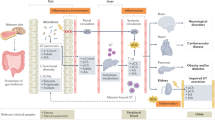
Abstract
More than 850 million individuals worldwide, accounting for 10–15% of the adult population, are estimated to have chronic kidney disease. Each of these individuals is host to tens of trillions of microorganisms that are collectively referred to as microbiota — a dynamic ecosystem that both influences host health and is itself influenced by changes in the host. Available evidence supports the existence of functional connections between resident microorganisms and kidney health that are altered in the context of specific kidney diseases, including acute kidney injury, chronic kidney disease and renal stone disease. Moreover, promising data from preclinical studies suggest that targeting of gut microbial pathways may provide new therapeutic opportunities for the treatment of kidney disease. This Roadmap describes current understanding of the mechanisms by which microorganisms regulate host organ function, the effects of kidney disease on the gut microbiome, and how these insights may contribute to the development of microbe-targeted therapeutics. We highlight key knowledge gaps that remain to be addressed and strategies for addressing these, outlining both the promise and the potential pitfalls of leveraging our understanding of the gut microbiota to better understand and treat kidney disease.
Key points
-
A bidirectional relationship exists between microorganisms and kidney disease: microorganisms can influence host health, and are themselves influenced by changes in host health.
-
The relationship between microorganisms and renal disease is likely to be at least somewhat specific to each disease type.
-
Further studies are needed to determine the prognostic value of gut microorganisms in kidney disease.
-
Available evidence suggests that interactions occur between microorganisms and drugs used to treat kidney disease; further studies are needed to determine the nature and consequences of these interactions.
-
There is also promise in the possibility of targeting gut microorganisms as a therapeutic strategy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hill, N. R. et al. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS ONE 11, e0158765 (2016).
Snelson, M. et al. A renal clinician’s guide to the gut microbiota. J. Ren. Nutr. 30, 384–395 (2020).
Lambert, K. et al. Targeting the gut microbiota in kidney disease: the future in renal nutrition and metabolism. J. Ren. Nutr. 33, S30–S39 (2023).
Stanford, J. et al. The gut microbiota profile of adults with kidney disease and kidney stones: a systematic review of the literature. BMC Nephrol. 21, 215 (2020).
Holle, J. et al. Inflammation in children with CKD linked to gut dysbiosis and metabolite imbalance. J. Am. Soc. Nephrol. 33, 2259–2275 (2022).
Holle, J. et al. Gut microbiome alterations precede graft rejection in kidney transplantation patients. Am. J. Transpl. 18, S1600–S6135 (2025).
Vaziri, N. D. et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 83, 308–315 (2013).
Nishiyama, K. et al. Chronic kidney disease after 5/6 nephrectomy disturbs the intestinal microbiota and alters intestinal motility. J. Cell Physiol. 234, 6667–6678 (2019).
Mishima, E. et al. Canagliflozin reduces plasma uremic toxins and alters the intestinal microbiota composition in a chronic kidney disease mouse model. Am. J. Physiol. Renal Physiol. 315, F824–F833 (2018).
Vaziri, N. D., Yuan, J. & Norris, K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am. J. Nephrol. 37, 1–6 (2013).
Chaves, L. D. et al. Chronic kidney disease, uremic milieu, and its effects on gut bacterial microbiota dysbiosis. Am. J. Physiol. Renal Physiol. 315, F487–F502 (2018).
Wong, J. et al. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 39, 230–237 (2014).
Wang, X. et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 69, 2131–2142 (2020).
Laiola, M. et al. Toxic microbiome and progression of chronic kidney disease: insights from a longitudinal CKD-Microbiome Study. Gut https://doi.org/10.1136/gutjnl-2024-334634 (2025).
Noel, S. et al. Gut microbiota-immune system interactions during acute kidney injury. Kidney360 2, 528–531 (2021).
Noel, S. et al. Metagenomic sequencing reveals distinct gut microbiome profiles in patients with AKI compared to CKD and normals: KPMP project. Am. Soc. Neph. https://doi.org/10.1681/ASN.2024k2n81acd (2024).
Mishima, E. et al. Evaluation of the impact of gut microbiota on uremic solute accumulation by a CE-TOFMS-based metabolomics approach. Kidney Int. 92, 634–645 (2017).
Avery, E. G. et al. Quantifying the impact of gut microbiota on inflammation and hypertensive organ damage. Cardiovasc. Res. 119, 1441–1452 (2023).
Jang, H. R. et al. Early exposure to germs modifies kidney damage and inflammation after experimental ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 297, F1457–F1465 (2009).
Al, K. F. et al. Multi-site microbiota alteration is a hallmark of kidney stone formation. Microbiome 11, 263 (2023).
Moore, B. N. et al. Commensal microbiota regulate aldosterone. Am. J. Physiol. Renal Physiol. 326, F1032–F1038 (2024).
Moore, B. N. & Pluznick, J. L. Commensal microbiota regulate renal gene expression in a sex-specific manner. Am. J. Physiol. Renal Physiol. 324, F511–F520 (2023).
Xu, J. et al. Microbes regulate glomerular filtration rate in health and chronic kidney disease in mice. Prerit at bioRxiv https://doi.org/10.1101/2025.04.08.647647 (2025).
Gupta, N. et al. Targeted inhibition of gut microbial trimethylamine N-oxide production reduces renal tubulointerstitial fibrosis and functional impairment in a murine model of chronic kidney disease. Arterioscler. Thromb. Vasc. Biol. 40, 1239–1255 (2020).
