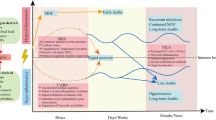
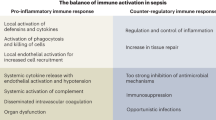
Abstract
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. Treatments that influence this dysregulated host response are sparse. The immunopathophysiology of sepsis entails overzealous inflammation causing acute organ dysfunction, as well as a profound and/or persistent anti-inflammatory response that increases susceptibility to secondary infection. The immune response in sepsis is under the influence of various endogenous and exogenous factors, including genetic makeup, age, sex, comorbidities, metabolism, prior microbial exposure and medications. The consequent heterogeneity of the syndrome hampers immunomodulatory treatment strategies that rely on a ‘one-size-fits-all’ approach. A precision medicine approach is therefore warranted. Balanced application of prognostic- and predictive-enrichment strategies is instrumental to achieve precision medicine. Phenotyping of patients using clinical, physiological, microbiological and/or molecular (‘omics’) data enables the identification of more homogeneous patient subgroups. Several studies suggest that such approaches can be used to tailor adjunctive immunomodulatory therapies in patients with sepsis. As well as repurposing existing drugs to treat sepsis, new drugs aimed at restoring immune homeostasis are under investigation. New clinical trial methodologies, including flexible platform trials, Bayesian statistics and embedding trials in health care systems are increasingly being used to keep pace with rapid developments in the field of sepsis immunobiology and ultimately to improve clinical outcomes.
Key points
-
Sepsis is a highly heterogenous syndrome resulting from a dysregulated immune response that is characterized by varying degrees of inflammation and immune suppression.
-
Immunomodulatory treatment strategies that use a ‘one-size-fits-all approach’ have consistently failed to attenuate organ dysfunction or reduce mortality in sepsis, underscoring the need for a better understanding of the host response and theragnostic biomarkers to guide appropriate therapeutic interventions.
-
Harnessing concepts such as disease tolerance, resilience, resolution and trained immunity may shed new perspectives on the dysregulated host response in sepsis, moving away from the simplified hyperinflammatory versus immunosuppressive paradigm.
-
Unsupervised clustering methods and machine learning can assign patients with sepsis into distinct subgroups on the basis of various demographic, clinical, physiological and/or molecular data, with the ultimate goal of enabling precision medicine — appropriate treatment for individual patients, at the right dose and at the optimal time.
-
Several retrospective analyses indicate that precision medicine may be feasible and effective in sepsis, but prospective studies are required to demonstrate clinical benefit.
-
Overcoming the limitations of traditional trial designs and adopting innovative approaches such as flexible platform trials, Bayesian methods and embedded trials could help to advance the delivery of effective, timely treatments to improve the outcomes of patients with sepsis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Angus, D. C. & van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 369, 2063 (2013).
Rudd, K. E. et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study. Lancet 395, 200–211 (2020).
Stevenson, E. K., Rubenstein, A. R., Radin, G. T., Wiener, R. S. & Walkey, A. J. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit. Care Med. 42, 625–631 (2014).
Fleischmann-Struzek, C. et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 46, 1552–1562 (2020).
Yende, S. et al. Risk of cardiovascular events in survivors of severe sepsis. Am. J. Respir. Crit. Care Med. 189, 1065–1074 (2014).
Inghammar, M. et al. Long-term mortality and hospital readmissions among survivors of sepsis in Sweden: a population-based cohort study. Open. Forum Infect. Dis. 11, ofae331 (2024).
Fleischmann-Struzek, C. et al. Epidemiology and costs of postsepsis morbidity, nursing care dependency, and mortality in Germany, 2013 to 2017. JAMA Netw. Open. 4, e2134290 (2021).
Rahmel, T. et al. Long-term mortality and outcome in hospital survivors of septic shock, sepsis, and severe infections: the importance of aftercare. PLoS ONE 15, e0228952 (2020).
Gotts, J. E. & Matthay, M. A. Sepsis: pathophysiology and clinical management. BMJ 353, i1585 (2016).
Cajander, S. et al. Profiling the dysregulated immune response in sepsis: overcoming challenges to achieve the goal of precision medicine. Lancet Respir. Med. 12, 305–322 (2024).
Joffre, J., Hellman, J., Ince, C. & Ait-Oufella, H. Endothelial responses in sepsis. Am. J. Respir. Crit. Care Med. 202, 361–370 (2020).
Shankar-Hari, M. et al. Reframing sepsis immunobiology for translation: towards informative subtyping and targeted immunomodulatory therapies. Lancet Respir. Med. 12, 323–336 (2024).
Cazalis, M. A. et al. Early and dynamic changes in gene expression in septic shock patients: a genome-wide approach. Intensive Care Med. Exp. 2, 20 (2014).
Xiao, W. et al. A genomic storm in critically injured humans. J. Exp. Med. 208, 2581–2590 (2011).
Calvano, S. E. et al. A network-based analysis of systemic inflammation in humans. Nature 437, 1032–1037 (2005).
Tang, B. M., Huang, S. J. & McLean, A. S. Genome-wide transcription profiling of human sepsis: a systematic review. Crit. Care 14, R237 (2010).
Ferlito, M. et al. Effect of cross-tolerance between endotoxin and TNF-alpha or IL-1beta on cellular signaling and mediator production. J. Leukoc. Biol. 70, 821–829 (2001).
Wiersinga, W. J. & van der Poll, T. Immunopathophysiology of human sepsis. EBioMedicine 86, 104363 (2022).
van der Poll, T., Shankar-Hari, M. & Wiersinga, W. J. The immunology of sepsis. Immunity 54, 2450–2464 (2021).
Bauer, M. & Wetzker, R. The cellular basis of organ failure in sepsis-signaling during damage and repair processes. Med. Klin. Intensivmed. Notfmed 115, 4–9 (2020).
Fattahi, F., Zetoune, F. S. & Ward, P. A. Complement as a major inducer of harmful events in infectious sepsis. Shock 54, 595–605 (2020).
Chousterman, B. G., Swirski, F. K. & Weber, G. F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 39, 517–528 (2017).
Zhang, Y. Y. & Ning, B. T. Signaling pathways and intervention therapies in sepsis. Signal. Transduct. Target. Ther. 6, 407 (2021).
Kox, M., Waalders, N. J. B., Kooistra, E. J., Gerretsen, J. & Pickkers, P. Cytokine levels in critically ill patients with COVID-19 and other conditions. JAMA 324, 1565–1567 (2020).
Iba, T., Levi, M. & Levy, J. H. Intracellular communication and immunothrombosis in sepsis. J. Thromb. Haemost. 20, 2475–2484 (2022).
Larsen, R. et al. A central role for free heme in the pathogenesis of severe sepsis. Sci. Transl. Med. 2, 51ra71 (2010).
Karakike, E. & Giamarellos-Bourboulis, E. J. Macrophage activation-like syndrome: a distinct entity leading to early death in sepsis. Front. Immunol. 10, 55 (2019).
