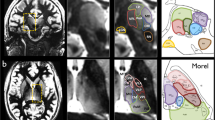
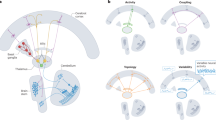
Abstract
The thalamus has a key role in mediating cortical–subcortical interactions but is often neglected in neuroimaging studies, which mostly focus on changes in cortical structure and activity. One of the main reasons for the thalamus being overlooked is that the delineation of individual thalamic nuclei via neuroimaging remains controversial. Indeed, neuroimaging atlases vary substantially regarding which thalamic nuclei are included and how their delineations were established. Here, we review current and emerging methods for thalamic nuclei segmentation in neuroimaging data and consider the limitations of existing techniques in terms of their research and clinical applicability. We address these challenges by proposing a roadmap to improve thalamic nuclei segmentation in human neuroimaging and, in turn, harmonize research approaches and advance clinical applications. We believe that a collective effort is required to achieve this. We hope that this will ultimately lead to the thalamic nuclei being regarded as key brain regions in their own right and not (as often currently assumed) as simply a gateway between cortical and subcortical regions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Usrey, W. M. & Sherman, S. M. In: The Cerebral Cortex and Thalamus (eds Usrey, W. M. & Sherman, S. M.) 3–10 (Oxford Univ. Press, 2023). An updated and comprehensive volume on thalamic nuclei and their contributions to cortical mechanisms.
Jones, E. G. The Thalamus (Cambridge Univ. Press, 2007).
Halassa, M. M. & Sherman, S. M. Thalamocortical circuit motifs: a general framework. Neuron 103, 762–770 (2019).
Krauth, A. et al. A mean three-dimensional atlas of the human thalamus: generation from multiple histological data. Neuroimage 49, 2053–2062 (2010).
Tourdias, T., Saranathan, M., Levesque, I. R., Su, J. & Rutt, B. K. Visualization of intra-thalamic nuclei with optimized white-matter-nulled MPRAGE at 7T. Neuroimage 84, 534–545 (2014).
Segobin, S. & Pitel, A. L. The specificity of thalamic alterations in Korsakoff’s syndrome: implications for the study of amnesia. Neurosci. Biobehav. Rev. 130, 292–300 (2021).
Kumar, V. J., Scheffler, K., Hagberg, G. E. & Grodd, W. Quantitative susceptibility mapping of the basal ganglia and thalamus at 9.4 Tesla. Front. Neuroanat. 15, 725731 (2021).
Morel, A., Magnin, M. & Jeanmonod, D. Multiarchitectonic and stereotactic atlas of the human thalamus. J. Comp. Neurol. 387, 588–630 (1997). Most probabilistic atlases that incorporate histological data are derived from this histological atlas.
Niemann, K., Mennicken, V. R., Jeanmonod, D. & Morel, A. The Morel stereotactic atlas of the human thalamus: atlas-to-MR registration of internally consistent canonical model. Neuroimage 12, 601–616 (2000).
Schaltenbrand, G. & Wahren, W. Atlas for Stereotaxy of the Human Brain (Thieme, 1977).
Chakravarty, M. M., Bertrand, G., Hodge, C. P., Sadikot, A. F. & Collins, D. L. The creation of a brain atlas for image guided neurosurgery using serial histological data. Neuroimage 30, 359–376 (2006).
Sadikot, A. et al. Creation of computerized 3D MRI-integrated atlases of the human basal ganglia and thalamus. Front. Syst. Neurosci. 5, 71 (2011).
Iglehart, C., Monti, M., Cain, J., Tourdias, T. & Saranathan, M. A systematic comparison of structural-, structural connectivity-, and functional connectivity-based thalamus parcellation techniques. Brain Struct. Funct. 225, 1631–1642 (2020).
Hwang, K., Shine, J. M., Cole, M. W. & Sorenson, E. Thalamocortical contributions to cognitive task activity. eLife 11, e81282 (2022).
Antonucci, L. A. et al. Flexible and specific contributions of thalamic subdivisions to human cognition. Neurosci. Biobehav. Rev. 124, 35–53 (2021).
Kumar, V. J., Beckmann, C. F., Scheffler, K. & Grodd, W. Relay and higher-order thalamic nuclei show an intertwined functional association with cortical-networks. Commun. Biol. 5, 1187 (2022).