Wang, M. et al. The gut microbial metabolite trimethylamine N -oxide, incident CKD, and kidney function decline. J. Am. Soc. Nephrol. 35, 749–760 (2024).
Andrikopoulos, P. et al. Evidence of a causal and modifiable relationship between kidney function and circulating trimethylamine N-oxide. Nat. Commun. 14, 5843 (2023).
Witkowski, M., Weeks, T. L. & Hazen, S. L. Gut microbiota and cardiovascular disease. Circ. Res. 127, 553–570 (2020).
Holle, J. et al. Gut dysbiosis contributes to TMAO accumulation in CKD. Nephrol. Dial. Transpl. 39, 1923–1926 (2024).
Gryp, T. et al. Gut microbiota generation of protein-bound uremic toxins and related metabolites is not altered at different stages of chronic kidney disease. Kidney Int. 97, 1230–1242 (2020).
Agudelo, J. & Miller, A. W. A perspective on the metabolic potential for microbial contributions to urolithiasis. Kidney360 2, 1170–1173 (2021).
Miller, A. W. et al. Mechanisms of the intestinal and urinary microbiome in kidney stone disease. Nat. Rev. Urol. 19, 695–707 (2022).
Stepanova, N. Role of impaired oxalate homeostasis in cardiovascular disease in patients with end-stage renal disease: an opinion article. Front. Pharmacol. 12, 692429 (2021).
Nazzal, L., Puri, S. & Goldfarb, D. S. Enteric hyperoxaluria: an important cause of end-stage kidney disease. Nephrol. Dialysis Transplant. 31, 375–382 (2015).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Tang, W. H. W. & Hazen, S. L. Unraveling the complex relationship between gut microbiome and cardiovascular diseases. Circulation 149, 1543–1545 (2024).
Johnson, A. J. et al. Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host Microbe 25, 789–802.e5 (2019).
Mailing, L. J. et al. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc. Sport. Sci. Rev. 47, 75–85 (2019).
Vandecruys, M. et al. Revitalizing the gut microbiome in chronic kidney disease: a comprehensive exploration of the therapeutic potential of physical activity. Toxins 16, 242 (2024).
Maier, L. et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555, 623–628 (2018).
Biruete, A. et al. Phosphate binders and nonphosphate effects in the gastrointestinal tract. J. Ren. Nutr. 30, 4–10 (2020).
Asnicar, F. et al. Blue poo: impact of gut transit time on the gut microbiome using a novel marker. Gut 70, 1665–1674 (2021).
O’Donnell, J. A. et al. The gut microbiome and hypertension. Nat. Rev. Nephrol. 19, 153–167 (2023).
Byndloss, M. et al. The gut microbiota and diabetes: research, translation, and clinical applications-2023 diabetes, Diabetes Care, and Diabetologia Expert Forum. Diabetes Care 47, 1491–1508 (2024).
Teixeira, R. R. et al. Gut microbiota profile of patients on peritoneal dialysis: comparison with household contacts. Eur. J. Clin. Nutr. 77, 90–97 (2022).
Poesen, R. et al. The influence of CKD on colonic microbial metabolism. J. Am. Soc. Nephrol. 27, 1389–1399 (2016).
Wiese, G. N. et al. Gut microbiota and uremic retention solutes in adults with moderate CKD: a 6-day controlled feeding study. J. Ren. Nutr. 34, 26–34 (2023).
Wu, G. et al. A core microbiome signature as an indicator of health. Cell 187, 6550–6565.e11 (2024).
Liu, F. et al. Sex-specific dysbiotic bladder microbiome in CKD uncovered via high-throughput sequencing and culture. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-3407275/v1 (2023).
Hrbacek, J. et al. Bladder microbiota are associated with clinical conditions that extend beyond the urinary tract. Microorganisms 10, 874 (2022).
Kachroo, N. et al. Meta-analysis of clinical microbiome studies in urolithiasis reveal age, stone composition, and study location as the predominant factors in urolithiasis-associated microbiome composition. Mbio 12, e02007-21 (2021).
Liang, J. & Liu, Y. Animal models of kidney disease: challenges and perspectives. Kidney360 4, 1479–1493 (2023).
Mirzayi, C. et al. Reporting guidelines for human microbiome research: the STORMS checklist. Nat. Med. 27, 1885–1892 (2021).
Abdill, R. J., Adamowicz, E. M. & Blekhman, R. Public human microbiome data are dominated by highly developed countries. PLoS Biol. 20, e3001536 (2022).
Muralitharan, R. R. et al. Guidelines for microbiome studies in renal physiology. Am. J. Physiol. Renal Physiol. 325, F345–F362 (2023).
Wensel, C. R. et al. Next-generation sequencing: insights to advance clinical investigations of the microbiome. J. Clin. Invest. 132, e154944 (2022).
Nasko, D. J. et al. RefSeq database growth influences the accuracy of k-mer-based lowest common ancestor species identification. Genome Biol. 19, 165 (2018).
Smith, R. H. et al. Investigating the impact of database choice on the accuracy of metagenomic read classification for the rumen microbiome. Anim. Microbiome 4, 57 (2022).
Mills, S. et al. Precision nutrition and the microbiome, part I: current state of the science. Nutrients 11, 1468 (2019).
Sommer, F. & Bäckhed, F. The gut microbiota — masters of host development and physiology. Nat. Rev. Microbiol. 11, 227–238 (2013).
Walter, J. & Ley, R. The human gut microbiome: ecology and recent evolutionary changes. Annu. Rev. Microbiol. 65, 411–429 (2011).
