Abstract
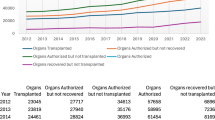
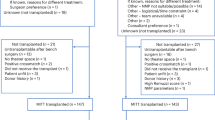
The global shortage of suitable donor kidneys is the primary challenge in kidney transplantation, and it is exacerbated by ageing donors with increased numbers of health issues. Improving organ assessment, preservation and conditioning could enhance organ utilization and patient outcomes. Hypothermic machine perfusion (HMP) is associated with better results than static cold storage by reducing delayed graft function and improving short-term graft survival, especially in kidneys recovered from marginal-quality donors. Although HMP is useful for organ preservation, it is difficult to assess organ viability during HMP because of the reduced metabolic activity at low temperatures, and the adoption of HMP has faced logistical challenges. The addition of oxygen during HMP is aimed at reducing ischaemia–reperfusion injury, but has shown mixed results in kidney transplantation, often depending on the duration of perfusion, although some studies found that the addition of oxygen improved outcomes in higher-risk donors. Normothermic machine perfusion helps to restore kidney function by delivering oxygen and nutrients at body temperature, potentially reducing ischaemia–reperfusion injury. Early studies suggest its safety, but clinical benefits remain unproven. Normothermic machine perfusion also holds promise for assessing organ viability pre-transplantation by enabling real-time evaluation. In this Review, we will summarize the different methods of kidney preservation, providing details of the effect that each method has on graft and patient outcomes and the strengths and limitations of each method.
Key points
-
The primary limitation in kidney transplantation globally is the scarcity of suitable donor organs, which is worsening with an ageing donor population with increasing co-morbidities; therefore, we must focus on improving donor organ assessment, preservation and conditioning.
-
Hypothermic machine perfusion (HMP) has demonstrated clear benefits over static cold storage in reducing delayed graft function and improving graft survival and is the new clinical standard in kidney preservation, but organ viability cannot be assessed during HMP.
-
Continuous hypothermic oxygenated machine perfusion demonstrated improved 1-year kidney function and reduced rejection rates compared with HMP alone; however, use of hypothermic oxygenated machine perfusion for a brief period immediately before transplantation showed no benefit over static cold storage alone.
-
Normothermic machine perfusion (NMP) is aimed at restoring aerobic respiration and metabolic activity in donor kidneys by providing oxygen and nutrients at physiological temperatures; early studies show that both NMP and normothermic regional perfusion (an in situ method before donor organs are procured) are safe and feasible.
-
Ex situ NMP holds potential for assessing kidney viability before transplantation by restoring metabolic activity and enabling real-time evaluation of biomarkers, tissue samples and imaging data.
-
Ex situ NMP also provides a promising platform for delivering targeted therapies to isolated donor kidneys before transplantation, enabling real-time intervention and therapeutic modulation of organ function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Summers, D. M. et al. Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet 376, 1303–1311 (2010).
Summers, D. M. et al. Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: a cohort study. Lancet 381, 727–734 (2013).
Querard, A. H. et al. Comparison of survival outcomes between expanded criteria donor and standard criteria donor kidney transplant recipients: a systematic review and meta-analysis. Transpl. Int. 29, 403–415 (2016).
Husain, S. A. et al. Association between declined offers of deceased donor kidney allograft and outcomes in kidney transplant candidates. JAMA Netw. Open. 2, e1910312 (2019).
Pippias, M. et al. Temporal trends in the quality of deceased donor kidneys and kidney transplant outcomes in Europe: an analysis by the ERA-EDTA Registry. Nephrol. Dial. Transpl. 37, 175–186 (2021).
Statistics and Clinical Research, NHS Blood and Transplant. Summary of activity and kidney activity. https://www.odt.nhs.uk/statistics-and-reports/annual-activity-report/ (NHSBT, 2025).
OPTN. Donor: donation year by organs recovered. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/# (accessed 11 July 2025).
The UK’s Clinical Study Registry. Statins for improving organ outcome in transplantation. https://www.isrctn.com/ISRCTN11440354 (2021).
Malinoski, D. et al. Hypothermia or machine perfusion in kidney donors. N. Engl. J. Med. 388, 418–426 (2023).
Damman, J. et al. Systemic complement activation in deceased donors is associated with acute rejection after renal transplantation in the recipient. Transplantation 92, 163–169 (2011).