Wen, H. et al. Pulvinar response profiles and connectivity patterns to object domains. J. Neurosci. 43, 812–826 (2023).
Dadar, M., Fonov, V. S. & Collins, D. L. A comparison of publicly available linear MRI stereotaxic registration techniques. Neuroimage 174, 191–200 (2018).
Klein, A. et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 46, 786–802 (2009).
Mai, J. K. & Majtanik, M. Toward a common terminology for the thalamus. Front. Neuroanat. 12, 114 (2019). A paper that underlines the importance of a common nomenclature and discusses how it can be achieved.
Deoni, S. C. L., Josseau, M. J. C., Rutt, B. K. & Peters, T. M. Visualization of thalamic nuclei on high resolution, multi-averaged T1 and T2 maps acquired at 1.5T. Hum. Brain Mapp. 25, 353–359 (2005).
Deoni, S. C. L., Rutt, B. K., Parrent, A. G. & Peters, T. M. Segmentation of thalamic nuclei using a modified k-means clustering algorithm and high-resolution quantitative magnetic resonance imaging at 1.5T. Neuroimage 34, 117–126 (2007).
Traynor, C. R., Barker, G. J., Crum, W. R., Williams, S. C. R. & Richardson, M. P. Segmentation of the thalamus in MRI based on T1 and T2. Neuroimage 56, 939–950 (2011).
Mulder, M. J., Keuken, M. C., Bazin, P.-L., Alkemade, A. & Forstmann, B. U. Size and shape matter: the impact of voxel geometry on the identification of small nuclei. PLoS One 14, e0215382 (2019).
Iglesias, J. E. & Sabuncu, M. R. Multi-atlas segmentation of biomedical images: a survey. Med. Image Anal. 24, 205–219 (2015).
Iglesias, J. E. et al. A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. Neuroimage 183, 314–326 (2018).
Fischl, B. FreeSurfer. Neuroimage 62, 774–781 (2012).
Wang, H. et al. Multi-atlas segmentation with joint label fusion. IEEE Trans. Pattern Anal. Mach. Intell. 35, 611–623 (2013).
Su, J. H. et al. Thalamus Optimized Multi Atlas Segmentation (THOMAS): fast, fully automated segmentation of thalamic nuclei from structural MRI. Neuroimage 194, 272–282 (2019).
Saranathan, M., Iglehart, C., Monti, M., Tourdias, T. & Rutt, B. In vivo high-resolution structural MRI-based atlas of human thalamic nuclei. Sci. Data 8, 275 (2021).
Saranathan, M., Tourdias, T., Bayram, E., Ghanouni, P. & Rutt, B. K. Optimization of white-matter-nulled magnetization prepared rapid gradient echo (MP-RAGE) imaging. Magn. Reson. Med. 73, 1786–1794 (2015).
Sudhyadhom, A., Haq, I. U., Foote, K. D., Okun, M. S. & Bova, F. J. A high resolution and high contrast MRI for differentiation of subcortical structures for DBS targeting: the Fast Gray Matter Acquisition T1 Inversion Recovery (FGATIR). Neuroimage 47, T44–T52 (2009).
Brun, G. et al. Automatic segmentation of deep grey nuclei using a high-resolution 7 T magnetic resonance imaging atlas-Quantification of T1 values in healthy volunteers. Eur. J. Neurosci. 55, 438–460 (2022).
Datta, R. et al. Fast automatic segmentation of thalamic nuclei from MP2RAGE acquisition at 7 Tesla. Magn. Reson. Med. 85, 2781–2790 (2021).
Weiskopf, N. et al. Quantitative multi-parameter mapping of R1, PD*, MT, and R2* at 3 T: a multi-center validation. Front. Neurosci. 7, 95 (2013).
Caan, M. W. A. et al. MP2RAGEME: T1, T2*, and QSM mapping in one sequence at 7 tesla. Hum. Brain Mapp. 40, 1786–1798 (2019).
Alkemade, A. et al. 7 Tesla MRI followed by histological 3D reconstructions in whole-brain specimens. Front. Neuroanat. 14, 536838 (2020).
Alkemade, A. et al. A unified 3D map of microscopic architecture and MRI of the human brain. Sci. Adv. 8, eabj7892 (2022).