Meijers, B., Evenepoel, P. & Anders, H. J. Intestinal microbiome and fitness in kidney disease. Nat. Rev. Nephrol. 15, 531–545 (2019).
Mulle, J. G., Sharp, W. G. & Cubells, J. F. The gut microbiome: a new frontier in autism research. Curr. Psychiatry Rep. 15, 337 (2013).
Pluznick, J. L. The gut microbiota in kidney disease. Science 369, 1426–1427 (2020).
Harrison, M. A. et al. Production of p-cresol by decarboxylation of p-HPA by all five lineages of Clostridioides difficile provides a growth advantage. Front. Cell. Infect. Microbiol. 11, 757599 (2021).
Fishbane, S. N. & Nigwekar, S. Phosphate absorption and hyperphosphatemia management in kidney disease: a physiology-based review. Kidney Med. 3, 1057–1064 (2021).
Weiner, I. D., Mitch, W. E. & Sands, J. M. Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin. J. Am. Soc. Nephrol. 10, 1444–1458 (2015).
Tonelli, M., Karumanchi, S. A. & Thadhani, R. Epidemiology and mechanisms of uremia-related cardiovascular disease. Circulation 133, 518–536 (2016).
Chen, Y. et al. Kidney clearance of secretory solutes is associated with progression of CKD: the CRIC study. J. Am. Soc. Nephrol. 31, 817–827 (2020).
Chen, Y. et al. Association of tubular solute clearances with the glomerular filtration rate and complications of chronic kidney disease: the chronic renal insufficiency cohort study. Nephrol. Dial. Transpl. 36, 1271–1281 (2020).
Lim, Y. J. et al. Uremic toxins in the progression of chronic kidney disease and cardiovascular disease: mechanisms and therapeutic targets. Toxins 13, 142 (2021).
Owada, S. et al. Indoxyl sulfate reduces superoxide scavenging activity in the kidneys of normal and uremic rats. Am. J. Nephrol. 28, 446–454 (2008).
Niwa, T. Indoxyl sulfate is a nephro-vascular toxin. J. Ren. Nutr. 20, S2–S6 (2010).
Liu, W. C., Tomino, Y. & Lu, K. C. Impacts of indoxyl sulfate and p-cresol sulfate on chronic kidney disease and mitigating effects of AST-120. Toxins 10, 367 (2018).
Al-mansouri, A. et al. Assessment of treatment burden and its impact on quality of life in dialysis-dependent and pre-dialysis chronic kidney disease patients. Res. Soc. Adm. Pharm. 17, 1937–1944 (2021).
Tang, W. H. et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 116, 448–455 (2015).
Meijers, B. K. et al. p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin. J. Am. Soc. Nephrol. 5, 1182–1189 (2010).
Ren, X. et al. Plasma metabolomics of dietary intake of protein-rich foods and kidney disease progression in children. J. Ren. Nutr. 34, 95–104 (2024).
Liu, J. et al. Effect of dapagliflozin on proteomics and metabolomics of serum from patients with type 2 diabetes. Diabetol. Metab. Syndr. 15, 251 (2023).
Koeth, R. A. et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19, 576–585 (2013).
Tang, W. H. et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 368, 1575–1584 (2013).
Saito, Y. et al. Identification of phenol- and p-cresol-producing intestinal bacteria by using media supplemented with tyrosine and its metabolites. FEMS Microbiol. Ecol. 94, fiy125 (2018).
Whittaker, R. Evolution and measurement of species diversity. Taxon 21, 213–251 (1972).
Breiman, L. Random forests. Mach. Learn. 45, 5–32 (2001).
Knights, D. et al. Human-associated microbial signatures: examining their predictive value. Cell Host Microbe 10, 292–296 (2011).
Hu, X. et al. Characterizing the gut microbiota in patients with chronic kidney disease. Postgrad. Med. 132, 495–505 (2020).
Ren, Z. et al. Alterations of the human gut microbiome in chronic kidney disease. Adv. Sci. 7, 2001936 (2020).
Yu, W. et al. The gut microbiome in differential diagnosis of diabetic kidney disease and membranous nephropathy. Ren. Fail. 42, 1100–1110 (2020).
Khasnobish, A. et al. Dysbiosis in the salivary microbiome associated with IgA nephropathy — a Japanese cohort study. Microbes Env. 36, ME21006 (2021).
Du, X. et al. Alteration of gut microbial profile in patients with diabetic nephropathy. Endocrine 73, 71–84 (2021).
Yu, B. et al. The gut microbiome in microscopic polyangiitis with kidney involvement: common and unique alterations, clinical association and values for disease diagnosis and outcome prediction. Ann. Transl. Med. 9, 1286 (2021).
Xiang, L. et al. Prediction of the occurrence of calcium oxalate kidney stones based on clinical and gut microbiota characteristics. World J. Urol. 40, 221–227 (2022).
Shi, X. et al. Alterations of gut microbial pathways and virulence factors in hemodialysis patients. Front. Cell Infect. Microbiol. 12, 904284 (2022).
Dong, Y. et al. Development and validation of diagnostic models for immunoglobulin A nephropathy based on gut microbes. Front. Cell Infect. Microbiol. 12, 1059692 (2022).
Tang, Y. et al. Aberrant gut microbiome contributes to barrier dysfunction, inflammation, and local immune responses in IgA nephropathy. Kidney Blood Press. Res. 48, 261–276 (2023).
Chen, T. H. et al. Exploring the relevance between gut microbiota-metabolites profile and chronic kidney disease with distinct pathogenic factor. Microbiol. Spectr. 11, e0280522 (2023).