Nijboer, W. N. et al. Effect of brain death on gene expression and tissue activation in human donor kidneys. Transplantation 78, 978–986 (2004).
Poppelaars, F. & Seelen, M. A. Complement-mediated inflammation and injury in brain dead organ donors. Mol. Immunol. 84, 77–83 (2017).
Vieira, R. F. et al. 17β-Estradiol protects against lung injuries after brain death in male rats. J. Heart Lung Transpl. 37, 1381–1387 (2018).
Kelpke, S. S. et al. Sodium nitrite protects against kidney injury induced by brain death and improves post-transplant function. Kidney Int. 82, 304–313 (2012).
Aubert, O. et al. Disparities in acceptance of deceased donor kidneys between the United States and France and estimated effects of increased US acceptance. JAMA Intern. Med. 179, 1365–1374 (2019).
van Ittersum, F. J. et al. Increased risk of graft failure and mortality in Dutch recipients receiving an expanded criteria donor kidney transplant. Transpl. Int. 30, 14–28 (2017).
Chen, S., Chen, L. & Jiang, H. Prognosis and risk factors of chronic kidney disease progression in patients with diabetic kidney disease and non-diabetic kidney disease: a prospective cohort CKD-ROUTE study. Ren. Fail. 44, 1309–1318 (2022).
Eurotransplant. Annual Report https://www.eurotransplant.org/wp-content/uploads/2024/06/ETP_AR2023_LowRes.pdf (2023).
OPTN. Donor, kidney, age and BMI. https://optn.transplant.hrsa.gov/data/view-data-reports/build-advanced/ (accessed 11 July 2025).
NHS Blood and Transplant. Annual Report on Kidney Transplantation. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/34295/nhsbt-kidney-transplantation-report-2324.pdf (2024).
Mico-Carnero, M. et al. A potential route to reduce ischemia/reperfusion injury in organ preservation. Cells 11, 2763 (2022).
Wang, G. et al. Analyzing cell-type-specific dynamics of metabolism in kidney repair. Nat. Metab. 4, 1109–1118 (2022).
Rabbani, N. & Thornalley, P. J. Hexokinase-2 glycolytic overload in diabetes and ischemia-reperfusion injury. Trends Endocrinol. Met. 30, 419–431 (2019).
Martin, J. L. et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat. Metab. 1, 966–974 (2019).
Bernardi, P. et al. Identity, structure, and function of the mitochondrial permeability transition pore: controversies, consensus, recent advances, and future directions. Cell Death Differ. 30, 1869–1885 (2023).
Vance, R. E. How DNA sensing drives inflammation. N. Engl. J. Med. 391, 1456–1458 (2024).
Chung, K. W. et al. Mitochondrial damage and activation of the STING pathway lead to renal inflammation and fibrosis. Cell Metab. 30, 784–799.e5 (2019).
Dondelinger, Y. et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 7, 971–981 (2014).
Weissenbacher, A. et al. Hemodynamics and metabolic parameters in normothermic kidney preservation are linked with donor factors, perfusate cells, and cytokines. Front. Med. 8, 801098 (2021).
Zaza, G. et al. Proteomics reveals specific biological changes induced by the normothermic machine perfusion of donor kidneys with a significant up-regulation of Latexin. Sci. Rep. 13, 5920 (2023).
de Haan, M. J. A. et al. A cell-free nutrient-supplemented perfusate allows four-day ex vivo metabolic preservation of human kidneys. Nat. Commun. 15, 3818 (2024).
Kawamura, M. et al. Normothermic ex vivo kidney perfusion preserves mitochondrial and graft function after warm ischemia and is further enhanced by AP39. Nat. Commun. 15, 8086 (2024).
Collins, G. M., Bravo-Shugarman, M. & Terasaki, P. I. Kidney preservation for transportation. Initial perfusion and 30 hours’ ice storage. Lancet 2, 1219–1222 (1969).
Calne, R. Y., Pegg, D. E., Pryse-Davies, J. & Brown, F. L. Renal preservation by ice-cooling: an experimental study relating to kidney transplantation from cadavers. Br. Med. J. 2, 651–655 (1963).