Duan, Y., Li, X. & Xi, Y. Thalamus segmentation from diffusion tensor magnetic resonance imaging. Int. J. Biomed. Imaging 2007, 90216 (2007).
Jonasson, L. et al. A level set method for segmentation of the thalamus and its nuclei in DT-MRI. Signal. Process. 87, 309–321 (2007).
Rittner, L., Lotufo, R. A., Campbell, J. & Pike, G. B. In 2010 IEEE International Symposium on Biomedical Imaging: From Nano to Macro 1173–1176 (2010).
Wiegell, M. R., Tuch, D. S., Larsson, H. B. W. & Wedeen, V. J. Automatic segmentation of thalamic nuclei from diffusion tensor magnetic resonance imaging. Neuroimage 19, 391–401 (2003).
Kumar, V., Mang, S. & Grodd, W. Direct diffusion-based parcellation of the human thalamus. Brain Struct. Funct. 220, 1619–1635 (2015).
Mang, S. C., Busza, A., Reiterer, S., Grodd, W. & Klose, A. U. Thalamus segmentation based on the local diffusion direction: a group study. Magn. Reson. Med. 67, 118–126 (2012).
Ziyan, U., Tuch, D. & Westin, C.-F. Segmentation of thalamic nuclei from DTI using spectral clustering. Med. Image Comput. Comput. Interv. 9, 807–814 (2006).
Ziyan, U. & Westin, C.-F. Joint segmentation of thalamic nuclei from a population of diffusion tensor MR images. Med. Image Comput. Comput. Interv. 11, 279–286 (2008).
Najdenovska, E. et al. In-vivo probabilistic atlas of human thalamic nuclei based on diffusion-weighted magnetic resonance imaging. Sci. Data 5, 180270 (2018).
Battistella, G. et al. Robust thalamic nuclei segmentation method based on local diffusion magnetic resonance properties. Brain Struct. Funct. 222, 2203–2216 (2017).
Behrens, T. E. J. et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 6, 750–757 (2003).
O’Muircheartaigh, J. et al. Clustering probabilistic tractograms using independent component analysis applied to the thalamus. Neuroimage 54, 2020–2032 (2011).
Calamante, F. et al. Super-resolution track-density imaging of thalamic substructures: comparison with high-resolution anatomical magnetic resonance imaging at 7.0T. Hum. Brain Mapp. 34, 2538–2548 (2013).
Basile, G. A. et al. In vivo super-resolution track-density imaging for thalamic nuclei identification. Cereb. Cortex 31, 5613–5636 (2021).
Stough, J. V. et al. Automatic method for thalamus parcellation using multi-modal feature classification. Med. Image Comput. Comput. Interv. 17, 169–176 (2014).
Semedo, C. et al. In Medical Image Computing and Computer Assisted Intervention – MICCAI 2018 (eds Frangi, A. F., Schnabel, J. A., Davatzikos, C., Alberola-López, C. & Fichtinger, G.) 383–391 (Springer International Publishing, 2018).
Huang, S. Y. et al. Connectome 2.0: developing the next-generation ultra-high gradient strength human MRI scanner for bridging studies of the micro-, meso- and macro-connectome. Neuroimage 243, 118530 (2021).
Behrens, T. E. J., Berg, H. J., Jbabdi, S., Rushworth, M. F. S. & Woolrich, M. W. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 34, 144–155 (2007).
Johansen-Berg, H. et al. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb. Cortex 15, 31–39 (2005).
Basile, G. A. et al. In vivo probabilistic atlas of white matter tracts of the human subthalamic area combining track density imaging and optimized diffusion tractography. Brain Struct. Funct. 227, 2647–2665 (2022).
Maier-Hein, K. H. et al. The challenge of mapping the human connectome based on diffusion tractography. Nat. Commun. 8, 1349 (2017).
Shine, J. M., Lewis, L. D., Garrett, D. D. & Hwang, K. The impact of the human thalamus on brain-wide information processing. Nat. Rev. Neurosci. 24, 416–430 (2023). Highlights the role of the thalamus in a large number of human brain functional signatures.
Mezer, A., Yovel, Y., Pasternak, O., Gorfine, T. & Assaf, Y. Cluster analysis of resting-state fMRI time series. Neuroimage 45, 1117–1125 (2009).
O’Muircheartaigh, J., Keller, S. S., Barker, G. J. & Richardson, M. P. White matter connectivity of the thalamus delineates the functional architecture of competing thalamocortical systems. Cereb. Cortex 25, 4477–4489 (2015).
