Abstract
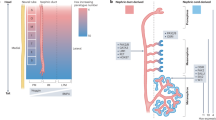
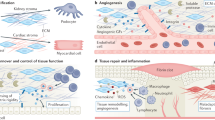
The kidney is one of the most complex organs in the body. It is made up of thousands of patterned epithelial and endothelial tubules that work together to maintain body chemistry. Precise spatial integration of these different cell types is essential for the organ to function optimally. A complex and heterogeneous network of cells collectively referred to as ‘stroma’ lies between the epithelial and endothelial tubules. A growing body of evidence suggests that the stroma mediates communication between the epithelia and endothelia, and functions to support a variety of processes during kidney development and in the adult kidney, with implications for disease. However, stromal cells remain far less well defined than the epithelia and endothelia, and we understand only a fraction of their functions, leading some to refer to the stroma as the ‘dark matter’ of the kidney. In this Review, we discuss the developmental origins of the stroma and describe current understanding of its roles in the growth and patterning of the renal epithelia and endothelia, and in the maintenance and repair of the adult organ. Finally, we highlight critical questions that remain unanswered and the resources that will be required to answer them so that we can fully understand the function of these enigmatic cells.
Key points
-
The kidney stroma is a heterogeneous population of vascular mural cells, fibroblasts, smooth muscle and leukocytes that arise from at least three unique cellular lineages.
-
The different stromal cell types produce a wide array a signalling proteins, small molecules and metabolites, extracellular matrix and hormones that create regional microenvironments in the kidney.
-
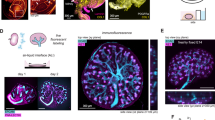
Studies using genetic mouse models and kidney organoids have revealed essential roles for the stroma in the development of nephrons and the vasculature.
-
Growing evidence indicates that the stroma of the adult organ is as diverse as that of the embryo, suggesting that it might have essential roles in tissue maintenance, repair and disease progression.
-
The development of new tools is expected to uncover additional roles for the stroma in both the embryonic and adult organ.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Webster, A. C. et al. Chronic kidney disease. Lancet 389, 1238–1252 (2017).
Yamashita, N. & Kramann, R. Mechanisms of kidney fibrosis and routes towards therapy. Trends Endocrinol. Metab. 35, 31–48 (2024).
Leggatt, G. P. et al. A role for genetic modifiers in tubulointerstitial kidney diseases. Genes 14, 1582 (2023).
Plikus, M. V. et al. Fibroblasts: origins, definitions, and functions in health and disease. Cell 184, 3852–3872 (2021).
Buechler, M. B. et al. Cross-tissue organization of the fibroblast lineage. Nature 593, 575–579 (2021).
Buechler, M. B. & Turley, S. J. A short field guide to fibroblast function in immunity. Semin. Immunol. 35, 48–58 (2018).
Kaissling, B. & Le Hir, M. The renal cortical interstitium: morphological and functional aspects. Histochem. Cell Biol. 130, 247–262 (2008).
Qi, W. et al. The renal cortical fibroblast in renal tubulointerstitial fibrosis. Int. J. Biochem. Cell Biol. 38, 1–5 (2006).
Lemley, K. V. & Kriz, W. Anatomy of the renal interstitium. Kidney Int. 39, 370–381 (1991).
Kobayashi, H. et al. Distinct subpopulations of FOXD1 stroma-derived cells regulate renal erythropoietin. J. Clin. Invest. 126, 1926–1938 (2016).
Maxwell, P. H. et al. Identification of the renal erythropoietin-producing cells using transgenic mice. Kidney Int. 44, 1149–1162 (1993).
Plotkin, M. D. & Goligorsky, M. S. Mesenchymal cells from adult kidney support angiogenesis and differentiate into multiple interstitial cell types including erythropoietin-producing fibroblasts. Am. J. Physiol. Ren. Physiol. 291, F902–F912 (2006).
Bachmann, S., Le Hir, M. & Eckardt, K. U. Co-localization of erythropoietin mRNA and ecto-5′-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietin. J. Histochem. Cytochem. 41, 335–341 (1993).
Yamazaki, S. et al. A mouse model of adult-onset anaemia due to erythropoietin deficiency. Nat. Commun. 4, 1950 (2013).