Belzer, F. O., Ashby, B. S., Gulyassy, P. F. & Powell, M. Successful seventeen-hour preservation and transplantation of human-cadaver kidney. N. Engl. J. Med. 278, 608–610 (1968).
Hosgood, S. A. & Nicholson, M. L. First in man renal transplantation after ex vivo normothermic perfusion. Transplantation 92, 735–738 (2011).
Fondevila, C. et al. Liver transplant using donors after unexpected cardiac death: novel preservation protocol and acceptance criteria. Am. J. Transpl. 7, 1849–1855 (2007).
Levy, M. N. Oxygen consumption and blood flow in the hypothermic, perfused kidney. Am. J. Physiol. 197, 1111–1114 (1959).
Boudjema, K. et al. Effect of oxidized and reduced glutathione in liver preservation. Transplantation 50, 948–951 (1990).
Gores, G. J., Nieminen, A. L., Wray, B. E., Herman, B. & Lemasters, J. J. Intracellular pH during “chemical hypoxia” in cultured rat hepatocytes. Protection by intracellular acidosis against the onset of cell death. J. Clin. Invest. 83, 386–396 (1989).
Kosieradzki, M. et al. Prognostic significance of free radicals: mediated injury occurring in the kidney donor. Transplantation 75, 1221–1227 (2003).
Bonventre, J. V. & Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Invest. 121, 4210–4221 (2011).
Zhao, H., Alam, A., Soo, A. P., George, A. J. T. & Ma, D. Ischemia-reperfusion injury reduces long term renal graft survival: mechanism and beyond. EBioMedicine 28, 31–42 (2018).
Moers, C. et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 360, 7–19 (2009).
Jochmans, I. et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann. Surg. 252, 756–764 (2010).
Gallinat, A. et al. Machine perfusion versus static cold storage in expanded criteria donor kidney transplantation: 3-year follow-up data. Transpl. Int. 26, E52–E53 (2013).
Peng, P. et al. Hypothermic machine perfusion versus static cold storage in deceased donor kidney transplantation: a systematic review and meta-analysis of randomized controlled trials. Artif. Organs 43, 478–489 (2019).
Jiao, B. et al. Hypothermic machine perfusion reduces delayed graft function and improves one-year graft survival of kidneys from expanded criteria donors: a meta-analysis. PLoS ONE 8, e81826 (2013).
Tingle, S. J. et al. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst. Rev. 3, CD011671 (2019).
Tingle, S. J. et al. Hypothermic machine perfusion is superior to static cold storage in deceased donor kidney transplantation: a meta-analysis. Clin. Transpl. 34, e13814 (2020).
Tingle, S. J. et al. Normothermic and hypothermic machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst. Rev. 7, CD011671 (2024).
Watson, C. J. et al. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: a UK multicenter randomized controlled trial. Am. J. Transpl. 10, 1991–1999 (2010).
Summers, D. M. et al. Cold pulsatile machine perfusion versus static cold storage for kidneys donated after circulatory death: a multicenter randomized controlled trial. Transplantation 104, 1019–1025 (2020).
Groen, H. et al. Cost-effectiveness of hypothermic machine preservation versus static cold storage in renal transplantation. Am. J. Transpl. 12, 1824–1830 (2012).
Chang, A., Schaubel, D. E., Chen, M., Abt, P. L. & Bittermann, T. Trends and outcomes of hypothermic machine perfusion preservation of kidney allografts in simultaneous liver and kidney transplantation in the United States. Transpl. Int. 35, 10345 (2022).
Tedesco-Silva, H. J. et al. Randomized trial of machine perfusion versus cold storage in recipients of deceased donor kidney transplants with high incidence of delayed graft function. Transpl. Direct 3, e155 (2017).
de Sandes-Freitas, T. V. et al. The impact of hypothermic pulsatile machine perfusion versus static cold storage: a donor-matched paired analysis in a scenario of high incidence of delayed kidney graft function. Ann. Transpl. 25, e927010 (2020).
Chatauret, N. et al. Mechanistic analysis of nonoxygenated hypothermic machine perfusion’s protection on warm ischemic kidney uncovers greater eNOS phosphorylation and vasodilation. Am. J. Transpl. 14, 2500–2514 (2014).
Jochmans, I. et al. The prognostic value of renal resistance during hypothermic machine perfusion of deceased donor kidneys. Am. J. Transpl. 11, 2214–2220 (2011).