Toulmin, H. et al. Specialization and integration of functional thalamocortical connectivity in the human infant. Proc. Natl Acad. Sci. USA 112, 6485–6490 (2015).
Yuan, R. et al. Functional topography of the thalamocortical system in human. Brain Struct. Funct. 221, 1971–1984 (2016).
Zhang, D. et al. Intrinsic functional relations between human cerebral cortex and thalamus. J. Neurophysiol. 100, 1740–1748 (2008).
Zhang, D., Snyder, A. Z., Shimony, J. S., Fox, M. D. & Raichle, M. E. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb. Cortex 20, 1187–1194 (2010).
Ji, B. et al. Dynamic thalamus parcellation from resting‐state fMRI data. Hum. Brain Mapp. 37, 954–967 (2016).
Kumar, V. J., van Oort, E., Scheffler, K., Beckmann, C. F. & Grodd, W. Functional anatomy of the human thalamus at rest. Neuroimage 147, 678–691 (2017). Compares and contrasts parcellations of the thalamus obtained from structural versus functional connectivity data and shows that there is no one-to-one mapping between them.
Kim, D., Park, B. & Park, H. Functional connectivity‐based identification of subdivisions of the basal ganglia and thalamus using multilevel independent component analysis of resting state fMRI. Hum. Brain Mapp. 34, 1371–1385 (2013).
Hale, J. R. et al. Comparison of functional thalamic segmentation from seed-based analysis and ICA. Neuroimage 114, 448–465 (2015).
Zhang, S. & Li, C.-S. R. Functional connectivity parcellation of the human thalamus by independent component analysis. Brain Connect. 7, 602–616 (2017).
Setzer, B. et al. A temporal sequence of thalamic activity unfolds at transitions in behavioral arousal state. Nat. Commun. 13, 5442 (2022).
Tregidgo, H. F. J. et al. Accurate Bayesian segmentation of thalamic nuclei using diffusion MRI and an improved histological atlas. Neuroimage 274, 120129 (2023). An optimized segmentation procedure using T1w MRI and DWI data that is available within the new FreeSurfer pipeline.
Yan, C. et al. Segmenting thalamic nuclei from manifold projections of multi-contrast MRI. Proceedings of SPIE https://doi.org/10.48550/arXiv.2301.06114 (2023).
Majdi, M. S. et al. Automated thalamic nuclei segmentation using multi-planar cascaded convolutional neural networks. Magn. Reson. Imaging 73, 45–54 (2020).
Umapathy, L., Keerthivasan, M. B., Zahr, N. M., Bilgin, A. & Saranathan, M. Convolutional neural network based frameworks for fast automatic segmentation of thalamic nuclei from native and synthesized contrast structural MRI. Neuroinformatics 20, 651–664 (2022). Describes the use of deep-learning procedures to enhance thalamic nuclei segmentation procedures, arguably the method of the future.
Shao, M. et al. Evaluating the impact of MR image harmonization on thalamus deep network segmentation. Proc. SPIE Int. Soc. Opt. Eng. 12032, 120320H (2022).
Setsompop, K., Feinberg, D. A. & Polimeni, J. R. Rapid brain MRI acquisition techniques at ultra-high fields. NMR Biomed. 29, 1198–1221 (2016).
Williams, B., Nguyen, D., Vidal, J. P. & Saranathan, M. Thalamic nuclei segmentation from T1-weighted MRI: unifying and benchmarking state-of-the-art methods. Imaging Neurosci. 2, 1–16 (2024).
Jaimes, C. et al. Probabilistic tractography-based thalamic parcellation in healthy newborns and newborns with congenital heart disease. J. Magn. Reson. Imaging 47, 1626–1637 (2018).
Jakab, A. et al. Mental development is associated with cortical connectivity of the ventral and nonspecific thalamus of preterm newborns. Brain Behav. 10, e01786 (2020).
Lidauer, K. et al. Subcortical and hippocampal brain segmentation in 5-year-old children: validation of FSL-FIRST and FreeSurfer against manual segmentation. Eur. J. Neurosci. 56, 4619–4641 (2022).
Hashempour, N. et al. A novel approach for manual segmentation of the amygdala and hippocampus in neonate MRI. Front. Neurosci. 13, 1025 (2019).