Rubio, I. et al. Current gaps in sepsis immunology: new opportunities for translational research. Lancet Infect. Dis. 19, e422–e436 (2019).
Venet, F. & Monneret, G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 14, 121–137 (2018).
Finfer, S., Venkatesh, B., Hotchkiss, R. S. & Sasson, S. C. Lymphopenia in sepsis-an acquired immunodeficiency? Immunol. Cell Biol. 101, 535–544 (2023).
Boomer, J. S. et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306, 2594–2605 (2011).
Leijte, G. P. et al. Monocytic HLA-DR expression kinetics in septic shock patients with different pathogens, sites of infection and adverse outcomes. Crit. Care 24, 110 (2020).
Patera, A. C. et al. Frontline science: defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1. J. Leukoc. Biol. 100, 1239–1254 (2016).
Guignant, C. et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit. Care 15, R99 (2011).
Cavaillon, J. M., Adrie, C., Fitting, C. & Adib-Conquy, M. Reprogramming of circulatory cells in sepsis and SIRS. J. Endotoxin Res. 11, 311–320 (2005).
Bouras, M., Asehnoune, K. & Roquilly, A. Contribution of dendritic cell responses to sepsis-induced immunosuppression and to susceptibility to secondary pneumonia. Front. Immunol. 9, 2590 (2018).
Berton, R. R. et al. Sepsis leads to lasting changes in phenotype and function of naive CD8 T cells. PLoS Pathog. 19, e1011720 (2023).
Wu, D., Shi, Y., Zhang, H. & Miao, C. Epigenetic mechanisms of Immune remodeling in sepsis: targeting histone modification. Cell Death Dis. 14, 112 (2023).
Uhel, F. et al. Early expansion of circulating granulocytic myeloid-derived suppressor cells predicts development of nosocomial infections in patients with sepsis. Am. J. Respir. Crit. Care Med. 196, 315–327 (2017).
Schrijver, I. T. et al. Myeloid-derived suppressor-like cells as a prognostic marker in critically ill patients: insights from experimental endotoxemia and intensive care patients. Cells 13, 314–2024.
Coudereau, R. et al. Immature neutrophils and myeloid-derived suppressor cells in sepsis: differences in occurrence kinetics. Crit. Care 28, 7 (2024).
Darden, D. B. et al. Single-cell RNA-seq of human myeloid-derived suppressor cells in late sepsis reveals multiple subsets with unique transcriptional responses: a pilot study. Shock 55, 587–595 (2021).
Venet, F. et al. Increased circulating regulatory T cells (CD4+CD25+CD127−) contribute to lymphocyte anergy in septic shock patients. Intensive Care Med. 35, 678–686 (2009).
Landelle, C. et al. Low monocyte human leukocyte antigen-DR is independently associated with nosocomial infections after septic shock. Intensive Care Med. 36, 1859–1866 (2010).
Monneret, G. et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 32, 1175–1183 (2006).
Adigbli, D. et al. Early persistent lymphopenia and risk of death in critically ill patients with and without sepsis. Shock 61, 197–203 (2024).
Drewry, A. M. et al. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock 42, 383–391 (2014).
Retter, A., Singer, M. & Annane, D. The NET effect: neutrophil extracellular traps — a potential key component of the dysregulated host immune response in sepsis. Crit. Care 29, 59 (2025).
Coupland, L. A. et al. Plasma dynamics of neutrophil extracellular traps and cell-free DNA in septic and nonseptic vasoplegic shock: a prospective comparative observational cohort study. Shock 62, 193–200 (2024).
Shi, Y. et al. Treg and neutrophil extracellular trap interaction contributes to the development of immunosuppression in sepsis. JCI Insight 9, e180132.
Cavaillon, J. M. & Annane, D. Compartmentalization of the inflammatory response in sepsis and SIRS. J. Endotoxin Res. 12, 151–170 (2006).
Conway Morris, A., Rynne, J. & Shankar-Hari, M. Compartmentalisation of immune responses in critical illness: does it matter? Intensive Care Med. 48, 1617–1620 (2022).
Cavaillon, J. M., Chousterman, B. G. & Skirecki, T. Compartmentalization of the inflammatory response during bacterial sepsis and severe COVID-19. J. Intensive Med. 4, 326–340 (2024).
de Brabander, J. et al. Persistent alveolar inflammatory response in critically ill patients with COVID-19 is associated with mortality. Thorax 78, 912–921 (2023).
Jouan, Y., Baranek, T., Si-Tahar, M., Paget, C. & Guillon, A. Lung compartmentalization of inflammatory biomarkers in COVID-19-related ARDS. Crit. Care 25, 120 (2021).
Neyton, L. P. A. et al. Distinct pulmonary and systemic effects of dexamethasone in severe COVID-19. Nat. Commun. 15, 5483 (2024).
Morrell, E. D. et al. PD-L1 and PD-1 are associated with clinical outcomes and alveolar immune cell activation in ARDS. Am. J. Respir. Cell Mol. Biol. 71, 534–545.
Dugernier, T. L. et al. Compartmentalization of the inflammatory response during acute pancreatitis: correlation with local and systemic complications. Am. J. Respir. Crit. Care Med. 168, 148–157 (2003).
Li, Z. et al. The pathogenesis of ischemia-reperfusion induced acute kidney injury depends on renal neutrophil recruitment whereas sepsis-induced AKI does not. Front. Immunol. 13, 843782 (2022).
Wen, X. et al. Time-dependent effects of histone deacetylase inhibition in sepsis-associated acute kidney injury. Intensive Care Med. Exp. 8, 9 (2020).
Kuwabara, S., Goggins, E. & Okusa, M. D. The pathophysiology of sepsis-associated AKI. Clin. J. Am. Soc. Nephrol. 17, 1050–1069 (2022).
Zarbock, A. et al. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat. Rev. Nephrol. 19, 401–417 (2023).
Lee, K., Jang, H. R. & Rabb, H. Lymphocytes and innate immune cells in acute kidney injury and repair. Nat. Rev. Nephrol. 20, 789–805 (2024).
Zager, R. A., Johnson, A. C., Hanson, S. Y. & Lund, S. Ischemic proximal tubular injury primes mice to endotoxin-induced TNF-α generation and systemic release. Am. J. Physiol. Renal Physiol. 289, F289–F297 (2005).
Zager, R. A., Johnson, A. C., Hanson, S. Y. & Lund, S. Acute nephrotoxic and obstructive injury primes the kidney to endotoxin-driven cytokine/chemokine production. Kidney Int. 69, 1181–1188 (2006).
Mar, D. et al. Heterogeneity of epigenetic changes at ischemia/reperfusion- and endotoxin-induced acute kidney injury genes. Kidney Int. 88, 734–744 (2015).
Bauer, M., Ermolaeva, M., Singer, M., Wetzker, R. & Soares, M. P. Hormesis as an adaptive response to infection. Trends Mol. Med. 30, 633–641 (2024).
Weis, S. et al. Metabolic adaptation establishes disease tolerance to sepsis. Cell 169, 1263–1275.e14 (2017).