Nath, J. et al. Metabolic differences between cold stored and machine perfused porcine kidneys: a 1H NMR based study. Cryobiology 74, 115–120 (2017).
Tozzi, M. et al. Impact of static cold storage VS hypothermic machine preservation on ischemic kidney graft: inflammatory cytokines and adhesion molecules as markers of ischemia/reperfusion tissue damage. Our preliminary results. Int. J. Surg. 11, S110–S114 (2013).
Zhang, Y. et al. Hypothermic machine perfusion decreases renal cell apoptosis during ischemia/reperfusion injury via the Ezrin/AKT pathway. Artif. Organs 40, 129–135 (2016).
Meister, F. A. et al. Decrease of renal resistance during hypothermic oxygenated machine perfusion is associated with early allograft function in extended criteria donation kidney transplantation. Sci. Rep. 10, 17726 (2020).
Lentine, K. L. et al. OPTN/SRTR 2023 annual data report: kidney. Am. J. Transpl. 25, S22–S137 (2025).
Sandal, S. et al. Renal resistance thresholds during hypothermic machine perfusion and transplantation outcomes — a retrospective cohort study. Transpl. Int. 31, 658–669 (2018).
Guzzi, F., Knight, S. R., Ploeg, R. J. & Hunter, J. P. A systematic review to identify whether perfusate biomarkers produced during hypothermic machine perfusion can predict graft outcomes in kidney transplantation. Transpl. Int. 33, 590–602 (2020).
Moers, C. et al. The value of machine perfusion perfusate biomarkers for predicting kidney transplant outcome. Transplantation 90, 966–973 (2010).
Nagelschmidt, M. et al. Lipid peroxidation products in machine perfusion of older donor kidneys. J. Surg. Res. 180, 337–342 (2013).
Hendriks, K. D. W. et al. Renal temperature reduction progressively favors mitochondrial ROS production over respiration in hypothermic kidney preservation. J. Transl. Med. 17, 265 (2019).
van Rijn, R. et al. Hypothermic machine perfusion in liver transplantation — a randomized trial. N. Engl. J. Med. 384, 1391–1401 (2021).
Jochmans, I. et al. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): a randomised, double-blind, paired, phase 3 trial. Lancet 396, 1653–1662 (2020).
Husen, P. et al. Oxygenated end-hypothermic machine perfusion in expanded criteria donor kidney transplant: a randomized clinical trial. JAMA Surg. 156, 517–525 (2021).
Houtzager, J. H. E. et al. The use of the oxygenated Airdrive™ machine perfusion system in kidney graft preservation: a clinical pilot study. Eur. Surg. Res. 61, 153–162 (2020).
Ravaioli, M. et al. Successful dual kidney transplantation after hypothermic oxygenated perfusion of discarded human kidneys. Am. J. Case Rep. 18, 1009–1013 (2017).
Ravaioli, M. et al. Hypothermic oxygenated new machine perfusion system in liver and kidney transplantation of extended criteria donors: first Italian clinical trial. Sci. Rep. 10, 6063 (2020).
Pool, M. B. F. et al. Prolonged ex-vivo normothermic kidney perfusion: the impact of perfusate composition. PLoS ONE 16, e0251595 (2021).
Hosgood, S. A., Elliott, T. R., Jordan, N. P. & Nicholson, M. L. The effects of free heme on functional and molecular changes during ex vivo normothermic machine perfusion of human kidneys. Front. Immunol. 13, 849742 (2022).
Greite, R. et al. Free heme and hemopexin in acute kidney injury after cardiopulmonary bypass and transient renal ischemia. Clin. Transl. Sci. 16, 2729–2743 (2023).
Minor, T. et al. First-in-man controlled rewarming and normothermic perfusion with cell-free solution of a kidney prior to transplantation. Am. J. Transpl. 20, 1192–1195 (2020).
Zlatev, H., von Horn, C., Kaths, M., Paul, A. & Minor, T. Clinical use of controlled oxygenated rewarming of kidney grafts prior to transplantation by ex vivo machine perfusion. A pilot study. Eur. J. Clin. Invest. 52, e13691 (2022).
Nicholson, M. L. & Hosgood, S. A. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am. J. Transpl. 13, 1246–1252 (2013).