Kaneko, K. et al. Lineage tracing analysis defines erythropoietin-producing cells as a distinct subpopulation of resident fibroblasts with unique behaviors. Kidney Int. 102, 280–292 (2022).
Kragesteen, B. K. et al. The transcriptional and regulatory identity of erythropoietin producing cells. Nat. Med. 29, 1191–1200 (2023).
Li, H. et al. Comprehensive single-cell transcriptional profiling defines shared and unique epithelial injury responses during kidney fibrosis. Cell Metab. 34, 1977–1998.e9 (2022).
Rudman-Melnick, V. et al. Single-cell sequencing dissects the transcriptional identity of activated fibroblasts and identifies novel persistent distal tubular injury patterns in kidney fibrosis. Sci. Rep. 14, 439 (2024).
Barwinska, D. et al. Molecular characterization of the human kidney interstitium in health and disease. Sci. Adv. 7, eabd3359 (2021).
Lake, B. B. et al. An atlas of healthy and injured cell states and niches in the human kidney. Nature 619, 585–594 (2023).
Kuppe, C. et al. Decoding myofibroblast origins in human kidney fibrosis. Nature 589, 281–286 (2021).
Sims, D. E. The pericyte — a review. Tissue Cell 18, 153–174 (1986).
Schiller, B. & Moran, J. Experimental glomerulosclerosis: Defektheilung of the kidney. Artif. Organs 20, 445–450 (1996).
Yosypiv, I. V. Renin-angiotensin system in mammalian kidney development. Pediatr. Nephrol. 36, 479–489 (2021).
Yamaguchi, H., Gomez, R. A. & Sequeira-Lopez, M. L. S. Renin cells, from vascular development to blood pressure sensing. Hypertension 80, 1580–1589 (2023).
Hurtado, R., Bub, G. & Herzlinger, D. The pelvis-kidney junction contains HCN3, a hyperpolarization-activated cation channel that triggers ureter peristalsis. Kidney Int. 77, 500–508 (2010).
Mulţescu, R., Georgescu, D., Geavlete, A., Geavlete, B. in Retrograde Ureteroscopy Ch. 2 (ed. Geavlete, A.) 7–19 (Academic Press. 2016).
Kitching, A. R. & Hickey, M. J. Immune cell behaviour and dynamics in the kidney — insights from in vivo imaging. Nat. Rev. Nephrol. 18, 22–37 (2022).
Kurts, C., Ginhoux, F. & Panzer, U. Kidney dendritic cells: fundamental biology and functional roles in health and disease. Nat. Rev. Nephrol. 16, 391–407 (2020).
Zimmerman, K. A. et al. Single-cell RNA sequencing identifies candidate renal resident macrophage gene expression signatures across species. J. Am. Soc. Nephrol. 30, 767–781 (2019).
Zimmerman, K. A. et al. Kidney resident macrophages in the rat have minimal turnover and replacement by blood monocytes. Am. J. Physiol. Ren. Physiol. 321, F162–F169 (2021).
Chew, C. et al. Kidney resident macrophages have distinct subsets and multifunctional roles. Matrix Biol. 127, 23–37 (2024).
Ide, S. et al. Yolk-sac-derived macrophages progressively expand in the mouse kidney with age. Elife 9, e51756 (2020).
Puranik, A. S. et al. Kidney-resident macrophages promote a proangiogenic environment in the normal and chronically ischemic mouse kidney. Sci. Rep. 8, 13948 (2018).
Zimmerman, K. A. et al. Tissue-resident macrophages promote renal cystic disease. J. Am. Soc. Nephrol. 30, 1841–1856 (2019).
Lever, J. M. et al. Resident macrophages reprogram toward a developmental state after acute kidney injury. JCI Insight 4, e125503 (2019).
Garcia-Estan, J. & Roman, R. J. Role of renal interstitial hydrostatic pressure in the pressure diuresis response. Am. J. Physiol. 256, F63–F70 (1989).
Park, H. C. et al. Renal capsule as a stem cell niche. Am. J. Physiol. Ren. Physiol. 298, F1254–F1262 (2010).
Korin, B. et al. The renal capsule: a vibrant and adaptive cell environment of the kidney in homeostasis and aging. Preprint at bioRxiv https://doi.org/10.1101/2023.05.11.540033 (2023).
Michailova, K., Wassilev, W. & Wedel, T. Scanning and transmission electron microscopic study of visceral and parietal peritoneal regions in the rat. Ann. Anat. 181, 253–260 (1999).