Cai, F. et al. Systematic microbiome dysbiosis is associated with IgA nephropathy. Microbiol. Spectr. 11, e0520222 (2023).
Jiang, Y. et al. Combination of the gut microbiota and clinical indicators as a potential index for differentiating idiopathic membranous nephropathy and minimal change disease. Ren. Fail. 45, 2209392 (2023).
Tang, S. et al. Guild-level signature of gut microbiome for diabetic kidney disease. mBio 15, e0073524 (2024).
Lee, A. M. et al. Using machine learning to identify metabolomic signatures of pediatric chronic kidney disease etiology. J. Am. Soc. Nephrol. 33, 375–386 (2022).
Hu, J. et al. Location-specific oral microbiome possesses features associated with CKD. Kidney Int. Rep. 3, 193–204 (2017).
Yang, Y. et al. The genetics of urinary microbiome, an exploration of the trigger in calcium oxalate stone. Front. Genet. 14, 1260278 (2023).
NIH/FDA. BEST (Biomarkers, EndpointS, and other Tools) Resource. ncbi.nlm.nih.gov https://www.ncbi.nlm.nih.gov/books/NBK326791/ (FDA, 2016).
Bennett, M. & Devarajan, P. in Biomarkers of Kidney Disease (ed. Edelstein, C.) 1–24 (Academic, 2011).
Yu, Y. et al. Assessing and mitigating batch effects in large-scale omics studies. Genome Biol. 25, 254 (2024).
Agudelo, J. et al. Delineating the role of the urinary metabolome in the lithogenesis of calcium-based kidney stones. Urology 167, 49–55 (2022).
Vaswani, A. et al. Attention is all you need. In 31st Conference on Neural Information Processing Systems (NIPS) (eds. Guyon, I. et al.) https://papers.nips.cc/paper_files/paper/2017/hash/3f5ee243547dee91fbd053c1c4a845aa-Abstract.html (2017).
Theodosiou, A. A. & Read, R. C. Artificial intelligence, machine learning and deep learning: potential resources for the infection clinician. J. Infect. 87, 287–294 (2023).
Trepka, K. R. et al. Pharma[e]cology: how the gut microbiome contributes to variations in drug response. Annu. Rev. Pharmacol. Toxicol. 65, 355–373 (2024).
Wallace, B. D. et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 330, 831–835 (2010).
Haiser, H. J. et al. Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science 341, 295–298 (2013).
Simpson, J. B. et al. Metagenomics combined with activity-based proteomics point to gut bacterial enzymes that reactivate mycophenolate. Gut Microbes 14, 2107289 (2022).
Yang, T. et al. Identification of a gut commensal that compromises the blood pressure-lowering effect of ester angiotensin-converting enzyme inhibitors. Hypertension 79, 1591–1601 (2022).
Kyoung, J. & Yang, T. Depletion of the gut microbiota enhances the blood pressure-lowering effect of captopril: implication of the gut microbiota in resistant hypertension. Hypertens. Res. 45, 1505–1510 (2022).
Vallon, V. & Verma, S. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu. Rev. Physiol. 83, 503–528 (2021).
Billing, A. M. et al. Metabolic communication by SGLT2 inhibition. Circulation 149, 860–884 (2024).
Chrysopoulou, M. & Rinschen, M. M. Metabolic rewiring and communication: an integrative view of kidney proximal tubule function. Annu. Rev. Physiol. 86, 405–427 (2024).
Szymczak-Pajor, I. et al. The gut microbiota-related antihyperglycemic effect of metformin. Pharmaceuticals 18, 55 (2025).
Nigam, S. K. The SLC22 transporter family: a paradigm for the impact of drug transporters on metabolic pathways, signaling, and disease. Annu. Rev. Pharmacol. Toxicol. 58, 663–687 (2018).
Hakimi, S., Dutta, P. & Layton, A. T. Renal calcium and magnesium handling during pregnancy: modeling and analysis. Am. J. Physiol. Renal Physiol. 327, F77–F90 (2024).
Stadt, M. M. & Layton, A. T. A modeling analysis of whole body potassium regulation on a high-potassium diet: proximal tubule and tubuloglomerular feedback effects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 326, R401–R415 (2024).
Jariwala, P. B. et al. Discovering the microbial enzymes driving drug toxicity with activity-based protein profiling. ACS Chem. Biol. 15, 217–225 (2020).
Rinschen, M. M. et al. Accelerated lysine metabolism conveys kidney protection in salt-sensitive hypertension. Nat. Commun. 13, 4099 (2022).
Andrade-Oliveira, V. et al. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J. Am. Soc. Nephrol. 26, 1877–1888 (2015).
Emal, D. et al. Depletion of gut microbiota protects against renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 28, 1450–1461 (2017).
Nakade, Y. et al. Gut microbiota-derived D-serine protects against acute kidney injury. JCI Insight 3, e97957 (2018).
Zhu, H. et al. The probiotic L. casei Zhang slows the progression of acute and chronic kidney disease. Cell Metab. 33, 1926–1942.e8 (2021).
Yang, Z. et al. The prevention effect of Limosilactobacillus reuteri on acute kidney injury by regulating gut microbiota. Microbiol. Immunol. 68, 213–223 (2024).
Gharaie, S. et al. Microbiome modulation after severe acute kidney injury accelerates functional recovery and decreases kidney fibrosis. Kidney Int. 104, 470–491 (2023).