Rahmel, T. et al. An open-label, randomized controlled trial to assess a ketogenic diet in critically ill patients with sepsis. Sci. Transl. Med. 16, eadn9285 (2024).
Pearce, E. L., Poffenberger, M. C., Chang, C. H. & Jones, R. G. Fueling immunity: insights into metabolism and lymphocyte function. Science 342, 1242454 (2013).
Soto-Heredero, G., Gomez de Las Heras, M. M., Gabande-Rodriguez, E., Oller, J. & Mittelbrunn, M. Glycolysis — a key player in the inflammatory response. FEBS J. 287, 3350–3369 (2020).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020).
Cheng, S. C. et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat. Immunol. 17, 406–413 (2016).
Shi, W., Cassmann, T. J., Bhagwate, A. V., Hitosugi, T. & Ip, W. K. E. Lactic acid induces transcriptional repression of macrophage inflammatory response via histone acetylation. Cell Rep. 43, 113746 (2024).
Zhang, D. et al. Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–580 (2019).
Nolt, B. et al. Lactate and immunosuppression in sepsis. Shock 49, 120–125 (2018).
Vandewalle, J. et al. Combined glucocorticoid resistance and hyperlactatemia contributes to lethal shock in sepsis. Cell Metab. 33, 1763–1776.e5 (2021).
Levy, B., Gibot, S., Franck, P., Cravoisy, A. & Bollaert, P. E. Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet 365, 871–875 (2005).
Iwashyna, T. J., Ely, E. W., Smith, D. M. & Langa, K. M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304, 1787–1794 (2010).
Iwashyna, T. J., Cooke, C. R., Wunsch, H. & Kahn, J. M. Population burden of long-term survivorship after severe sepsis in older Americans. J. Am. Geriatr. Soc. 60, 1070–1077 (2012).
Wang, H. E. et al. Long-term mortality after community-acquired sepsis: a longitudinal population-based cohort study. BMJ Open 4, e004283 (2014).
Fleischmann-Struzek, C. et al. Functional dependence following intensive care unit-treated sepsis: three-year follow-up results from the prospective mid-German sepsis cohort (MSC). Lancet Reg. Health Eur. 46, 101066 (2024).
Torres, J. S. S. et al. Sepsis and post-sepsis syndrome: a multisystem challenge requiring comprehensive care and management-a review. Front. Med. 12, 1560737 (2025).
Lu, X., Yang, Y. M. & Lu, Y. Q. Immunosenescence: a critical factor associated with organ injury after sepsis. Front. Immunol. 13, 917293 (2022).
Darden, D. B. et al. Biomarker evidence of the persistent inflammation, immunosuppression and catabolism syndrome (PICS) in chronic critical illness (CCI) after surgical sepsis. Ann. Surg. 274, 664–673 (2021).
Barrios, E. L. et al. Unique lymphocyte transcriptomic profiles in septic patients with chronic critical illness. Front. Immunol. 15, 1478471 (2024).
Elek, Z. et al. Persistent sepsis-induced transcriptomic signatures in signaling pathways of peripheral blood leukocytes: a pilot study. Hum. Immunol. 84, 600–608 (2023).
Mageau, A. et al. High incidence of immune-mediated inflammatory diseases in sepsis survivors: a nationwide exposed-nonexposed epidemiological study. J. Intern. Med. 295, 242–252 (2024).
Lira Chavez, F. M. et al. Restoring the infected powerhouse: mitochondrial quality control in sepsis. Redox Biol. 68, 102968 (2023).
Medzhitov, R., Schneider, D. S. & Soares, M. P. Disease tolerance as a defense strategy. Science 335, 936–941 (2012).
Soares, M. P., Gozzelino, R. & Weis, S. Tissue damage control in disease tolerance. Trends Immunol. 35, 483–494 (2014).
Leitner, B. P. et al. Tissue-specific reprogramming of glutamine metabolism maintains tolerance to sepsis. PLoS ONE 18, e0286525 (2023).
Wang, A. et al. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell 166, 1512–1525.e12 (2016).
London, N. R. et al. Targeting Robo4-dependent slit signaling to survive the cytokine storm in sepsis and influenza. Sci. Transl. Med. 2, 23ra19 (2010).
Ahuja, S. K. et al. Immune resilience despite inflammatory stress promotes longevity and favorable health outcomes including resistance to infection. Nat. Commun. 14, 3286 (2023).
Beane, A. & Shankar-Hari, M. Long-term ill health in sepsis survivors: an ignored health-care challenge? Lancet 404, 1178–1180 (2024).
Jundi, B. et al. Inflammation resolution circuits are uncoupled in acute sepsis and correlate with clinical severity. JCI Insight 6, e148866 (2021).
Levy, B. D., Clish, C. B., Schmidt, B., Gronert, K. & Serhan, C. N. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2, 612–619 (2001).
Basil, M. C. & Levy, B. D. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 16, 51–67 (2016).
Sugimoto, M. A., Sousa, L. P., Pinho, V., Perretti, M. & Teixeira, M. M. Resolution of inflammation: what controls its onset? Front. Immunol. 7, 160 (2016).
Jordan, P. M. & Werz, O. Specialized pro-resolving mediators: biosynthesis and biological role in bacterial infections. FEBS J. 289, 4212–4227 (2022).
Ferrucci, L. & Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522 (2018).
Rocheteau, P. et al. Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nat. Commun. 6, 10145 (2015).
Eming, S. A., Wynn, T. A. & Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 356, 1026–1030 (2017).
Powell, R. E., Soares, M. P. & Weis, S. What’s new in intensive care: disease tolerance. Intensive Care Med. 49, 1235–1237 (2023).
Thomas-Ruddel, D. et al. Epirubicin for the treatment of sepsis and septic shock (EPOS-1): study protocol for a randomised, placebo-controlled phase IIa dose-escalation trial. BMJ Open. 14, e075158 (2024).
Foster, S. L., Hargreaves, D. C. & Medzhitov, R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447, 972–978 (2007).
Haak, B. W. & Wiersinga, W. J. The role of the gut microbiota in sepsis. Lancet Gastroenterol. Hepatol. 2, 135–143 (2017).
Keith, J. W. & Pamer, E. G. Enlisting commensal microbes to resist antibiotic-resistant pathogens. J. Exp. Med. 216, 10–19 (2019).
Kullberg, R. F. J. et al. Association between butyrate-producing gut bacteria and the risk of infectious disease hospitalisation: results from two observational, population-based microbiome studies. Lancet Microbe 5, 100864 (2024).
Adelman, M. W. et al. The gut microbiome’s role in the development, maintenance, and outcomes of sepsis. Crit. Care 24, 278 (2020).
Ngo, V. L. et al. Intestinal microbiota programming of alveolar macrophages influences severity of respiratory viral infection. Cell Host Microbe 32, 335–348.e8 (2024).
Schuijt, T. J. et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 65, 575–583 (2016).
Schulthess, J. et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity 50, 432–445.e7 (2019).
Seufert, A. L. et al. Enriched dietary saturated fatty acids induce trained immunity via ceramide production that enhances severity of endotoxemia and clearance of infection. Elife 11, e76744 (2022).