Tutunji, R. et al. Thalamic volume and dimensions on MRI in the pediatric population: normative values and correlations: (a cross sectional study). Eur. J. Radiol. 109, 27–32 (2018).
Turesky, T. K., Vanderauwera, J. & Gaab, N. Imaging the rapidly developing brain: current challenges for MRI studies in the first five years of life. Dev. Cogn. Neurosci. 47, 100893 (2021).
Gousias, I. S. et al. Magnetic resonance imaging of the newborn brain: manual segmentation of labelled atlases in term-born and preterm infants. Neuroimage 62, 1499–1509 (2012).
Morey, R. A. et al. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage 45, 855–866 (2009).
Pomponio, R. et al. Harmonization of large MRI datasets for the analysis of brain imaging patterns throughout the lifespan. Neuroimage 208, 116450 (2020).
Choi, E. Y. et al. Thalamic nuclei atrophy at high and heterogenous rates during cognitively unimpaired human aging. Neuroimage 262, 119584 (2022).
Pfefferbaum, A., Sullivan, E. V., Zahr, N. M., Pohl, K. M. & Saranathan, M. Multi-atlas thalamic nuclei segmentation on standard T1-weighed MRI with application to normal aging. Hum. Brain Mapp. 44, 612–628 (2023).
Schmahmann, J. D. Vascular syndromes of the thalamus. Stroke 34, 2264–2278 (2003).
Carlesimo, G. A., Lombardi, M. G. & Caltagirone, C. Vascular thalamic amnesia: a reappraisal. Neuropsychologia 49, 777–789 (2011).
Golden, E. C., Graff-Radford, J., Jones, D. T. & Benarroch, E. E. Mediodorsal nucleus and its multiple cognitive functions. Neurology 87, 2161–2168 (2016).
Pergola, G. et al. Quantitative assessment of chronic thalamic stroke. AJNR Am. J. Neuroradiol. 34, E51–E55 (2013).
Percheron, G. The anatomy of the arterial supply of the human thalamus and its use for the interpretation of the thalamic vascular pathology. Z. Neurol. 205, 1–13 (1973).
Danet, L. et al. Thalamic amnesia after infarct: the role of the mammillothalamic tract and mediodorsal nucleus. Neurology 85, 2107–2115 (2015).
Hwang, K., Shine, J. M., Bruss, J., Tranel, D. & Boes, A. Neuropsychological evidence of multi-domain network hubs in the human thalamus. eLife 10, e69480 (2021).
Harding, A., Halliday, G., Caine, D. & Kril, J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain 123, 141–154 (2000).
Segobin, S. et al. Dissociating thalamic alterations in alcohol use disorder defines specificity of Korsakoff’s syndrome. Brain 142, 1458–1470 (2019).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991).
Bernstein, A. S., Rapcsak, S. Z., Hornberger, M. & Saranathan, M.; Alzheimer’s Disease Neuroimaging Initiative.Structural changes in thalamic nuclei across prodromal and clinical Alzheimer’s disease. J. Alzheimers Dis. 82, 361–371 (2021).
Forno, G. et al. Thalamic nuclei changes in early and late onset Alzheimer’s disease. Curr. Res. Neurobiol. 4, 100084 (2023).
Azevedo, C. J. et al. Thalamic atrophy in multiple sclerosis: a magnetic resonance imaging marker of neurodegeneration throughout disease. Ann. Neurol. 83, 223–234 (2018).
Planche, V. et al. White-matter-nulled MPRAGE at 7T reveals thalamic lesions and atrophy of specific thalamic nuclei in multiple sclerosis. Mult. Scler. 26, 987–992 (2020).
Alemán-Gómez, Y. et al. Multimodal magnetic resonance imaging depicts widespread and subregion specific anomalies in the thalamus of early-psychosis and chronic schizophrenia patients. Schizophr. Bull. 49, 196–207 (2023).
Henry, R. G. et al. Connecting white matter injury and thalamic atrophy in clinically isolated syndromes. J. Neurol. Sci. 282, 61–66 (2009).
Ontaneda, D. et al. Deep grey matter injury in multiple sclerosis: a NAIMS consensus statement. Brain 144, 1974–1984 (2021).
Kuchcinski, G. et al. Thalamic alterations remote to infarct appear as focal iron accumulation and impact clinical outcome. Brain 140, 1932–1946 (2017).