Li, H.-B. et al. Faecalibacterium prausnitzii attenuates CKD via butyrate-renal GPR43 axis. Circulation Res. 131, e120–e134 (2022).
Shankaranarayanan, D. & Raj, D. S. Gut microbiome and kidney disease: reconciling optimism and skepticism. Clin. J. Am. Soc. Nephrol. 17, 1694–1696 (2022).
He, M. et al. Gut microbial metabolites SCFAs and chronic kidney disease. J. Transl. Med. 22, 172 (2024).
Tang, Z., Yu, S. & Pan, Y. The gut microbiome tango in the progression of chronic kidney disease and potential therapeutic strategies. J. Transl. Med. 21, 689 (2023).
Gao, B. et al. Butyrate producing microbiota are reduced in chronic kidney diseases. Sci. Rep. 11, 23530 (2021).
Guo, X. et al. Novel metabolites to improve glomerular filtration rate estimation. Kidney Blood Press. Res. 48, 287–296 (2023).
Yamaguchi, Y. et al. Plasma metabolites associated with chronic kidney disease and renal function in adults from the Baltimore longitudinal study of aging. Metabolomics 17, 1–11 (2021).
Peng, H. et al. A metabolomics study of metabolites associated with the glomerular filtration rate. BMC Nephrol. 24, 105 (2023).
Li, T. et al. Consistency of metabolite associations with measured glomerular filtration rate in children and adults. Clin. Kidney J. 17, sfae108 (2024).
Roberts, A. B. et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat. Med. 24, 1407–1417 (2018).
Graboski, A. L. et al. Mechanism-based inhibition of gut microbial tryptophanases reduces serum indoxyl sulfate. Cell Chem. Biol. 30, 1402–1413.e7 (2023).
Winkler, M. K. H. & van Loosdrecht, M. C. M. Intensifying existing urban wastewater. Science 375, 377–378 (2022).
Candry, P. et al. Tailoring polyvinyl alcohol-sodium alginate (PVA-SA) hydrogel beads by controlling crosslinking pH and time. Sci. Rep. 12, 20822 (2022).
Godfrey, B. et al. Co-immobilization of AOA strains with Anammox bacteria in three different synthetic bio-granules maintained under two substrate-level conditions. Chemosphere 342, 140192 (2023).
Gottshall, E. Y. et al. Sustained nitrogen loss in a symbiotic association of comammox Nitrospira and anammox bacteria. Water Res. 202, 117426 (2021).
Landreau, M. et al. Immobilization of active ammonia-oxidizing archaea in hydrogel beads. npj Clean Water 4, 43 (2021).
Li, B. et al. Mainstream nitrogen removal from low temperature and low ammonium strength municipal wastewater using hydrogel-encapsulated comammox and anammox. Water Res. 242, 120303 (2023).
Saingam, P. et al. Towards an effective delivery system of a microbial sink of the uremic toxin, p-cresol; an in vitro study with Thauera aminoaromatica S2. Front. Microbiol. 16, 1577556 (2025).
Ogawa, T. et al. Oral administration of Bifidobacterium longum in a gastro-resistant seamless capsule decreases serum phosphate levels in patients receiving haemodialysis. Nephrol. Dialysis Transplant. 5, 373–374 (2012).
Wang, I.-K. et al. The effect of probiotics on serum levels of cytokine and endotoxin in peritoneal dialysis patients: a randomised, double-blind, placebo-controlled trial. Benef. Microbes 6, 423–430 (2015).
Soleimani, A. et al. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 91, 435–442 (2017).
Taki, K., Takayama, F. & Niwa, T. Beneficial effects of bifidobacteria in a gastroresistant seamless capsule on hyperhomocysteinemia in hemodialysis patients. J. Ren. Nutr. 15, 77–80 (2005).
Ikizler, T. A. et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am. J. Kidney Dis. 76, S1–S107 (2020).
Stanford, J. et al. High-diversity plant-based diet and gut microbiome, plasma metabolome, and symptoms in adults with CKD. Clin. J. Am. Soc. Nephrol. 20, 619–631 (2025).
Felizardo, R. J. F. et al. Gut microbial metabolite butyrate protects against proteinuric kidney disease through epigenetic- and GPR109a-mediated mechanisms. FASEB J. 33, 11894–11908 (2019).
Li, Y. J. et al. Dietary fiber protects against diabetic nephropathy through short-chain fatty acid-mediated activation of G protein-coupled receptors GPR43 and GPR109A. J. Am. Soc. Nephrol. 31, 1267–1281 (2020).
Li, Y. J. et al. Short-chain fatty acids directly exert anti-inflammatory responses in podocytes and tubular epithelial cells exposed to high glucose. Front. Cell Dev. Biol. 11, 1182570 (2023).
Corte-Iglesias, V. et al. Propionate and butyrate counteract renal damage and progression to chronic kidney disease. Nephrol. Dial. Transpl. 40, 133–150 (2024).
Lobel, L. et al. Diet posttranslationally modifies the mouse gut microbial proteome to modulate renal function. Science 369, 1518–1524 (2020).
Linares, L. et al. Epidemiology and outcomes of multiple antibiotic-resistant bacterial infection in renal transplantation. Transpl. Proc. 39, 2222–2224 (2007).
Magruder, M. et al. Gut uropathogen abundance is a risk factor for development of bacteriuria and urinary tract infection. Nat. Commun. 10, 5521 (2019).
Shimasaki, T. et al. Increased relative abundance of Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae within the gut microbiota is associated with risk of bloodstream infection in long-term acute care hospital patients. Clin. Infect. Dis. 68, 2053–2059 (2019).