Rijkse, E. et al. Safety and feasibility of 2 h of normothermic machine perfusion of donor kidneys in the Eurotransplant Senior Program. BJS Open. 5, zraa024 (2021).
Mazilescu, L. I. et al. Normothermic ex vivo kidney perfusion for human kidney transplantation: first North American results. Transplantation 106, 1852–1859 (2022).
Chandak, P. et al. Dissemination of a novel organ perfusion technique: ex vivo normothermic perfusion of deceased donor kidneys. Artif. Organs 43, E308–E319 (2019).
Hosgood, S. A., Thompson, E., Moore, T., Wilson, C. H. & Nicholson, M. L. Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors. Br. J. Surg. 105, 388–394 (2018).
Leighton, P., Hosgood, S. A., Butler, A. J. & Nicholson, M. L. Use of a double-J stent during ex vivo normothermic machine perfusion of human kidneys. Am. J. Transpl. 20, 1754–1755 (2020).
Georgiades, F., Hosgood, S. A., Butler, A. J. & Nicholson, M. L. Use of ex vivo normothermic machine perfusion after normothermic regional perfusion to salvage a poorly perfused DCD kidney. Am. J. Transpl. 19, 3415–3419 (2019).
Pearson, R. et al. Viability assessment and utilization of declined donor kidneys with rhabdomyolysis using ex vivo normothermic perfusion without preimplantation biopsy. Am. J. Transpl. 21, 1317–1321 (2021).
Pearson, R., Wubetu, J., Jackson, A. & Kingsmore, D. Living donor kidney transplant following nephrectomy for renal artery stenosis with arterial reconstruction and viability assessment using ex vivo normothermic perfusion. BMJ Case Rep. 14, e245273 (2021).
Nicholson, M. & Hosgood, S. Preoperative assessment of renal transplant ureteric blood supply using ex vivo normothermic perfusion. Transplantation 99, e166 (2015).
Hosgood, S. A., Saeb-Parsy, K., Hamed, M. O. & Nicholson, M. L. Successful transplantation of human kidneys deemed untransplantable but resuscitated by ex vivo normothermic machine perfusion. Am. J. Transpl. 16, 3282–3285 (2016).
Hosgood, S. A. & Nicholson, M. L. The first clinical case of intermediate ex vivo normothermic perfusion in renal transplantation. Am. J. Transpl. 14, 1690–1692 (2014).
Hosgood, S. A. et al. Normothermic machine perfusion versus static cold storage in donation after circulatory death kidney transplantation: a randomized controlled trial. Nat. Med. 29, 1511–1519 (2023).
Dumbill R, K. S. et al. Prolonged normothermic perfusion of the kidney: a historically controlled, phase 1 cohort study. Nat. Commun. 16, 4584 (2025).
Oniscu, G. C. et al. Improved organ utilization and better transplant outcomes with in situ normothermic regional perfusion in controlled donation after circulatory death. Transplantation 107, 438–448 (2023).
Hessheimer, A. J. et al. Abdominal normothermic regional perfusion in controlled donation after circulatory determination of death liver transplantation: outcomes and risk factors for graft loss. Am. J. Transpl. 22, 1169–1181 (2022).
Klein Nulend, R. et al. Normothermic machine perfusion and normothermic regional perfusion of DCD kidneys before transplantation: a systematic review. Transplantation 109, 362–375 (2025).
Boteon, Y. L. et al. The economic impact of machine perfusion technology in liver transplantation. Artif. Organs 46, 191–200 (2022).
De Deken, J., Kocabayoglu, P. & Moers, C. Hypothermic machine perfusion in kidney transplantation. Curr. Opin. Organ. Transpl. 21, 294–300 (2016).
Verstraeten, L. & Jochmans, I. Sense and sensibilities of organ perfusion as a kidney and liver viability assessment platform. Transpl. Int. 35, 10312 (2022).
Mulvey, J. F. et al. Perfusate proteomes provide biological insight into oxygenated versus standard hypothermic machine perfusion in kidney transplantation. Ann. Surg. 278, 676–682 (2023).
Hamelink, T. L. et al. Renal normothermic machine perfusion: the road toward clinical implementation of a promising pretransplant organ assessment tool. Transplantation 106, 268–279 (2022).