Miller, M. et al. Antibiotics, sedatives, and catecholamines further compromise sepsis-induced immune suppression in peripheral blood mononuclear cells. Crit. Care Med. 52, 596–606 (2024).
Cata, J. P., Wang, H., Gottumukkala, V., Reuben, J. & Sessler, D. I. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br. J. Anaesth. 110, 690–701 (2013).
Kiers, D. et al. Short-term hypoxia dampens inflammation in vivo via enhanced adenosine release and adenosine 2B receptor stimulation. EBioMedicine 33, 144–156 (2018).
Wheeler, D. S. et al. The immunomodulatory effects of albumin in vitro and in vivo. Adv. Pharmacol. Sci. 2011, 691928 (2011).
Stolk, R. et al. Norepinephrine dysregulates the immune response and compromises host defense during sepsis. Am. J. Respir. Crit. Care Med. 202, 830–842 (2020).
Stolk, R. F., Kox, M. & Pickkers, P. Noradrenaline drives immunosuppression in sepsis: clinical consequences. Intensive Care Med. 46, 1246–1248 (2020).
Babouee Flury, B., Andrey, D. & Kohler, P. Antibiotics’ collateral effects on the gut microbiota in the selection of ESKAPE pathogens. CMI Commun. 1, 100012 (2024).
Theriot, C. M. et al. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to clostridium difficile infection. Nat. Commun. 5, 3114 (2014).
Drummond, R. A. et al. Long-term antibiotic exposure promotes mortality after systemic fungal infection by driving lymphocyte dysfunction and systemic escape of commensal bacteria. Cell Host Microbe 30, 1020–1033.e6 (2022).
Boukerb, A. M. et al. Inter-kingdom signaling of stress hormones: sensing, transport and modulation of bacterial physiology. Front. Microbiol. 12, 690942 (2021).
Llitjos, J. F. et al. Enhancing sepsis biomarker development: key considerations from public and private perspectives. Crit. Care 28, 238 (2024).
Barichello, T., Generoso, J. S., Singer, M. & Dal-Pizzol, F. Biomarkers for sepsis: more than just fever and leukocytosis — a narrative review. Crit. Care 26, 14 (2022).
Povoa, P. et al. How to use biomarkers of infection or sepsis at the bedside: guide to clinicians. Intensive Care Med. 49, 142–153 (2023).
Pierrakos, C., Velissaris, D., Bisdorff, M., Marshall, J. C. & Vincent, J. L. Biomarkers of sepsis: time for a reappraisal. Crit. Care 24, 287 (2020).
Matsumoto, H. et al. The clinical importance of a cytokine network in the acute phase of sepsis. Sci. Rep. 8, 13995 (2018).
Zhang, Y., Li, X., Zhang, X., Wang, T. & Zhang, X. Progress in the study of pentraxin-3 (PTX-3) as a biomarker for sepsis. Front. Med. 11, 1398024 (2024).
Carcillo, J. A. et al. A systemic inflammation mortality risk assessment contingency table for severe sepsis. Pediatr. Crit. Care Med. 18, 143–150 (2017).
He, L., Guo, C., Su, Y. & Ding, N. The relationship between serum ferritin level and clinical outcomes in sepsis based on a large public database. Sci. Rep. 13, 8677 (2023).
Albrecht, E. A. & Ward, P. A. Complement-induced impairment of the innate immune system during sepsis. Curr. Infect. Dis. Rep. 7, 349–354 (2005).
Riedemann, N. C. et al. Increased C5a receptor expression in sepsis. J. Clin. Invest. 110, 101–108 (2002).
Khater, W. S., Salah-Eldeen, N. N., Khater, M. S. & Saleh, A. N. Role of suPAR and lactic acid in diagnosing sepsis and predicting mortality in elderly patients. Eur. J. Microbiol. Immunol. 6, 178–185 (2016).
Adami, M. E. et al. qSOFA combined with suPAR for early risk detection and guidance of antibiotic treatment in the emergency department: a randomized controlled trial. Crit. Care 28, 42 (2024).
Theobald, V. et al. Triggering receptor expressed on myeloid cells-1 in sepsis, and current insights into clinical studies. Crit. Care 28, 17 (2024).
Torres, L. K., Pickkers, P. & van der Poll, T. Sepsis-induced immunosuppression. Annu. Rev. Physiol. 84, 157–181 (2022).
Fumeaux, T. & Pugin, J. Role of interleukin-10 in the intracellular sequestration of human leukocyte antigen-DR in monocytes during septic shock. Am. J. Respir. Crit. Care Med. 166, 1475–1482 (2002).
Marie, C., Cavaillon, J. M. & Losser, M. R. Elevated levels of circulating transforming growth factor-β 1 in patients with the sepsis syndrome. Ann. Intern. Med. 125, 520–521 (1996).
Liu, M. et al. Serum sPD-L1, upregulated in sepsis, may reflect disease severity and clinical outcomes in septic patients. Scand. J. Immunol. 85, 66–72 (2017).
Yuan, Y., Yan, D., Han, G., Gu, G. & Ren, J. Complement C3 depletion links to the expansion of regulatory T cells and compromises T-cell immunity in human abdominal sepsis: a prospective pilot study. J. Crit. Care 28, 1032–1038 (2013).
Docke, W. D. et al. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat. Med. 3, 678–681 (1997).
Bodinier, M. et al. Monocyte trajectories endotypes are associated with worsening in septic patients. Front. Immunol. 12, 795052 (2021).
Seok, Y. et al. Delta neutrophil index: a promising diagnostic and prognostic marker for sepsis. Shock 37, 242–246 (2012).
Demaret, J. et al. Marked alterations of neutrophil functions during sepsis-induced immunosuppression. J. Leukoc. Biol. 98, 1081–1090 (2015).
Drifte, G., Dunn-Siegrist, I., Tissieres, P. & Pugin, J. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit. Care Med. 41, 820–832 (2013).
Seymour, C. W. et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA 321, 2003–2017 (2019).
Molinari, L. et al. Distribution of acute and chronic kidney disease across clinical phenotypes for sepsis. Chest 166, 480–490 (2024).
Bruse, N. et al. Clinical sepsis phenotypes in critically ill COVID-19 patients. Crit. Care 26, 244 (2022).
Bruse, N. Clinical phenotyping uncovers heterogeneous associations between corticosteroid treatment and survival in critically ill (COVD-19) patients. Intensive Care Med. 50, 1884–1886 (2024).
Gardlund, B. et al. Six subphenotypes in septic shock: latent class analysis of the PROWESS Shock study. J. Crit. Care 47, 70–79 (2018).
Zhang, Z., Zhang, G., Goyal, H., Mo, L. & Hong, Y. Identification of subclasses of sepsis that showed different clinical outcomes and responses to amount of fluid resuscitation: a latent profile analysis. Crit. Care 22, 347 (2018).
Zador, Z., Landry, A., Cusimano, M. D. & Geifman, N. Multimorbidity states associated with higher mortality rates in organ dysfunction and sepsis: a data-driven analysis in critical care. Crit. Care 23, 247 (2019).
