.org/10.1038/s41581-025-00950-5
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41581-025-00950-5
This article is cited by
-
Visceral fat metabolism score and cardiovascular-kidney-metabolic syndrome stages in all-cause and cause-specific mortality: insights from UK Biobank
Nutrition & Metabolism (2026)
-
Adipokine networks in diabetic kidney disease: mechanistic insights and therapeutic implications
Lipids in Health and Disease (2026)
-
Triglyceride-glucose index, a silent predictor for osteosarcopenic adiposity occurrence and risk of cardiovascular and all-cause mortality
Diabetology & Metabolic Syndrome (2025)