Linck, P. A. et al. Neurodegeneration of the substantia nigra after ipsilateral infarct: MRI R2* mapping and relationship to clinical outcome. Radiology 291, 438–448 (2019).
Tamura, A. et al. Thalamic atrophy following cerebral infarction in the territory of the middle cerebral artery. Stroke 22, 615–618 (1991).
Moon, Y., Han, S.-H. & Moon, W.-J. Patterns of brain iron accumulation in vascular dementia and Alzheimer’s dementia using quantitative susceptibility mapping imaging. J. Alzheimers Dis. 51, 737–745 (2016).
Blyau, S. et al. Differential vulnerability of thalamic nuclei in multiple sclerosis. Mult. Scler. J. 29, 295–300 (2023).
Magliozzi, R. et al. “Ependymal‐in” gradient of thalamic damage in progressive multiple sclerosis. Ann. Neurol. 92, 670–685 (2022).
Lee, J.-S., Heo, D.-Y., Choi, K.-H. & Kim, H.-J. Impact of the ventricle size on alzheimer’s disease progression: a retrospective longitudinal study. Dement. Neurocogn. Disord. 23, 95–106 (2024).
Oliveira, L. M., Nitrini, R. & Román, G. C. Normal-pressure hydrocephalus: a critical review. Dement. Neuropsychol. 13, 133–143 (2019).
Johnstone, E., Frith, C. D., Crow, T. J., Husband, J. & Kreel, L. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet 308, 924–926 (1976).
Van Erp, T. G. M. et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol. Psychiatry 21, 547–553 (2016).
Pergola, G., Selvaggi, P., Trizio, S., Bertolino, A. & Blasi, G. The role of the thalamus in schizophrenia from a neuroimaging perspective. Neurosci. Biobehav. Rev. 54, 57–75 (2015).
Pergola, G. et al. Grey matter volume patterns in thalamic nuclei are associated with familial risk for schizophrenia. Schizophr. Res. 180, 13–20 (2017).
Honea, R. A. et al. Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol. Psychiatry 63, 465–474 (2008).
Cooper, D., Barker, V., Radua, J., Fusar-Poli, P. & Lawrie, S. M. Multimodal voxel-based meta-analysis of structural and functional magnetic resonance imaging studies in those at elevated genetic risk of developing schizophrenia. Psychiatry Res. Neuroimaging 221, 69–77 (2014).
Akudjedu, T. N. et al. Progression of neuroanatomical abnormalities after first-episode of psychosis: a 3-year longitudinal sMRI study. J. Psychiatr. Res. 130, 137–151 (2020).
Cobia, D. J., Smith, M. J., Wang, L. & Csernansky, J. G. Longitudinal progression of frontal and temporal lobe changes in schizophrenia. Schizophr. Res. 139, 1–6 (2012).
Gaser, C., Nenadic, I., Buchsbaum, B. R., Hazlett, E. A. & Buchsbaum, M. S. Ventricular enlargement in schizophrenia related to volume reduction of the thalamus, striatum, and superior temporal cortex. Am. J. Psychiatry 161, 154–156 (2004).
Guo, J. Y. et al. Longitudinal regional brain volume loss in schizophrenia: relationship to antipsychotic medication and change in social function. Schizophr. Res. 168, 297–304 (2015).
Borghei, A., Piracha, A. & Sani, S. Prevalence and anatomical characteristics of the human massa intermedia. Brain Struct. Funct. 226, 471–480 (2021).
Trzesniak, C. et al. Adhesio interthalamica alterations in schizophrenia spectrum disorders: a systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 877–886 (2011).
Cassel, J.-C. et al. The reuniens and rhomboid nuclei of the thalamus: a crossroads for cognition-relevant information processing? Neurosci. Biobehav. Rev. 126, 338–360 (2021).
Schiff, N. D. et al. Thalamic deep brain stimulation in traumatic brain injury: a phase 1, randomized feasibility study. Nat. Med. 29, 3162–3174 (2023).
Wong, J. K. et al. Deep brain stimulation in essential tremor: targets, technology, and a comprehensive review of clinical outcomes. Expert. Rev. Neurother. 20, 319–331 (2020).
Middlebrooks, E. H., He, X., Grewal, S. S. & Keller, S. S. Neuroimaging and thalamic connectomics in epilepsy neuromodulation. Epilepsy Res. 182, 106916 (2022).