Yehya, N. et al. Temperature trajectory sub-phenotypes and the immuno-inflammatory response in pediatric sepsis. Shock 57, 645–651 (2022).
Bhavani, S. V. et al. Identifying novel sepsis subphenotypes using temperature trajectories. Am. J. Respir. Crit. Care Med. 200, 327–335 (2019).
Xu, Z. et al. Sepsis subphenotyping based on organ dysfunction trajectory. Crit. Care 26, 197 (2022).
Leng, F. et al. Novel cortisol trajectory sub-phenotypes in sepsis. Crit. Care 28, 290 (2024).
Calfee, C. S. et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir. Med. 2, 611–620 (2014).
Sinha, P., Meyer, N. J. & Calfee, C. S. Biological phenotyping in sepsis and acute respiratory distress syndrome. Annu. Rev. Med. 74, 457–471 (2023).
Sinha, P. et al. Identifying molecular phenotypes in sepsis: an analysis of two prospective observational cohorts and secondary analysis of two randomised controlled trials. Lancet Respir. Med. 11, 965–974 (2023).
Calfee, C. S. et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir. Med. 6, 691–698 (2018).
Maddali, M. V. et al. Validation and utility of ARDS subphenotypes identified by machine-learning models using clinical data: an observational, multicohort, retrospective analysis. Lancet Respir. Med. 10, 367–377 (2022).
Famous, K. R. et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am. J. Respir. Crit. Care Med. 195, 331–338 (2017).
Heijnen, N. F. L. et al. Biological subphenotypes of acute respiratory distress syndrome show prognostic enrichment in mechanically ventilated patients without acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 203, 1503–1511 (2021).
Kudo, D. et al. Coagulation phenotypes in sepsis and effects of recombinant human thrombomodulin: an analysis of three multicentre observational studies. Crit. Care 25, 114 (2021).
Wong, H. R. et al. Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med. 7, 34 (2009).
Wong, H. R. et al. Validation of a gene expression-based subclassification strategy for pediatric septic shock. Crit. Care Med. 39, 2511–2517 (2011).
Davenport, E. E. et al. Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir. Med. 4, 259–271 (2016).
Burnham, K. L. et al. Shared and distinct aspects of the sepsis transcriptomic response to fecal peritonitis and pneumonia. Am. J. Respir. Crit. Care Med. 196, 328–339 (2017).
Cano-Gamez, E. et al. An immune dysfunction score for stratification of patients with acute infection based on whole-blood gene expression. Sci. Transl. Med. 14, eabq4433 (2022).
Scicluna, B. P. et al. Classification of patients with sepsis according to blood genomic endotype: a prospective cohort study. Lancet Respir. Med. 5, 816–826 (2017).
Sweeney, T. E. et al. Unsupervised analysis of transcriptomics in bacterial sepsis across multiple datasets reveals three robust clusters. Crit. Care Med. 46, 915–925 (2018).
Sweeney, T. E. et al. Validation of inflammopathic, adaptive, and coagulopathic sepsis endotypes in coronavirus disease 2019. Crit. Care Med. 49, e170–e178 (2021).
Rautanen, A. et al. Genome-wide association study of survival from sepsis due to pneumonia: an observational cohort study. Lancet Respir. Med. 3, 53–60 (2015).
Scherag, A. et al. Genetic factors of the disease course after sepsis: a genome-wide study for 28day mortality. EBioMedicine 12, 239–246 (2016).
Hernandez-Beeftink, T. et al. A genome-wide association study of survival in patients with sepsis. Crit. Care 26, 341 (2022).
Rogers, A. J. et al. Plasma metabolites in early sepsis identify distinct clusters defined by plasma lipids. Crit. Care Explor. 3, e0478 (2021).
Mi, Y. et al. High-throughput mass spectrometry maps the sepsis plasma proteome and differences in patient response. Sci. Transl. Med. 16, eadh0185 (2024).
van Amstel, R. B. E. et al. Uncovering heterogeneity in sepsis: a comparative analysis of subphenotypes. Intensive Care Med. 49, 1360–1369 (2023).
Neyton, L. P. A. et al. Host and microbe blood metagenomics reveals key pathways characterizing critical illness phenotypes. Am. J. Respir. Crit. Care Med. 209, 805–815 (2024).
Wong, H. R. Intensive care medicine in 2050: precision medicine. Intensive Care Med. 43, 1507–1509 (2017).
Prescott, H. C., Calfee, C. S., Thompson, B. T., Angus, D. C. & Liu, V. X. Toward smarter lumping and smarter splitting: rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am. J. Respir. Crit. Care Med. 194, 147–155 (2016).
Stanski, N. L. & Wong, H. R. Prognostic and predictive enrichment in sepsis. Nat. Rev. Nephrol. 16, 20–31 (2020).
Shankar-Hari, M. & Rubenfeld, G. D. Population enrichment for critical care trials: phenotypes and differential outcomes. Curr. Opin. Crit. Care 25, 489–497 (2019).
Pourmand, A., Whiteside, T., Yamane, D., Rashed, A. & Mazer-Amirshahi, M. The controversial role of corticosteroids in septic shock. Am. J. Emerg. Med. 37, 1353–1361 (2019).
Antcliffe, D. B. et al. Transcriptomic signatures in sepsis and a differential response to steroids. from the VANISH randomized trial. Am. J. Respir. Crit. Care Med. 199, 980–986 (2019).
Scicluna, B. P. & Baillie, J. K. The search for efficacious new therapies in sepsis needs to embrace heterogeneity. Am. J. Respir. Crit. Care Med. 199, 936–938 (2019).
Yao, L. et al. Gene expression scoring of immune activity levels for precision use of hydrocortisone in vasodilatory shock. Shock 57, 384–391 (2022).
Group, R. C. et al. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 384, 693–704 (2021).
Sinha, P. et al. Latent class analysis reveals COVID-19-related acute respiratory distress syndrome subgroups with differential responses to corticosteroids. Am. J. Respir. Crit. Care Med. 204, 1274–1285 (2021).
Galvan-Roman, J. M. et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: an observational study. J. Allergy Clin. Immunol. 147, 72–80 e78 (2021).
Practical, P. T. I. & investigators, R.-C The rise of adaptive platform trials in critical care. Am. J. Respir. Crit. Care Med. 209, 491–496 (2024).
Meisel, C. et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am. J. Respir. Crit. Care Med. 180, 640–648 (2009).
Shibata, M. et al. Myeloid-derived suppressor cells: cancer, autoimmune diseases, and more. Oncotarget 13, 1273–1285 (2022).
Opal, S. M. et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The interleukin-1 receptor antagonist sepsis investigator group. Crit. Care Med. 25, 1115–1124 (1997).
Shakoory, B. et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit. Care Med. 44, 275–281 (2016).
Meyer, N. J. et al. Mortality benefit of recombinant human interleukin-1 receptor antagonist for sepsis varies by initial interleukin-1 receptor antagonist plasma concentration. Crit. Care Med. 46, 21–28 (2018).