Taur, Y. et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis. 55, 905–914 (2012).
Ghani, R. et al. Disease prevention not decolonization: a model for fecal microbiota transplantation in patients colonized with multidrug-resistant organisms. Clin. Infect. Dis. 72, 1444–1447 (2021).
Mangalea, M. R. et al. Decolonization and pathogen reduction approaches to prevent antimicrobial resistance and healthcare-associated infections. Emerg. Infect. Dis. 30, 1069 (2024).
Roman, Y. M. The role of uric acid in human health: insights from the uricase gene. J. Pers. Med. 13, 1409 (2023).
Krishnan, E. Reduced glomerular function and prevalence of gout: NHANES 2009-10. PLoS ONE 7, e50046 (2012).
Sorensen, L. B. Role of the intestinal tract in the elimination of uric acid. Arthritis Rheum. 8, 694–706 (1965).
Vargas-Santos, A. B. & Neogi, T. Management of gout and hyperuricemia in CKD. Am. J. Kidney Dis. 70, 422–439 (2017).
Liu, Y. et al. A widely distributed gene cluster compensates for uricase loss in hominids. Cell 186, 3400–3413 e20 (2023).
Kasahara, K. et al. Gut bacterial metabolism contributes to host global purine homeostasis. Cell Host Microbe 31, 1038–1053.e10 (2023).
Moe, O. W. Kidney stones: pathophysiology and medical management. Lancet 367, 333–344 (2006).
Filler, G. et al. In focus: perplexing increase of urinary stone disease in children, adolescent and young adult women and its economic impact. Front. Med. 10, 1272900 (2023).
Vo, A. K. et al. Measuring quality of life in patients with kidney stone disease: is it the future in endourology? Curr. Opin. Urol. 34, 91–97 (2024).
Hatch, M. et al. Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int. 69, 691–698 (2006).
Campieri, C. et al. Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int. 60, 1097–1105 (2001).
Lieske, J. C. et al. Use of a probiotic to decrease enteric hyperoxaluria. Kidney Int. 68, 1244–1249 (2005).
Goldfarb, D. S., Modersitzki, F. & Asplin, J. R. A randomized, controlled trial of lactic acid bacteria for idiopathic hyperoxaluria. Clin. J. Am. Soc. Nephrol. 2, 745–749 (2007).
Lieske, J. C. et al. Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int. 78, 1178–1185 (2010).
Siener, R. et al. Dietary hyperoxaluria is not reduced by treatment with lactic acid bacteria. J. Transl. Med. 11, 306 (2013).
Tavasoli, S. et al. Effect of a probiotic supplement containing Lactobacillus acidophilus and Bifidobacterium animalis lactis on urine oxalate in calcium stone formers with hyperoxaluria: a randomized, placebo-controlled, double-blind and in-vitro trial. Urol. J. 19, 179–188 (2021).
Hoppe, B. et al. A randomised phase I/II trial to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Pediatr. Nephrol. 32, 781–790 (2017).
Hoppe, B. et al. Efficacy and safety of Oxalobacter formigenes to reduce urinary oxalate in primary hyperoxaluria. Nephrol. Dial. Transpl. 26, 3609–3615 (2011).
Milliner, D., Hoppe, B. & Groothoff, J. A randomised phase II/III study to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Urolithiasis 46, 313–323 (2018).
Mukherjee, S. D. et al. Complex system modeling reveals oxalate homeostasis is driven by diverse oxalate-degrading bacteria. eLife 14, RP104121 (2025).
Duranton, F. et al. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 23, 1258–1270 (2012).
Waikar, S. S. et al. Association of urinary oxalate excretion with the risk of chronic kidney disease progression. JAMA Intern. Med. 179, 542–551 (2019).
Ermer, T. et al. Oxalate, inflammasome, and progression of kidney disease. Curr. Opin. Nephrol. Hypertens. 25, 363–371 (2016).
Pfau, A. et al. High oxalate concentrations correlate with increased risk for sudden cardiac death in dialysis patients. J. Am. Soc. Nephrol. 32, 2375–2385 (2021).
Choy, W. H. et al. Deficient butyrate metabolism in the intestinal microbiome is a potential risk factor for recurrent kidney stone disease. Urolithiasis 52, 38 (2024).
Zampini, A. et al. Defining dysbiosis in patients with urolithiasis. Sci. Rep. 9, 5425 (2019).
Agudelo, J. et al. Cefazolin shifts the kidney microbiota to promote a lithogenic environment. Nat. Commun. 15, 10509 (2024).
Chesnaye, N. C. et al. Differences in the epidemiology, management and outcomes of kidney disease in men and women. Nat. Rev. Nephrol. 20, 7–20 (2024).
de la Cuesta-Zuluaga, J. et al. Age- and sex-dependent patterns of gut microbial diversity in human adults. mSystems 4, e00261-19 (2019).
Perez, M. et al. A synthetic consortium of 100 gut commensals modulates the composition and function in a colon model of the microbiome of elderly subjects. Gut Microbes 13, 1–19 (2021).
El Houari, A. et al. Development of an in vitro model of human gut microbiota for screening the reciprocal interactions with antibiotics, drugs, and xenobiotics. Front. Microbiol. 13, 828359 (2022).
Cheng, A. G. et al. Design, construction, and in vivo augmentation of a complex gut microbiome. Cell 185, 3617–3636.e19 (2022).
Wang, M. et al. Strain dropouts reveal interactions that govern the metabolic output of the gut microbiome. Cell 186, 2839–2852.e21 (2023).