Dick, S. A. et al. Three tissue resident macrophage subsets coexist across organs with conserved origins and life cycles. Sci. Immunol. 7, eabf7777 (2022).
Albertine, K. H. & O’Morchoe, C. C. Distribution and density of the canine renal cortical lymphatic system. Kidney Int. 16, 470–480 (1979).
Lee, H. W. et al. Expression of lymphatic endothelium-specific hyaluronan receptor LYVE-1 in the developing mouse kidney. Cell Tissue Res. 343, 429–444 (2011).
Kobayashi, A. et al. Identification of a multipotent self-renewing stromal progenitor population during mammalian kidney organogenesis. Stem Cell Rep. 3, 650–662 (2014).
Leuning, D. G. et al. The human kidney capsule contains a functionally distinct mesenchymal stromal cell population. PLoS ONE 12, e0187118 (2017).
Levinson, R. S. et al. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development 132, 529–539 (2005).
Yallowitz, A. R. et al. Hox10 genes function in kidney development in the differentiation and integration of the cortical stroma. PLoS ONE 6, e23410 (2011).
Kobayashi, A. et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3, 169–181 (2008).
Mugford, J. W. et al. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev. Biol. 324, 88–98 (2008).
James, R. G. et al. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development 133, 2995–3004 (2006).
Taguchi, A. et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14, 53–67 (2014).
Guillaume, R., Bressan, M. & Herzlinger, D. Paraxial mesoderm contributes stromal cells to the developing kidney. Dev. Biol. 329, 169–175 (2009).
Wilson, S. B. & Little, M. H. The origin and role of the renal stroma. Development 148, dev199886 (2021).
Bohnenpoll, T. et al. Tbx18 expression demarcates multipotent precursor populations in the developing urogenital system but is exclusively required within the ureteric mesenchymal lineage to suppress a renal stromal fate. Dev. Biol. 380, 25–36 (2013).
Mass, E. et al. Specification of tissue-resident macrophages during organogenesis. Science 353, 698–707 (2016).
Schulz, C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012).
Stamatiades, E. G. et al. Immune monitoring of trans-endothelial transport by kidney-resident macrophages. Cell 166, 991–1003 (2016).
Saxen, L. Organogenesis of the Kidney (Cambridge Univ. Press, 1987). [Series Eds Barlow, P. W., Green, P. B. & Wylie, C. C. Developmental and Cell Biology Series].
Packard, A., Klein, W. H. & Costantini, F. Ret signaling in ureteric bud epithelial cells controls cell movements, cell clustering and bud formation. Development 148, dev199386 (2021).
Riccio, P. et al. Ret and Etv4 promote directed movements of progenitor cells during renal branching morphogenesis. PLoS Biol. 14, e1002382 (2016).
Shakya, R., Watanabe, T. & Costantini, F. The role of GDNF/Ret signaling in ureteric bud cell fate and branching morphogenesis. Dev. Cell 8, 65–74 (2005).
Basson, M. A. et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev. Cell 8, 229–239 (2005).
Michos, O. et al. Kidney development in the absence of Gdnf and Spry1 requires Fgf10. PLoS Genet. 6, e1000809 (2010).
Batourina, E. et al. Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat. Genet. 27, 74–78 (2001).
Mendelsohn, C. et al. Stromal cells mediate retinoid-dependent functions essential for renal development. Development 126, 1139–1148 (1999).
Rosselot, C. et al. Non-cell-autonomous retinoid signaling is crucial for renal development. Development 137, 283–292 (2010).
Niederreither, K. et al. Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development 129, 3563–3574 (2002).
Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 134, 921–931 (2008).
Schuchardt, A. et al. Renal agenesis and hypodysplasia in ret-k− mutant mice result from defects in ureteric bud development. Development 122, 1919–1929 (1996).
Hatini, V. et al. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of winged helix transcription factor BF-2. Genes. Dev. 10, 1467–1478 (1996).
Song, R., Lopez, M. & Yosypiv, I. V. Foxd1 is an upstream regulator of the renin–angiotensin system during metanephric kidney development. Pediatr. Res. 82, 855–862 (2017).
Iosipiv, I. V. & Schroeder, M. A role for angiotensin II AT1 receptors in ureteric bud cell branching. Am. J. Physiol. Ren. Physiol. 285, F199–F207 (2003).