Kyriazopoulou, E. et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat. Med. 27, 1752–1760 (2021).
Ivashkiv, L. B. IFNγ: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 18, 545–558 (2018).
Casanova, J. L., MacMicking, J. D. & Nathan, C. F. Interferon-γ and infectious diseases: lessons and prospects. Science 384, eadl2016 (2024).
Leentjens, J. et al. Reversal of immunoparalysis in humans in vivo: a double-blind, placebo-controlled, randomized pilot study. Am. J. Respir. Crit. Care Med. 186, 838–845 (2012).
Payen, D. et al. Multicentric experience with interferon gamma therapy in sepsis induced immunosuppression. A case series. BMC Infect. Dis. 19, 931 (2019).
Kotsaki, A. et al. ImmunoSep (Personalised Immunotherapy in Sepsis) international double-blind, double-dummy, placebo-controlled randomised clinical trial: study protocol. BMJ Open. 12, e067251 (2022).
Leventogiannis, K. et al. Toward personalized immunotherapy in sepsis: the PROVIDE randomized clinical trial. Cell Rep. Med. 3, 100817 (2022).
Group, R. C. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis. Lancet 400, 359–368 (2022).
Brosius, F. C., Tuttle, K. R. & Kretzler, M. JAK inhibition in the treatment of diabetic kidney disease. Diabetologia 59, 1624–1627 (2016).
Hoisnard, L. et al. Adverse events associated with JAK inhibitors in 126,815 reports from the WHO pharmacovigilance database. Sci. Rep. 12, 7140 (2022).
Bittle, E. et al. Safety data for baricitinib use in children with Severe SARS-CoV-2 infection. Hosp. Pediatr. 15, e203–e208 (2025).
Jin, K. et al. Activation of AMP-activated protein kinase during sepsis/inflammation improves survival by preserving cellular metabolic fitness. FASEB J. 34, 7036–7057 (2020).
Gao, Y. et al. Metformin alleviates sepsis-associated myocardial injury by enhancing AMP-activated protein kinase/mammalian target of rapamycin signaling pathway-mediated autophagy. J. Cardiovasc. Pharmacol. 82, 308–317 (2023).
Fan, S. Y. et al. Metformin mitigates sepsis-induced acute lung injury and inflammation in young mice by suppressing the S100A8/A9-NLRP3-IL-1β signaling pathway. J. Inflamm. Res. 17, 3785–3799 (2024).
Gomez, H. et al. Association of metformin use during hospitalization and mortality in critically Ill adults with type 2 diabetes mellitus and sepsis. Crit. Care Med. 50, 935–944 (2022).
Li, Y. et al. Association of preadmission metformin use and prognosis in patients with sepsis and diabetes mellitus: a systematic review and meta-analysis. Front. Endocrinol. 12, 811776 (2021).
Liang, H. et al. Association of preadmission metformin use and mortality in patients with sepsis and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Crit. Care 23, 50 (2019).
Tan, K. et al. The association of premorbid metformin exposure with mortality and organ dysfunction in sepsis: a systematic review and meta-analysis. Crit. Care Explor. 1, e0009 (2019).
Owen, M. R., Doran, E. & Halestrap, A. P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 348, 607–614 (2000).
Shaw, R. J. et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 (2005).
Fullerton, M. D. et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 19, 1649–1654 (2013).
Zhou, G. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 (2001).
Madiraju, A. K. et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510, 542–546 (2014).
O’Neill, L. A. & Hardie, D. G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 493, 346–355 (2013).
Frauwirth, K. A. et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 (2002).
Dowling, R. J., Zakikhani, M., Fantus, I. G., Pollak, M. & Sonenberg, N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 67, 10804–10812 (2007).
Kalender, A. et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 11, 390–401 (2010).
Ben Sahra, I. et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 71, 4366–4372 (2011).
Hattori, Y., Suzuki, K., Hattori, S. & Kasai, K. Metformin inhibits cytokine-induced nuclear factor κB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 47, 1183–1188 (2006).
Xian, H. et al. Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation. Immunity 54, 1463–1477.e11 (2021).
Negrotto, L., Farez, M. F. & Correale, J. Immunologic effects of metformin and pioglitazone treatment on metabolic syndrome and multiple sclerosis. JAMA Neurol. 73, 520–528 (2016).
Lachmandas, E. et al. Metformin alters human host responses to mycobacterium tuberculosis in healthy subjects. J. Infect. Dis. 220, 139–150 (2019).
Kang, K. Y. et al. Metformin downregulates Th17 cells differentiation and attenuates murine autoimmune arthritis. Int. Immunopharmacol. 16, 85–92 (2013).
Sag, D., Carling, D., Stout, R. D. & Suttles, J. Adenosine 5’-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J. Immunol. 181, 8633–8641 (2008).
Liu, T. F., Vachharajani, V. T., Yoza, B. K. & McCall, C. E. NAD+-dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J. Biol. Chem. 287, 25758–25769 (2012).
Arai, M. et al. Metformin, an antidiabetic agent, suppresses the production of tumor necrosis factor and tissue factor by inhibiting early growth response factor-1 expression in human monocytes in vitro. J. Pharmacol. Exp. Ther. 334, 206–213 (2010).
Park, D. W. et al. Activation of AMPK enhances neutrophil chemotaxis and bacterial killing. Mol. Med. 19, 387–398 (2013).
Saraiva, I. E. et al. Metformin for sepsis-associated AKI: a protocol for the randomized clinical trial of the safety and feasibility of metformin as a treatment for sepsis-associated AKI (LiMiT AKI). BMJ Open. 14, e081120 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05979038 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06181422 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05572060 (2022).
Lim, E. H. T. et al. Complement activation in COVID-19 and targeted therapeutic options: a scoping review. Blood Rev. 57, 100995 (2023).
Annane, D., Grimaldi-Bensouda, L. & Fremeaux-Bacchic, V. Complement inhibition in severe COVID-19 - blocking C5a seems to be key: author’s reply. EClinicalMedicine 35, 100866 (2021).
Vlaar, A. P. J. et al. Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir. Med. 10, 1137–1146 (2022).
Bauer, M. et al. Efficacy and safety of vilobelimab (IFX-1), a novel monoclonal anti-C5a antibody, in patients with early severe sepsis or septic shock-a randomized, placebo-controlled, double-blind, multicenter, phase IIa trial (SCIENS Study). Crit. Care Explor. 3, e0577 (2021).
Hotchkiss, R. S. et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 27, 1230–1251 (1999).
de Jager, C. P. et al. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit. Care 14, R192 (2010).
Hofmeister, R. et al. Interleukin-7: physiological roles and mechanisms of action. Cytokine Growth Factor. Rev. 10, 41–60 (1999).
Unsinger, J. et al. Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis. J. Infect. Dis. 206, 606–616 (2012).
Unsinger, J. et al. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J. Immunol. 184, 3768–3779 (2010).
Delwarde, B. et al. Low interleukin-7 receptor messenger RNA expression is independently associated with day 28 mortality in septic shock patients. Crit. Care Med. 46, 1739–1746 (2018).