Woud, W. et al. Extracellular vesicles released during normothermic machine perfusion are associated with human donor kidney characteristics. Transplantation 106, S337 (2022).
Woud, W. W. et al. Extracellular vesicles released during normothermic machine perfusion are associated with human donor kidney characteristics. Transplantation 106, 2360–2369 (2022).
Jager, N. M. et al. Complement is activated during normothermic machine perfusion of porcine and human discarded kidneys. Front. Immunol. 13, 831371 (2022).
Lin, H. et al. Human transplant kidneys on normothermic machine perfusion display endocrine activity. Transpl. Direct 9, e1503 (2023).
Weissenbacher, A. et al. Urine recirculation prolongs normothermic kidney perfusion via more optimal metabolic homeostasis — a proteomics study. Am. J. Transpl. 21, 1740–1753 (2021).
Bontha, S. V., Maluf, D. G., Mueller, T. F. & Mas, V. R. Systems biology in kidney transplantation: the application of multi-omics to a complex model. Am. J. Transpl. 17, 11–21 (2017).
Hamelink, T. L. et al. Magnetic resonance imaging as a noninvasive adjunct to conventional assessment of functional differences between kidneys in vivo and during ex vivo normothermic machine perfusion. Am. J. Transpl. 24, 1761–1771 (2024).
van Smaalen, T. C., Hoogland, E. R. & van Heurn, L. W. Machine perfusion viability testing. Curr. Opin. Organ. Transpl. 18, 168–173 (2013).
Radajewska, A. et al. Mitoquinone alleviates donation after cardiac death kidney injury during hypothermic machine perfusion in rat model. Int. J. Mol. Sci. 24, 14772 (2023).
Sedigh, A. et al. Perfusion of porcine kidneys with macromolecular heparin reduces early ischemia reperfusion injury. Transplantation 103, 420–427 (2019).
Hamaoui, K. et al. Organ pretreatment with cytotopic endothelial localizing peptides to ameliorate microvascular thrombosis and perfusion deficits in ex vivo renal hemoreperfusion models. Transplantation 100, e128–e139 (2016).
Polyak, M. M., Arrington, B. O., Stubenbord, W. T. & Kinkhabwala, M. Prostaglandin E1 improves pulsatile preservation characteristics and early graft function in expanded criteria donor kidneys. ASAIO J. 44, M610–M612 (1998).
Diuwe, P. et al. The effect of the use of a TNF-alpha inhibitor in hypothermic machine perfusion on kidney function after transplantation. Contemp. Clin. Trials 59, 44–50 (2017).
Woodside, K. J. et al. Enhancing kidney function with thrombolytic therapy following donation after cardiac death: a multicenter quasi-blinded prospective randomized trial. Clin. Transpl. 29, 1173–1180 (2015).
DiRito, J. R. et al. Lysis of cold-storage-induced microvascular obstructions for ex vivo revitalization of marginal human kidneys. Am. J. Transpl. 21, 161–173 (2021).
Huang, W., Hickson, L. J., Eirin, A., Kirkland, J. L. & Lerman, L. O. Cellular senescence: the good, the bad and the unknown. Nat. Rev. Nephrol. 18, 611–627 (2022).
Kirkland, J. L. & Tchkonia, T. Senolytic drugs: from discovery to translation. J. Intern. Med. 288, 518–536 (2020).
He, A. et al. Renal inflamm-aging provokes intra-graft inflammation following experimental kidney transplantation. Am. J. Transpl. 22, 2529–2547 (2022).
Ferdinand, J. R. et al. Cytokine absorption during human kidney perfusion reduces delayed graft function-associated inflammatory gene signature. Am. J. Transpl. 21, 2188–2199 (2021).
van Willigenburg, H., de Keizer, P. L. J. & de Bruin, R. W. F. Cellular senescence as a therapeutic target to improve renal transplantation outcome. Pharmacol. Res. 130, 322–330 (2018).
Mylonas, K. J. et al. Cellular senescence inhibits renal regeneration after injury in mice, with senolytic treatment promoting repair. Sci. Transl. Med. 13, eabb0203 (2021).
Delaura, I. F. et al. Complement-targeting therapeutics for ischemia-reperfusion injury in transplantation and the potential for ex vivo delivery. Front. Immunol. 13, 1000172 (2022).