Gribouval, O. et al. Mutations in genes in the renin–angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat. Genet. 37, 964–968 (2005).
Knott, P. D., Thorpe, S. S. & Lamont, C. A. Congenital renal dysgenesis possibly due to captopril. Lancet 1, 451 (1989).
Sequeira-Lopez, M. L. et al. Vascular versus tubular renin: role in kidney development. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R650–R657 (2015).
Takahashi, N. et al. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J. Am. Soc. Nephrol. 16, 125–132 (2005).
Yosypiv, I. V. et al. Stromal prorenin receptor is critical for normal kidney development. Am. J. Physiol. Regul. Integr. Comp. Physiol. 316, R640–R650 (2019).
Song, R. et al. Deletion of the prorenin receptor from the ureteric bud causes renal hypodysplasia. PLoS ONE 8, e63835 (2013).
Miyazaki, Y. et al. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J. Clin. Invest. 105, 863–873 (2000).
Miyazaki, Y. et al. Evidence that bone morphogenetic protein 4 has multiple biological functions during kidney and urinary tract development. Kidney Int. 63, 835–844 (2003).
Brenner-Anantharam, A. et al. Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling. Development 134, 1967–1975 (2007).
Das, A. et al. Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nat. Cell Biol. 15, 1035–1044 (2013).
Carroll, T. J. et al. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell 9, 283–292 (2005).
Mao, Y., Francis-West & Irvine, K. D. Fat4/Dchs1 signaling between stromal and cap mesenchyme cells influences nephrogenesis and ureteric bud branching. Development 142, 2574–2585 (2015).
Bagherie-Lachidan, M. et al. Stromal Fat4 acts non-autonomously with Dchs1/2 to restrict the nephron progenitor pool. Development 142, 2564–2573 (2015).
Drake, K. A. et al. Transcription factors YAP/TAZ and SRF cooperate to specify renal myofibroblasts in the developing mouse kidney. J. Am. Soc. Nephrol. 33, 1694–1707 (2022).
Reginensi, A. et al. Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet. 9, e1003380 (2013).
Brown, A. C. et al. Role for compartmentalization in nephron progenitor differentiation. Proc. Natl Acad. Sci. USA 110, 4640–4645 (2013).
Fetting, J. L. et al. FOXD1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney. Development 141, 17–27 (2014).
Karner, C. M. et al. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development 138, 1247–1257 (2011).
Kumar, S. et al. ZEB2 controls kidney stromal progenitor differentiation and inhibits abnormal myofibroblast expansion and kidney fibrosis. JCI Insight 8, e158418 (2023).
Sato, M. et al. Targeted disruption of TGF-β1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J. Clin. Invest. 112, 1486–1494 (2003).
Kao, R. M. et al. Invasion of distal nephron precursors associates with tubular interconnection during nephrogenesis. J. Am. Soc. Nephrol. 23, 1682–1690 (2012).
Lopez-Garcia, I. et al. Epithelial tubule interconnection driven by HGF-Met signaling in the kidney. Proc. Natl Acad. Sci. USA 121, e2416887121 (2024).
Lindstrom, N. O. et al. Spatial transcriptional mapping of the human nephrogenic program. Dev. Cell 56, 2381–2398.e6 (2021).
Kopan, R., Cheng, H. T. & Surendran, K. Molecular insights into segmentation along the proximal–distal axis of the nephron. J. Am. Soc. Nephrol. 18, 2014–2020 (2007).
Georgas, K. et al. Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev. Biol. 332, 273–286 (2009).
Schneider, J. et al. Wnt signaling orients the proximal–distal axis of chick kidney nephrons. Development 142, 2686–2695 (2015).
Vanslambrouck, J. M. et al. Generation of proximal tubule-enhanced kidney organoids from human pluripotent stem cells. Nat. Protoc. 18, 3229–3252 (2023).
Chandrasekaran, V. et al. Generation and characterization of iPSC-derived renal proximal tubule-like cells with extended stability. Sci. Rep. 11, 11575 (2021).
Uchimura, K. et al. Human pluripotent stem cell-derived kidney organoids with improved collecting duct maturation and injury modeling. Cell Rep. 33, 108514 (2020).