Francois, B. et al. Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight 3, e98960 (2018).
Laterre, P. F. et al. Association of interleukin 7 Immunotherapy with lymphocyte counts among patients with severe coronavirus disease 2019 (COVID-19). JAMA Netw. Open. 3, e2016485 (2020).
Crausaz, M. et al. A novel virotherapy encoding human interleukin-7 improves ex vivo T lymphocyte functions in immunosuppressed patients with septic shock and critically ill COVID-19. Front. Immunol. 13, 939899 (2022).
Chang, K. et al. Targeting the programmed cell death 1: programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit. Care 18, R3 (2014).
Brahmamdam, P. et al. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J. Leukoc. Biol. 88, 233–240 (2010).
Kraehenbuehl, L., Weng, C. H., Eghbali, S., Wolchok, J. D. & Merghoub, T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 19, 37–50 (2022).
Shindo, Y. et al. Anti-PD-L1 peptide improves survival in sepsis. J. Surg. Res. 208, 33–39 (2017).
Rudick, C. P., Cornell, D. L. & Agrawal, D. K. Single versus combined immunoregulatory approach using PD-1 and CTLA-4 modulators in controlling sepsis. Expert. Rev. Clin. Immunol. 13, 907–919 (2017).
Hotchkiss, R. S. et al. Immune checkpoint inhibition in sepsis: a Phase 1b randomized study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of nivolumab. Intensive Care Med. 45, 1360–1371 (2019).
Liu, L. L., Skribek, M., Harmenberg, U. & Gerling, M. Systemic inflammatory syndromes as life-threatening side effects of immune checkpoint inhibitors: case report and systematic review of the literature. J. Immunother. Cancer 11, e005841 (2023).
Leibundgut-Landmann, S., Osorio, F., Brown, G. D. & Reis e Sousa, C. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood 112, 4971–4980 (2008).
Vannucci, L. et al. Immunostimulatory properties and antitumor activities of glucans. Int. J. Oncol. 43, 357–364 (2013).
Willment, J. A. et al. The human β-glucan receptor is widely expressed and functionally equivalent to murine dectin-1 on primary cells. Eur. J. Immunol. 35, 1539–1547 (2005).
van der Meer, J. W., Joosten, L. A., Riksen, N. & Netea, M. G. trained immunity: a smart way to enhance innate immune defence. Mol. Immunol. 68, 40–44 (2015).
Viana, J. P. M. et al. Glucans: a therapeutic alternative for sepsis treatment. J. Immunol. Res. 2024, 6876247 (2024).
Morris, T. et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J. Immunol. 183, 2089–2096 (2009).
Kiers, D. et al. A randomised trial on the effect of anti-platelet therapy on the systemic inflammatory response in human endotoxaemia. Thromb. Haemost. 117, 1798–1807 (2017).
Leijte, G. P. et al. Treatment with acetylsalicylic acid reverses endotoxin tolerance in humans in vivo: a randomized placebo-controlled study. Crit. Care Med. 47, 508–516 (2018).
Trauer, J. et al. Quantifying the effects of prior acetyl-salicylic acid on sepsis-related deaths: an individual patient data meta-analysis using propensity matching. Crit. Care Med. 45, 1871–1879 (2017).
Eisen, D. P. et al. Effect of aspirin on deaths associated with sepsis in healthy older people (ANTISEPSIS): a randomised, double-blind, placebo-controlled primary prevention trial. Lancet Respir. Med. 9, 186–195 (2021).
Johnstone, J. et al. Effect of probiotics on incident ventilator-associated pneumonia in critically Ill patients: a randomized clinical trial. JAMA 326, 1024–1033 (2021).
Litton, E. et al. Early and sustained lactobacillus plantarum probiotic therapy in critical illness: the randomised, placebo-controlled, restoration of gut microflora in critical illness trial (ROCIT). Intensive Care Med. 47, 307–315 (2021).
Biemond, J. J., McDonald, B. & Haak, B. W. Leveraging the microbiome in the treatment of sepsis: potential pitfalls and new perspectives. Curr. Opin. Crit. Care 29, 123–129 (2023).
Kim, S. G. et al. Microbiota-derived lantibiotic restores resistance against vancomycin-resistant enterococcus. Nature 572, 665–669 (2019).
Azevedo, A. S., Follain, G., Patthabhiraman, S., Harlepp, S. & Goetz, J. G. Metastasis of circulating tumor cells: favorable soil or suitable biomechanics, or both? Cell Adh. Migr. 9, 345–356 (2015).
Kim, S. M. et al. Fecal microbiota transplant rescues mice from human pathogen mediated sepsis by restoring systemic immunity. Nat. Commun. 11, 2354 (2020).
Zhang, L. et al. Protein modification by short-chain fatty acid metabolites in sepsis: a comprehensive review. Front. Immunol. 14, 1171834 (2023).
Riedemann, N. C. et al. Regulatory role of C5a in LPS-induced IL-6 production by neutrophils during sepsis. FASEB J. 18, 370–372 (2004).
Lindenkamp, C. et al. The activation of JAK/STAT signaling and the complement system modulate inflammation in the primary human dermal fibroblasts of PXE patients. Biomedicines 11, 2673 (2023).
Angus, D. C. et al. The integration of clinical trials with the practice of medicine: repairing a hohuse divided. JAMA 332, 153–162 (2024).
Angus, D. C. et al. The REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform for Community-acquired Pneumonia) study. rationale and design. Ann. Am. Thorac. Soc. 17, 879–891 (2020).
Practical Platform https://practicalplatform.org/.
Buell, K. G. et al. Individualized treatment effects of oxygen targets in mechanically ventilated critically Ill adults. JAMA 331, 1195–1204 (2024).
UPMC REMAP-COVID Group, on behalf of the REMAP-CAP Investigators Implementation of the randomized embedded multifactorial adaptive platform for COVID-19 (REMAP-COVID) trial in a US health system-lessons learned and recommendations. Trials 22, 100 (2021).
Pairo-Castineira, E. et al. Genetic mechanisms of critical illness in COVID-19. Nature 591, 92–98 (2021).
Keramati, F. et al. Systemic inflammation impairs myelopoiesis and interferon type I responses in humans. Nat. Immunol. 26, 737–747 (2025).