Pascal Andreu, V. et al. gutSMASH predicts specialized primary metabolic pathways from the human gut microbiota. Nat. Biotechnol. 41, 1416–1423 (2023).
Mei, X. et al. Genetically engineered Lactobacillus paracasei rescues colonic angiotensin converting enzyme 2 (ACE2) and attenuates hypertension in female Ace2 knock out rats. Pharmacol. Res. 196, 106920 (2023).
Guo, C. J. et al. Depletion of microbiome-derived molecules in the host using clostridium genetics. Science 366, eaav1282 (2019).
Rubin, B. E. et al. Species- and site-specific genome editing in complex bacterial communities. Nat. Microbiol. 7, 34–47 (2022).
Brödel, A. K. et al. In situ targeted base editing of bacteria in the mouse gut. Nature 632, 877–884 (2024).
Ali, N. et al. Advances in CRISPR-Cas systems for gut microbiome. Prog. Mol. Biol. Transl. Sci. 208, 59–81 (2024).
Kelly, C. R. et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am. J. Gastroenterol. 109, 1065–1071 (2014).
Cheng, Y.-W. et al. Fecal microbiota transplantation for the treatment of recurrent and severe Clostridium difficile infection in solid organ transplant recipients: a multicenter experience. Am. J. Transplant. 19, 501–511 (2019).
Peery, A. F. et al. AGA clinical practice guideline on fecal microbiota–based therapies for select gastrointestinal diseases. Gastroenterology 166, 409–434 (2024).
Carlson, P. E. Regulatory considerations for fecal microbiota transplantation products. Cell Host Microbe 27, 173–175 (2020).
Woodworth, M. H. et al. Fecal microbiota transplantation promotes reduction of antimicrobial resistance by strain replacement. Sci. Transl. Med. 15, eabo2750 (2023).
Tang, Q. et al. Current sampling methods for gut microbiota: a call for more precise devices. Front. Cell. Infect. Microbiol. 10, 151 (2020).
Zhang, X. & Figeys, D. Perspective and guidelines for metaproteomics in microbiome studies. J. Proteome Res. 18, 2370–2380 (2019).
James, K. R. et al. Distinct microbial and immune niches of the human colon. Nat. Immunol. 21, 343–353 (2020).
Anandakumar, H. et al. Segmental patterning of microbiota and immune cells in the murine intestinal tract. Gut Microbes 16, 2398126 (2024).
Shalon, D. et al. Profiling the human intestinal environment under physiological conditions. Nature 617, 581–591 (2023).
Folz, J. et al. Human metabolome variation along the upper intestinal tract. Nat. Metab. 5, 777–788 (2023).
Culver, R. N. et al. Improved mouse models of the small intestine microbiota using region-specific sampling from humans. Preprint at bioRxiv https://doi.org/10.1101/2024.04.24.590999 (2024).
Acknowledgements
The authors would like to acknowledge the workshop sponsored by the National Institutes of Health (NIH) on 28 and 29 May 2024 entitled “Gut Microbiota and Kidney Disease” and hosted by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The authors would specifically like to acknowledge D. Nihalani for helping to develop the workshop and bringing this expert panel together. They would also like to acknowledge other members of the NIDDK — P. Perrin, R. Lunsford, C. Maric-Bilkan, C. Mullins, C. J. Ketchum, and D. Gossett — who also helped to develop the workshop. The authors are grateful for funding that has supported their work: P.P.B. was supported by NIDDK grant K23 DK138239, and a research grant from Vedanta Biosciences, Inc.; K.L.P. was supported by NIH grant U24 DK127726; M.-K.H.W. was supported by NIH Katz Early Investigator Award R01 DK130815; S.L.H. was supported by NIH grants R01 HL172805, R01 HL103866 and P01 HL147823; J.A. was supported by the Urology Care Foundation grant UCF202-JA and ISAC award 22AU4279; A. Babiker was supported by an Antibacterial Resistance Leadership Group Early Faculty Seedling Award (NIAID) UM1 AI104681; D.D. was supported by NIH grants R35 GM142873 and R01 AT011396, the Stanford Microbiome Therapies Initiative and an OHF-ASN Foundation for Kidney Research Career Development Award; K.C.H. was supported by NIH grant RM1 GM135102; B.J. was supported by NHLBI grant R01 HL171401; A.W.M. was supported by NIDDK grant R01 DK121689; A.S. was supported by NIH grants R01 DK125256, U01 DK099914, and U01 DK099924; P.J.T. was supported by NIH grants R01 DK114034, R01 HL122593 and R01 CA255116; A.W.W. and the Rowett Institute were supported by core funding from the Scottish Government’s Rural and Environment Science and Analytical Services Division; N.W. was supported by the European Research Council grant 852796 under the European Union’s Horizon 2020 research and innovation programme, Corona-Stiftung grant S199/10080/2019, German Federal Ministry of Education and Research TAhRget grant 01EJ2202A and German Research Foundation (DFG) grant CRC 1470, 437531118; J.X. was supported by American Heart Association Career Development Award 23CDA1050485; T.Y. was supported by American Heart Association Career Development Award 852969, NIH grant R21 AG079357 and the University of Toledo Startup Fund; J.H. was supported by NIH grants UH3 TR003288, U2CTR004867, U01 DK133090, U24 DK114886, R01 DK133177 and R01 DK130815; M.R.R. is supported by NIH R35 GM152079; G.D.W. was supported by NIH grant R01 DK107566, the Center for Molecular Studies in Digestive and Liver Diseases under NIH grant P30 DK 050306, the PennCHOP MIcrobiome Program and the Penn Center for Nutritional Science and Medicine; H.R. was supported by NIDDK grants R01 DK123342 and R01 DK132278; M.H.W. was supported by NIAID grant K23 AI144036 