Aggleton, J. P., Pralus, A., Nelson, A. J. D. & Hornberger, M. Thalamic pathology and memory loss in early Alzheimer’s disease: moving the focus from the medial temporal lobe to Papez circuit. Brain 139, 1877–1890 (2016).
Craig, A. D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666 (2002).
Steullet, P. et al. The thalamic reticular nucleus in schizophrenia and bipolar disorder: role of parvalbumin-expressing neuron networks and oxidative stress. Mol. Psychiatry 23, 2057–2065 (2018).
El Khoueiry, C. et al. Developmental oxidative stress leads to T-type Ca2+ channel hypofunction in thalamic reticular nucleus of mouse models pertinent to schizophrenia. Mol. Psychiatry 27, 2042–2051 (2022).
Viviano, J. D. & Schneider, K. A. Interhemispheric interactions of the human thalamic reticular nucleus. J. Neurosci. 35, 2026–2032 (2015).
Schira, M. M. et al. HumanBrainAtlas: an in vivo MRI dataset for detailed segmentations. Brain Struct. Funct. 228, 1849–1863 (2023).
Boccardi, M. et al. Delphi definition of the EADC-ADNI harmonized protocol for hippocampal segmentation on magnetic resonance. Alzheimers Dement. 11, 126–138 (2015).
Baumeister, H. et al. Comparison of histological delineation of the entorhinal, perirhinal, ectorhinal, and parahippocampal cortices by different neuroanatomy laboratories. Alzheimers Dement 19, e076135 (2023).
Carter, P. et al. A demonstration of using formal consensus methods within guideline development; a case study. BMC Med. Res. Methodol. 21, 73 (2021).
Nasa, P., Jain, R. & Juneja, D. Delphi methodology in healthcare research: how to decide its appropriateness. World J. Methodol. 11, 116–129 (2021).
Vidal, J. P. et al. Robust thalamic nuclei segmentation from T1-weighted MRI using polynomial intensity transformation. Brain Struct. Funct. 229, 1087–1101 (2024).
Oxenford, S. et al. Lead-OR: a multimodal platform for deep brain stimulation surgery. eLife 11, e72929 (2022).
Fan, L. et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb. Cortex 26, 3508–3526 (2016).
van Oort, E. S. B. et al. Functional parcellation using time courses of instantaneous connectivity. Neuroimage 170, 31–40 (2018).
Danet, L. et al. Medial thalamic stroke and its impact on familiarity and recollection. eLife 6, e28141 (2017).
Pitel, A.-L. et al. Macrostructural abnormalities in Korsakoff syndrome compared with uncomplicated alcoholism. Neurology 78, 1330–1333 (2012).
Alegro, M. et al. In: IEEE Computer Society Conference on Computer Vision and Pattern Recognition Workshops 634–642 (IEEE Computer Society, 2016).
Alho, A. T. D. L. et al. Magnetic resonance diffusion tensor imaging for the pedunculopontine nucleus: proof of concept and histological correlation. Brain Struct. Funct. 222, 2547–2558 (2017).
Alho, E. J. L. et al. High thickness histological sections as alternative to study the three-dimensional microscopic human sub-cortical neuroanatomy. Brain Struct. Funct. 223, 1121–1132 (2018).
Mollink, J. et al. Evaluating fibre orientation dispersion in white matter: comparison of diffusion MRI, histology and polarized light imaging. Neuroimage 157, 561–574 (2017).
Sitek, K. R. et al. Mapping the human subcortical auditory system using histology, postmortem MRI and in vivo MRI at 7T. eLife 8, e48932 (2019).
Jorge, J. et al. Improved susceptibility-weighted imaging for high contrast and resolution thalamic nuclei mapping at 7T. Magn. Reson. Med. 84, 1218–1234 (2020).
Abosch, A., Yacoub, E., Ugurbil, K. & Harel, N. An assessment of current brain targets for deep brain stimulation surgery with susceptibility-weighted imaging at 7 tesla. Neurosurgery 67, 1745–1756 (2010).
Deshmane, A., Gulani, V., Griswold, M. A. & Seiberlich, N. Parallel MR imaging. J. Magn. Reson. Imaging 36, 55–72 (2012).