Cafferkey, J. & Shankar-Hari, M. Informative subtyping of patients with sepsis. Semin. Respir. Crit. Care Med. 45, 516–522 (2024).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed substantially to discussion of the content and wrote the article. M.K. and P.P. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.K. has received institutional funding (full institutional disclosure) from Reumafonds, Medisieve, Comentis, Abionic, CytoSorbents, Adrenomed, Inflammatix, SurvivX and 4TEEN4, as well as travel reimbursements and consulting fees from ARTCLINE, Atriva, AOP pharma, Inflammatix, SurvivX and 4TEEN4 outside the submitted work. M.B. has received travel reimbursements and consulting fees from ARTCLINE, BAYER, BRAHMS/ Thermo Fisher, Volition and research grants from DFG (German Research Council) and BMBF (Federal Ministry of Education and Research). L.D.J.B. has consulted for Aptarion, AstraZeneca, Bayer, CSL, Novartis, Volition and Bayer. He received an unrestricted grant from Volition. Consultancy fees and grants were paid to the institution. H.B. has received institutional funding from Becton Dickinson, Inflammatix, Octapharma, OSAsense, MeMed, Dutch Kidney Foundation, Dutch Research Council (NWO). T.C. reports grants from the Swiss Academy of Medical, Swiss Federal Institute of Technology Zurich and Horizon 2020 from the European Community; consulting, speaker’s bureau and data-safety-monitoring boards from Basilea, Cidara, Gilead Sciences, A. Menarini Pharma, MSD Merck Sharp & Dohme, Moderna, Novartis, Pfizer and Shionogi, all paid to his institution; and sponsorship to the International Sepsis Forum (ISF) by BD, Cytosorbents, Deepull, Gentian, Inflammatix, Inotrem, MeMed, Oxford Nanopore, RevImmune, Thermo Fisher Scientific and Volition. C.S.C. has consulted for Vasomune, Gen1e Life Sciences, Arrowhead, Cellenkos, Calcimedical, EnliTisa, Novartis, Aerogen, Fisher and Paykel, and Boehringer. She received grant funding from NIH, Roche Genentech and Quantum Leap Healthcare Collaborative (paid to the institution), all outside the submitted work other than NIH grant R35HL140026. B.G.C. received speaker fees from Baxter and was a member of an advisory board for Roche Diagnostics. L.P.G.D. reports institutional grants from HORIZON-Health (2021-2026) (ECRAID-Base, Grant agreement ID: 965313) and ZonMw (Immune Modulation Platform for Influenza Treatment (IMPRINT) project number 10140252210024 and NCOH Pandemic Preparedness Research Kickstarter project number 10710022210003). E.J.G.-B. reports honoraria and consultation fees from Abbott Products Operations, bioMérieux, Brahms GmbH, GSK and Sobi (granted to the National and Kapodistrian University of Athens); independent educational grants from AbbVie, InCyte, Novartis and UCB (granted to the National and Kapodistrian University of Athens) and from Abbott Products Operations, bioMérieux Inc, Johnson & Johnson, MSD and Swedish Orphan Biovitrum AB (granted to the Hellenic Institute for the Study of Sepsis); and funding from the Horizon 2020 European Grants ImmunoSep and RISCinCOVID and the Horizon Health grants EPIC-CROWN-2, POINT and Homi-Lung (granted to the Hellenic Institute for the Study of Sepsis). H.G. has received consulting fees from Trilinear Bioventures, Talphera, Novartis, speaker fees from bioMérieux and research grants from bioMérieux, Baxter, TES Pharma, the National Institutes of Health and the Department of Defense of the United States of America. M.G.N. is a Scientific Founder of Biotrip, Lemba, TTxD and Salvina Therapeutics. He is chairman of the Scientific Board of TTxD. He performed consultancy for Curevac and Primmune. M.O. has received research funding from Baxter and BioMérieux and consulted for AM-Pharma (paid to the institution). T.v.d.P. received funding from EU (FAIR) and the Dutch Ministry of Economic Affairs & Health Holland, and consultancy fees from Matisse, all paid to the institution. B.P.S. received funds from the European Society of Intensive Care Medicine (ESICM), Xjenza Malta Research Excellence Program (REP-2023-049; REP-2024-062) and a University of Malta Research Excellence Award 2023. All grants were paid to the institution. C.S. has consulted for Beckman Coulter, Edwards, Deepull, Inflammatix and Octapharma. He received grant funding from NIH and the Moore Foundation (paid to the institution). M.S.-H. declares that he is a Director of Research for the Intensive Care Society, Member of the MRC/NIHR EME Programme, Chair of the EME-NIHR Advanced Fellowship Committee, Council of International Sepsis Forum, receives funding for studies from the NIHR, Wellcome Trust, Medical Research Council, and has done advisory board activity either directly or indirectly through International Sepsis Forum for Biotest, Endpoint Health, Janssen, Pfizer, Aurobac, GSK and Santersus, with payments going into the unrestricted institutional research funds. M.S.-H. also acknowledges the programme grant named Time critical precision medicine for acute critical illness using treatable trait principles: TRAITS Programme (PMAS/21/08) from the Chief Scientist’s Office, Scotland. N.S. received grant funding from Bluejay Diagnostics, Inflammatix, CDC and NIH. He or his institution receives fees for lectures, presentations, speaker bureaus, manuscript writing or educational events from Lumos diagnostics. Stock options were offered to him from Prenosis. M.S. received grant funding from the Medical Research Council, UK National Institute for Health and Care Research (NIHR), Rosetrees Trust, UCL Therapeutic Acceleration Support Fund, Gentian, DSTL and the European Society of Intensive Care Medicine. He or his institution receives fees for consulting, advisory boards, data safety monitoring boards and speaking/chairing from AOP Pharma, Aptarion, BioMérieux, Biotest, Deltex Medical, deePull, Hemotune, Matisse, Roche Diagnostics, Safeguard Biosystems, Sanofi, Volition. He is past-president of the International Sepsis Forum (ISF) and current Sepsis Topic Advisor for NICE. F.V. has received travel reimbursement fees from Astra-Zeneca. A.P.J.V. received consultant fees from AM-Pharma, InflaRx and CSL Behring paid to the institution. L.A.v.V. was supported by the Netherlands Organisation for Health Research and Development ZonMW (Nederlandse Organisatie voor Wetenschappelijk Onderzoek NWO) VENI grant 09150161910033. S.W., is currently funded by the Deutsche Forschungsgemeinschaft, DFG, project number WE 4971/6-1, WE 4971/9-1 and the Federal Ministry of Education and Research (BMBF) project number 01EN2001. W.J.W. receives funding from EU (Eurostars) and Netherlands Organisation for Health Research and Development (ZonMw) in addition to consultancy fees from AstraZeneca and Shionogi outside the submitted work (all fees paid to the host institution). P.P. has received travel reimbursements and consulting fees from AM-Pharma, Adrenomed, EBI, Paion, Sphingotec and 4Teen4 outside the submitted work.
Peer review
Peer review information
Nature Reviews Nephrology thanks Monowar Aziz, Lyle Moldawer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Bayesian trial designs
-
Trial designs that use Bayesian inference to integrate prior knowledge with accumulating trial data, enabling adaptive decision making and real-time updates to probabilities of treatment efficacy or other outcomes.
- Jarisch–Herxheimer reaction
-
An acute, systemic inflammatory response that can occur after the initiation of antibiotic treatment.
- Quorum sensing
-
A cell-to-cell communication process used by bacteria to coordinate collective behaviours based on population density.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kox, M., Bauer, M., Bos, L.D.J. et al. The immunology of sepsis: translating new insights into clinical practice. Nat Rev Nephrol (2025). https://doi.org/10.1038/s41581-025-01004-6
Accepted:
Published:
DOI: https://doi.org/10.1038/s41581-025-01004-6