Shi, M. et al. Human ureteric bud organoids recapitulate branching morphogenesis and differentiate into functional collecting duct cell types. Nat. Biotechnol. 41, 252–261 (2023).
Tanigawa, S. et al. Generation of the organotypic kidney structure by integrating pluripotent stem cell-derived renal stroma. Nat. Commun. 13, 611 (2022).
Hum, S. et al. Ablation of the renal stroma defines its critical role in nephron progenitor and vasculature patterning. PLoS ONE 9, e88400 (2014).
Hurtado, R. et al. Pbx1-dependent control of VMC differentiation kinetics underlies gross renal vascular patterning. Development 142, 2653–2664 (2015).
Luo, P. M. et al. Stromal netrin 1 coordinates renal arteriogenesis and mural cell differentiation. Development 150, dev201884 (2023).
Liu, Y. et al. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J. Biol. Chem. 280, 9719–9727 (2005).
Yap, C. et al. Six shades of vascular smooth muscle cells illuminated by KLF4 (Kruppel-like factor 4). Arterioscler. Thromb. Vasc. Biol. 41, 2693–2707 (2021).
Honeycutt, S. E. et al. Netrin 1 directs vascular patterning and maturity in the developing kidney. Development 150, dev201886 (2023).
Selleri, L. et al. Requirement for Pbx1 in skeletal patterning and programming chondrocyte proliferation and differentiation. Development 128, 3543–3557 (2001).
Ficara, F. et al. Pbx1 regulates self-renewal of long-term hematopoietic stem cells by maintaining their quiescence. Cell Stem Cell 2, 484–496 (2008).
Winkler, E. A., Bell, R. D. & Zlokovic, B. V. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol. Neurodegener. 5, 32 (2010).
Lindblom, P. et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes. Dev. 17, 1835–1840 (2003).
Leveen, P. et al. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes. Dev. 8, 1875–1887 (1994).
Lindahl, P. et al. Paracrine PDGF-B/PDGF-Rβ signaling controls mesangial cell development in kidney glomeruli. Development 125, 3313–3322 (1998).
Krause, M. et al. Signaling during kidney development. Cells 4, 112–132 (2015).
Armulik, A., Genove, G. & Betsholtz, C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193–215 (2011).
Andrae, J., Gallini, R. & Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes. Dev. 22, 1276–1312 (2008).
Gaengel, K. et al. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 29, 630–638 (2009).
Hellstrom, M. et al. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047–3055 (1999).
Soriano, P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes. Dev. 8, 1888–1896 (1994).
Mohamed, T. & Sequeira-Lopez, M. L. S. Development of the renal vasculature. Semin. Cell Dev. Biol. 91, 132–146 (2019).
Sequeira Lopez, M. L. & Gomez, R. A. Development of the renal arterioles. J. Am. Soc. Nephrol. 22, 2156–2165 (2011).
Eming, S. A. et al. Regulation of the spatial organization of mesenchymal connective tissue: effects of cell-associated versus released isoforms of platelet-derived growth factor. Am. J. Pathol. 154, 281–289 (1999).
Wang, X. et al. YAP/TAZ orchestrate VEGF signaling during developmental angiogenesis. Dev. Cell 42, 462–478.e7 (2017).
Azad, T. et al. A LATS biosensor screen identifies VEGFR as a regulator of the Hippo pathway in angiogenesis. Nat. Commun. 9, 1061 (2018).
Kobayashi, S. et al. Vasculature is getting Hip(po): Hippo signaling in vascular development and disease. Dev. Cell 58, 2627–2640 (2023).
Neto, F. et al. YAP and TAZ regulate adherens junction dynamics and endothelial cell distribution during vascular development. Elife 7, e31037 (2018).
Kim, J. et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J. Clin. Invest. 127, 3441–3461 (2017).
Giampietro, C. et al. The actin-binding protein EPS8 binds VE-cadherin and modulates YAP localization and signaling. J. Cell Biol. 211, 1177–1192 (2015).
Choi, H. J. et al. Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nat. Commun. 6, 6943 (2015).
Yuan, H. T. et al. Expression of angiopoietin-1, angiopoietin-2, and the Tie-2 receptor tyrosine kinase during mouse kidney maturation. J. Am. Soc. Nephrol. 10, 1722–1736 (1999).