Glotz, D. et al. Safety and efficacy of eculizumab for the prevention of antibody-mediated rejection after deceased-donor kidney transplantation in patients with preformed donor-specific antibodies. Am. J. Transpl. 19, 2865–2875 (2019).
Berger, M., Lefaucheur, C. & Jordan, S. C. Update on C1 esterase inhibitor in human solid organ transplantation. Transplantation 103, 1763–1775 (2019).
Damman, J. et al. Targeting complement activation in brain-dead donors improves renal function after transplantation. Transpl. Immunol. 24, 233–237 (2011).
Schroppel, B. et al. Peritransplant eculizumab does not prevent delayed graft function in deceased donor kidney transplant recipients: results of two randomized controlled pilot trials. Am. J. Transpl. 20, 564–572 (2020).
Golshayan, D., Schwotzer, N., Fakhouri, F. & Zuber, J. Targeting the complement pathway in kidney transplantation. J. Am. Soc. Nephrol. 34, 1776–1792 (2023).
Harisa, G. I. et al. Gene-editing technology, from macromolecule therapeutics to organ transplantation: applications, limitations, and prospective uses. Int. J. Biol. Macromol. 253, 127055 (2023).
Yang, B., Hosgood, S. A. & Nicholson, M. L. Naked small interfering RNA of caspase-3 in preservation solution and autologous blood perfusate protects isolated ischemic porcine kidneys. Transplantation 91, 501–507 (2011).
Yang, C. et al. Cyclic helix B peptide in preservation solution and autologous blood perfusate ameliorates ischemia-reperfusion injury in isolated porcine kidneys. Transpl. Direct 1, e6 (2015).
Zheng, X. et al. Preventing renal ischemia-reperfusion injury using small interfering RNA by targeting complement 3 gene. Am. J. Transpl. 6, 2099–2108 (2006).
Stimmeder, S., Leber, B., Sucher, R. & Stiegler, P. Genetic modulation: future trends toward graft optimization during machine perfusion. Transplantation 108, 614–624 (2024).
de Ramon, L. et al. CD154-CD40 T-cell co-stimulation pathway is a key mechanism in kidney ischemia-reperfusion injury. Kidney Int. 88, 538–549 (2015).
Moser, M. A. et al. Protection of the transplant kidney from preservation injury by inhibition of matrix metalloproteinases. PLoS ONE 11, e0157508 (2016).
Zheng, X. et al. Attenuating ischemia-reperfusion injury in kidney transplantation by perfusing donor organs with siRNA cocktail solution. Transplantation 100, 743–752 (2016).
Thompson, E. R. et al. MicroRNA antagonist therapy during normothermic machine perfusion of donor kidneys. Am. J. Transpl. 22, 1088–1100 (2022).
Heikkila, P., Parpala, T., Lukkarinen, O., Weber, M. & Tryggvason, K. Adenovirus-mediated gene transfer into kidney glomeruli using an ex vivo and in vivo kidney perfusion system — first steps towards gene therapy of Alport syndrome. Gene Ther. 3, 21–27 (1996).
Brasile, L. et al. Transfection and transgene expression in a human kidney during ex vivo warm perfusion. Transpl. Proc. 34, 2624 (2002).
Yuzefovych, Y. et al. Genetic engineering of the kidney to permanently silence MHC transcripts during ex vivo organ perfusion. Front. Immunol. 11, 265 (2020).
Figueiredo, C., Horn, P. A., Blasczyk, R. & Seltsam, A. Regulating MHC expression for cellular therapeutics. Transfusion 47, 18–27 (2007).
Severi, A. A. & Akbari, B. CRISPR-Cas9 delivery strategies and applications: review and update. Genesis 62, e23598 (2024).
Lohmann, S. et al. Mesenchymal stromal cell treatment of donor kidneys during ex vivo normothermic machine perfusion: a porcine renal autotransplantation study. Am. J. Transpl. 21, 2348–2359 (2021).
Thompson, E. R. et al. Novel delivery of cellular therapy to reduce ischemia reperfusion injury in kidney transplantation. Am. J. Transpl. 21, 1402–1414 (2021).
Arcolino, F. O. et al. De novo SIX2 activation in human kidneys treated with neonatal kidney stem/progenitor cells. Am. J. Transpl. 22, 2791–2803 (2022).