and the US Centers for Disease Control and Prevention grant U54 CK000601; A.L.A. was supported by NIDDK grants K08 DK118176 and R01 DK138121, NCCIH grant R61 AT013008, DOD grant W81XWH2110644, the ISAC Award program, SUFU Foundation, Bristol Meyers Squibb Foundation, Cures Within Reach and the Urology Care Foundation; S.W. was supported by NIH grants R01 AI118807, R01 DK138912, R21 AI166263 and R21 AI171537 and Burroughs Wellcome Fund grant 1017880; M.M.R. was supported by DFG grants RI 2811/2-2 and SFB1192-project B10, Young Investigator Award NNF19OC0056043 from the Novo Nordisk Foundation, the Carlsberg Young Investigator fellowship and a grant by the Augustinusfonden, Denmark; A. Biruete was supported by NIH grant K12 TR004415 and the Showalter Trust Fund; A.H.A. was supported by NIH grants R01 DK107566, U24 DK060990, U24 DK137318 and UM1 TR004771; J.L.P. was supported by an American Heart Association Established Investigator Award and NIH grants R21 AG081683, R01 DK137762, R01 DK139021 and U54 DK137331.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
P.P.B. has received research funding from Vedanta Biosciences and consults for Nexilico and Boehringer Ingelheim. W.S.G. has received research funding from Merck, Sharpe & Dohme, and Astellas Pharmaceuticals. and serves on the scientific advisory boards of Empress Therapeutics, Freya Biosciences, Sail Biosciences and Seres Therapeutics. S.L.H. is a co-inventor on patents relating to diagnostics and therapeutics with a right to receive royalty payments for inventions or discoveries related to diagnostics or therapeutics from Cleveland Heart Lab, a fully owned subsidiary of Quest Diagnostics, and is a consultant for and receives research funds from Zehna Therapeutics. A. Babiker has served on a clinical advisory board for Beckman Coulter. M.A.F. is a co-founder of Kelonia and Revolution Medicines, a co-founder and director of Azalea Therapeutics, a member of the scientific advisory boards of the Chan Zuckerberg Initiative, NGM Biopharmaceuticals and TCG Labs/Soleil Labs, and an innovation partner at The Column Group. C.H. serves on the scientific advisory committee for Seres Therapeutics and Empress Therapeutics. K.K.-Z. has received honoraria from Fresenius Kabi. R.K. is a scientific advisory board member and consultant for BiomeSense, Inc., through which he has equity and receives income, is a scientific advisory board member and has equity in GenCirq, is a consultant for and receives income from DayTwo, has equity in and acts as a consultant for Cybele, is a co-founder of and has equity in Biota, Inc., and is a cofounder and scientific advisory board member of and has equity in Micronoma; the terms of these arrangements have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. A.W.M. has received funding from Coloplast and is a scientific advisory board member for the Oxalosis and Hyperoxaluria Foundation. H.R. is a scientific advisory board member for Renibus Therapeutics and Rapafusyn Pharmaceuticals. W.H.W.T. serves as consultant for Sequana Medical, Cardiol Therapeutics, Genomics plc, Zehna Therapeutics, WhiteSwell, Boston Scientific, CardiaTec Biosciences, Bristol Myers Squibb, Alleviant Medical, Alexion Pharmaceuticals, Salubris Biotherapeutics and BioCardia, and has received honoraria from Springer, Belvoir Media Group and the American Board of Internal Medicine. A.W.W. has a research grant from ZOE, Ltd. and consults for EnteroBiotix, Ltd. M.R.R. has received research funding from Merck and Lilly, and is a founder of Symberix, Inc. N.W. received speaker honoraria from Novartis and Bayer. G.D.W. is an advisory board member for Danone and BioCodex and receives research support from Intercept Pharmaceuticals. A.L.A. has received consulting fees from AbbVie, Inc., holds stock options in Watershed Medical and serves on advisory boards for GlaxoSmithKline and Desert Harvest. M.M.R. has received research funding from Novo Nordisk A/S, Copenhagen. H.A.H. is the co-founder, president and Chief Scientific Officer of Oxalo Therapeutics, and is a scientific advisory council member of Oxalosis and the Hyperoxaluria Foundation. A. Biruete has received honoraria from Ardelyx, FMC North America, Dialysis Clinic Inc. and the National Kidney Foundation, and is part of the NextGen Scientist Cohort of the National Dairy Council. All other authors declare that they have no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Sang-Kyung Jo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Disclaimer
This report does not represent the official view of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute of Allergy and Infectious Diseases (NIAID), the National Institutes of Health (NIH), the Department of Health and Human Services (HHS) or any part of the US Federal Government. No official support or endorsement of this article by the NIDDK, NIAID or NIH is intended or should be inferred. This content is solely the responsibility of the authors and does not necessarily represent the official views of the Centers for Disease Control and Prevention (CDC).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bloom, P.P., Garrett, W.S., Penniston, K.L. et al. Microbiota and kidney disease: the road ahead. Nat Rev Nephrol 21, 702–716 (2025). https://doi.org/10.1038/s41581-025-00988-5
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41581-025-00988-5
This article is cited by
-
CKD und Mikrobiom
Die Nephrologie (2026)