Yuan, H. T. et al. Angiopoietin-2 is a site-specific factor in differentiation of mouse renal vasculature. J. Am. Soc. Nephrol. 11, 1055–1066 (2000).
Maisonpierre, P. C. et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277, 55–60 (1997).
Suri, C. et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87, 1171–1180 (1996).
Zhang, Y. et al. Angiopoietin-Tie signaling pathway in endothelial cells: a computational model. iScience 20, 497–511 (2019).
Saharinen, P. et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell–cell and cell–matrix contacts. Nat. Cell Biol. 10, 527–537 (2008).
Sundberg, C. et al. Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab. Invest. 82, 387–401 (2002).
Jeansson, M. et al. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J. Clin. Invest. 121, 2278–2289 (2011).
Wakui, S. et al. Localization of Ang-1, -2, Tie-2, and VEGF expression at endothelial-pericyte interdigitation in rat angiogenesis. Lab. Invest. 86, 1172–1184 (2006).
Augustin, H. G. et al. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 10, 165–177 (2009).
Takabatake, Y. et al. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J. Am. Soc. Nephrol. 20, 1714–1723 (2009).
Tachibana, K. et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 393, 591–594 (1998).
Haege, S. et al. CXC chemokine receptor 7 (CXCR7) regulates CXCR4 protein expression and capillary tuft development in mouse kidney. PLoS ONE 7, e42814 (2012).
Boldajipour, B. et al. Control of chemokine-guided cell migration by ligand sequestration. Cell 132, 463–473 (2008).
Watanabe, E. et al. Stromal cell-derived factor 1 (SDF1) attenuates platelet-derived growth factor-B (PDGF-B)-induced vascular remodeling for adipose tissue expansion in obesity. Angiogenesis 23, 667–684 (2020).
Foo, S. S. et al. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124, 161–173 (2006).
Peuckert, C. et al. Multimodal Eph/Ephrin signaling controls several phases of urogenital development. Kidney Int. 90, 373–388 (2016).
Jin, S. et al. Notch signaling regulates platelet-derived growth factor receptor-β expression in vascular smooth muscle cells. Circ. Res. 102, 1483–1491 (2008).
Boyle, S. C., Liu, Z. & Kopan, R. Notch signaling is required for the formation of mesangial cells from a stromal mesenchyme precursor during kidney development. Development 141, 346–354 (2014).
Domenga, V. et al. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes. Dev. 18, 2730–2735 (2004).
Lin, E. E., Sequeira-Lopez, M. L. & Gomez, R. A. RBP-J in FOXD1+ renal stromal progenitors is crucial for the proper development and assembly of the kidney vasculature and glomerular mesangial cells. Am. J. Physiol. Ren. Physiol. 306, F249–F258 (2014).
Kusaba, T. et al. Renal involvement in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Clin. Nephrol. 67, 182–187 (2007).
Crawford, C. et al. An intact kidney slice model to investigate vasa recta properties and function in situ. Nephron Physiol. 120, p17–p31 (2012).
Chou, Y. H. et al. Update of pericytes function and their roles in kidney diseases. J. Formos. Med. Assoc. 123, 307–317 (2024).
Homma, K. et al. Rho-kinase contributes to pressure-induced constriction of renal microvessels. Keio J. Med. 63, 1–12 (2014).
Chaney, C. P. et al. Integration of spatial and single nucleus transcriptomics to map gene expression in the developing mouse kidney. Preprint at bioRxiv https://doi.org/10.1101/2024.12.18.629207 (2024).
Walker, K. A., Sims-Lucas, S. & Bates, C. M. Fibroblast growth factor receptor signaling in kidney and lower urinary tract development. Pediatr. Nephrol. 31, 885–895 (2016).
Finch, P. W. et al. Pattern of keratinocyte growth factor and keratinocyte growth factor receptor expression during mouse fetal development suggests a role in mediating morphogenetic mesenchymal-epithelial interactions. Dev. Dyn. 203, 223–240 (1995).
Mason, I. J. et al. FGF-7 (keratinocyte growth factor) expression during mouse development suggests roles in myogenesis, forebrain regionalisation and epithelial-mesenchymal interactions. Mech. Dev. 45, 15–30 (1994).
Qiao, J. et al. FGF-7 modulates ureteric bud growth and nephron number in the developing kidney. Development 126, 547–554 (1999).
Ohuchi, H. et al. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem. Biophys. Res. Commun. 277, 643–649 (2000).