Blondeel, J., Gilbo, N., De Bondt, S. & Monbaliu, D. Stem cell derived extracellular vesicles to alleviate ischemia-reperfusion injury of transplantable organs. a systematic review. Stem Cell Rev. Rep. 19, 2225–2250 (2023).
Rampino, T. et al. Extracellular vesicles derived from mesenchymal stromal cells delivered during hypothermic oxygenated machine perfusion repair ischemic/reperfusion damage of kidneys from extended criteria donors. Biology 11, 350 (2022).
Vallant, N., Wolfhagen, N., Sandhu, B., Hamaoui, K. & Papalois, V. Delivery of mesenchymal stem cells during hypothermic machine perfusion in a translational kidney perfusion study. Int. J. Mol. Sci. 25, 5038 (2024).
Gregorini, M. et al. Perfusion of isolated rat kidney with mesenchymal stromal cells/extracellular vesicles prevents ischaemic injury. J. Cell Mol. Med. 21, 3381–3393 (2017).
MacMillan, S., Hosgood, S. A. & Nicholson, M. L. Enzymatic blood group conversion of human kidneys during ex vivo normothermic machine perfusion. Br. J. Surg. 110, 133–137 (2023).
MacMillan, S. et al. Enzymatic conversion of human blood group A kidneys to universal blood group O. Nat. Commun. 15, 2795 (2024).
Sellers, M. T. et al. Normothermic regional perfusion experience of organ procurement organizations in the US. JAMA Netw. Open. 7, e2440130 (2024).
Thorlund, K., Haggstrom, J., Park, J. J. & Mills, E. J. Key design considerations for adaptive clinical trials: a primer for clinicians. BMJ 360, k698 (2018).
Loupy, A. et al. Prediction system for risk of allograft loss in patients receiving kidney transplants: international derivation and validation study. BMJ 366, l4923 (2019).
Wall, A. & Testa, G. The ethics surrounding normothermic regional perfusion in donors following circulatory death. Clin. Liver Dis. 23, e0193 (2024).
Olawade, D. B., Marinze, S., Qureshi, N., Weerasinghe, K. & Teke, J. Transforming organ donation and transplantation: strategies for increasing donor participation and system efficiency. Eur. J. Intern. Med. 133, 14–24 (2025).
Wight, J., Chilcott, J., Holmes, M. & Brewer, N. The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors. Health Technol. Assess. 7, 1–94 (2003).
Bond, M. et al. The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model. Health Technol. Assess. 13, 1–156 (2009). iii–iv, xi–xiv.
Gomez, V. et al. Economic impact of the introduction of machine perfusion preservation in a kidney transplantation program in the expanded donor era: cost-effectiveness assessment. Transpl. Proc. 44, 2521–2524 (2012).
Snyder, R. A., Moore, D. R. & Moore, D. E. More donors or more delayed graft function? A cost-effectiveness analysis of DCD kidney transplantation. Clin. Transpl. 27, 289–296 (2013).
Tedesco Silva, H. Jr. et al. Use of machine perfusion to increase the number of expanded criteria deceased donor kidney transplants: a pharmacoeconomic analysis. Transpl. Direct 10, e1668 (2024).
Acknowledgements
T.J.R. is supported by the Novo Nordisk Foundation Center for Stem Cell Medicine (reNEW, supported by Novo Nordisk Foundation grant (NNF21CC0073729)) and T.J.R. and C.M. are funded by the European Union. Views and opinions expressed are, however, those of the author(s) only and do not necessarily reflect those of the European Union or the European Research Council Executive Agency. Neither the European Union nor the granting authority can be held responsible for them. This work is supported by ERC grant (SPARK 101140863).
Author information
Authors and Affiliations
Consortia
Contributions
J.H. and S.H. researched data for the article. All authors contributed substantially to discussion of the content. J.H., S.H., C.M. and H.L. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Dianne McKay, who co-reviewed with Stephanie Almeida; Chris Callaghan; and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
34lives: http://34lives.com/
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hunter, J., Hosgood, S., Moers, C. et al. Improving outcomes in kidney transplantation through advances in donor organ perfusion. Nat Rev Nephrol (2025). https://doi.org/10.1038/s41581-025-00993-8
Accepted:
Published:
DOI: https://doi.org/10.1038/s41581-025-00993-8