Hains, D. et al. Role of fibroblast growth factor receptor 2 in kidney mesenchyme. Pediatr. Res. 64, 592–598 (2008).
Yu, J. et al. A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development 136, 161–171 (2009).
Boivin, F. J. & Bridgewater, D. β-Catenin in stromal progenitors controls medullary stromal development. Am. J. Physiol. Ren. Physiol. 314, F1177–F1187 (2018).
England, A. R. et al. Identification and characterization of cellular heterogeneity within the developing renal interstitium. Development 147, dev190108 (2020).
McCarthy, S. S., Karolak, M. & Oxburgh, L. Smad4 controls proliferation of interstitial cells in the neonatal kidney. Development 149, dev199984 (2022).
Stark, K. et al. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372, 679–683 (1994).
Kispert, A., Vainio, S. & McMahon, A. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 125, 4225–4234 (1998).
Itaranta, P. et al. Wnt-4 signaling is involved in the control of smooth muscle cell fate via Bmp-4 in the medullary stroma of the developing kidney. Dev. Biol. 293, 473–483 (2006).
DiRocco, D. P. et al. Wnt4/β-catenin signaling in medullary kidney myofibroblasts. J. Am. Soc. Nephrol. 24, 1399–1412 (2013).
Drake, K. A. et al. Stromal β-catenin activation impacts nephron progenitor differentiation in the developing kidney and may contribute to Wilms tumor. Development 147, dev189597 (2020).
D’Cruz, R. et al. Hedgehog signalling in Foxd1+ embryonic kidney stromal progenitors controls nephron formation via Cxcl12 and Wnt5a. J. Pathol. 261, 385–400 (2023).
Rowan, C. J. et al. Hedgehog-GLI signaling in Foxd1-positive stromal cells promotes murine nephrogenesis via TGFβ signaling. Development 145, dev159947 (2018).
Yu, J., Carroll, T. J. & McMahon, A. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129, 5301–5312 (2002).
Bohnenpoll, T. & Kispert, A. Ureter growth and differentiation. Semin. Cell Dev. Biol. 36, 21–30 (2014).
Kispert, A. Ureter development and associated congenital anomalies. Nat Rev Nephrol. 21, 366–382 (2025).
Grainger, N. et al. Identification and classification of interstitial cells in the mouse renal pelvis. J. Physiol. 598, 3283–3307 (2020).
Boivin, F. J. et al. Stromally expressed β-catenin modulates Wnt9b signaling in the ureteric epithelium. PLoS ONE 10, e0120347 (2015).
Cain, J. E. et al. GLI3 repressor controls functional development of the mouse ureter. J. Clin. Invest. 121, 1199–1206 (2011).
Mamo, T. M. et al. BMP4 uses several different effector pathways to regulate proliferation and differentiation in the epithelial and mesenchymal tissue compartments of the developing mouse ureter. Hum. Mol. Genet. 26, 3553–3563 (2017).
Caubit, X. et al. Teashirt 3 is necessary for ureteral smooth muscle differentiation downstream of SHH and BMP4. Development 135, 3301–3310 (2008).
Bush, K. T. et al. Development and differentiation of the ureteric bud into the ureter in the absence of a kidney collecting system. Dev. Biol. 298, 571–584 (2006).
Xu, P. X. et al. Six1 is required for the early organogenesis of mammalian kidney. Development 130, 3085–3094 (2003).
Nie, X. et al. SIX1 acts synergistically with TBX18 in mediating ureteral smooth muscle formation. Development 137, 755–765 (2010).
Airik, R. et al. Tbx18 regulates the development of the ureteral mesenchyme. J. Clin. Invest. 116, 663–674 (2006).
Weiss, A. C. et al. Permissive ureter specification by TBX18-mediated repression of metanephric gene expression. Development 150, dev201048 (2023).
Airik, R. et al. Hydroureternephrosis due to loss of Sox9-regulated smooth muscle cell differentiation of the ureteric mesenchyme. Hum. Mol. Genet. 19, 4918–4929 (2010).
Alasaadi, D. N. & Mayor, R. Mechanically guided cell fate determination in early development. Cell Mol. Life Sci. 81, 242 (2024).
Engler, A. J. et al. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Chen, W. C., Lin, H. H. & Tang, M. J. Regulation of proximal tubular cell differentiation and proliferation in primary culture by matrix stiffness and ECM components. Am. J. Physiol. Ren. Physiol. 307, F695–F707 (2014).
Melica, M. E. et al. Substrate stiffness modulates renal progenitor cell properties via a ROCK-mediated mechanotransduction mechanism. Cells 8, 1561 (2019).
Lacueva-Aparicio, A. et al. Role of extracellular matrix components and structure in new renal models in vitro. Front. Physiol. 13, 1048738 (2022).
Chakraborty, S. et al. Quantifying spatial patterns of tissue stiffness within the embryonic mouse kidney. Methods Mol. Biol. 2805, 171–186 (2024).
Combes, A. N. et al. Correction: single cell analysis of the developing mouse kidney provides deeper insight into marker gene expression and ligand–receptor crosstalk (doi:10.1242/dev.178673). Development 146, dev182162 (2019).
Zorn, A. M. & Wells, J. M. Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 25, 221–251 (2009).
Tabin, C. & Wolpert, L. Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Genes. Dev. 21, 1433–1442 (2007).
Maggiore, J. C. et al. A genetically inducible endothelial niche enables vascularization of human kidney organoids with multilineage maturation and emergence of renin expressing cells. Kidney Int. 106, 1086–1100 (2024).
Lipp, S. N. et al. 3D mapping reveals a complex and transient interstitial matrix during murine kidney development. J. Am. Soc. Nephrol. 32, 1649–1665 (2021).
Lipp, S. N. et al. FOXD1 is required for 3D patterning of the kidney interstitial matrix. Dev. Dyn. 252, 463–482 (2023).
Schrimpf, C. et al. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J. Am. Soc. Nephrol. 23, 868–883 (2012).
Kramann, R. et al. Gli1+ pericyte loss induces capillary rarefaction and proximal tubular injury. J. Am. Soc. Nephrol. 28, 776–784 (2017).
Schiessl, I. M. et al. Renal interstitial platelet-derived growth factor receptor-β cells support proximal tubular regeneration. J. Am. Soc. Nephrol. 29, 1383–1396 (2018).
Lemos, D. R. et al. Maintenance of vascular integrity by pericytes is essential for normal kidney function. Am. J. Physiol. Ren. Physiol. 311, F1230–F1242 (2016).
Tanaka, S., Portilla, D. & Okusa, M. D. Role of perivascular cells in kidney homeostasis, inflammation, repair and fibrosis. Nat. Rev. Nephrol. 19, 721–732 (2023).
Guo, C. et al. Crosstalk between proximal tubular epithelial cells and other interstitial cells in tubulointerstitial fibrosis after renal injury. Front. Endocrinol. 14, 1256375 (2023).
Sparks, M. A. et al. Vascular control of kidney epithelial transporters. Am. J. Physiol. Ren. Physiol. 320, F1080–F1092 (2021).
Bankir, L. & de Rouffignac, C. Urinary concentrating ability: insights from comparative anatomy. Am. J. Physiol. 249, R643–R666 (1985).
Swanson, R. A., Ying, W. & Kauppinen, T. M. Astrocyte influences on ischemic neuronal death. Curr. Mol. Med. 4, 193–205 (2004).
Rangel-Gomez, M. et al. Neuron–glial interactions: implications for plasticity, behavior, and cognition. J. Neurosci. 44, e1231242024 (2024).
Ding, H. et al. Inhibiting aerobic glycolysis suppresses renal interstitial fibroblast activation and renal fibrosis. Am. J. Physiol. Ren. Physiol. 313, F561–F575 (2017).
Shen, Y. et al. Tubule-derived lactate is required for fibroblast activation in acute kidney injury. Am. J. Physiol. Ren. Physiol. 318, F689–F701 (2020).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed substantially to discussion of the content and wrote the article. L.O. and T.J.C. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Sunder Sims-Lucas and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fusco, A.N., Oxburgh, L. & Carroll, T.J. The kidney stroma in development and disease. Nat Rev Nephrol 21, 756–777 (2025). https://doi.org/10.1038/s41581-025-00985-8
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41581-025-00985-8
This article is cited by
-
Developmentally inspired synthetic kidney engineering
Nature Biotechnology (2026)
-
Cell–cell crosstalk in kidney health and disease
Nature Reviews Nephrology (2025)