Marques, J. P. et al. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 49, 1271–1281 (2010).
Acknowledgements
The Thalamus Nuclei Neuroimaging Group (TANGO) consortium can be followed and reached via our website. S.S. was supported by the French National Institute for Health and Medical Research (INSERM), Label Excellence de la Région Normandie, the French National Agency for Research (ANR), the Fondation pour la Recherche Médicale (FRM; ING20140129160). R.A.M.H. was supported by H2020 Marie Skłodowska Curie Actions, Grant/Award Number: 101061988. V.J.K. was supported by the Deutsche Forschungsgemeinschaft, DFG SCHE 658/17. G.P. and A.L. were supported by RIPARTI – “assegni di RIcerca per riPARTire con le Imprese” initiative, APULIA REGION (POC PUGLIA FESR-FSE 2014 / 2020), Project Code 79ed97ad. G.P. was supported by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), “MNESYS, A multiscale integrated approach to the study of the nervous system in health and disease”) (PE0000006) – (DN. 1553 11.10.2022). T.T. was supported by University of Bordeaux’s IdEx “Investments for the Future” RRI programme “IMPACT” (IMaging for Precision medicine within A Collaborative Translational programme), and IHU Precision & Global Vascular Brain Health Institute, ANR-23-IAHU-000, which received financial support from the France 2030 programme. M.B.C. is funded by CIBM Center for Biomedical Imaging, a Swiss research centre of excellence founded and supported by CHUV, UNIL, EPFL, UNIGE and HUG, and also by Swiss National Science Foundation grants 205321-157040. A.-L.P. was supported by the INSERM, Label Excellence de la Région Normandie, the ANR, the FRM, the French Universitary Institute. A.A. was supported by ZonMW Open competition Grant 09120012110015 JPND/ZonMW (grant 73305113). M.S. was supported by National Institutes of Health NIBIB R01 EB032674. M.H. was supported by the National Institute for Health Research and the Medical Research Council.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed substantially to discussion of the content, and reviewed and/or edited the manuscript before submission. M.H. and S.S. wrote the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks Rosanna Olsen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
FMRIB Software Library: https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases
FreeSurfer software suite: https://surfer.nmr.mgh.harvard.edu/
Harmonized Hippocampal Protocol: http://www.hippocampal-protocol.net/SOPs/index.php
Hippocampal Subfields Group: https://hippocampalsubfields.com/
Human Brain Atlas: https://hba.neura.edu.au/methods/
Human Brainnetome Atlas: https://atlas.brainnetome.org/
Lead-DBS: https://www.lead-dbs.org/
TANGO: https://thalamicsegmentation.github.io/
THOMAS: https://github.com/thalamicseg
Glossary
- Bayesian inference
-
An analysis technique that involves the use of probabilities to infer a hypothesized outcome.
- Blood oxygen level-dependent (BOLD) signal
-
BOLD variations occur due to changes in deoxyhaemoglobin (which is paramagnetic) that are in turn caused by local changes in blood flow due to neuronal activity.
- Diffusion MRI
-
Refers to imaging the microscopic motion of water molecules. When magnetic field gradients are applied on either side of the refocusing pulse in a spin-echo experiment, motion will result in reduction of the refocused MRI signal, which can be quantified and related to the diffusion coefficient.
- Diffusion tensor imaging
-
(DTI). In diffusion MRI, by applying gradients in specific directions, information on preferential direction of motion can be inferred. One such method involves representing it as a tensor with the ellipsoid representing the three principal directions.
- Echo-planar imaging
-
An ultra-fast variant of gradient or spin-echo MRI, in which multiple (or even all) k-space lines are acquired within a single repetition time. Echo-planar imaging is used when very fast acquisitions are required. Its high temporal resolution allows imaging of rapid physiological processes with decreased motion artefacts.
- Fractional anisotropy
-
A scalar measurement from DTI that reflects the microstructural integrity of white matter tracts.
- Functional MRI
-
(fMRI). A means for depicting brain activity by measuring regional BOLD variations in the brain.
- Image registration
-
The mathematical operation that warps one image, called the source, to a target image. Registration can be rigid body, when the source and target are from the same brain, or non-linear, when matching a source to a template.
- Mesh-based representation
-
A method that generally involves partitioning an image into tiny polygons in a process called tessellation. This term is used in the field of computer vision to describe the representation of 3D objects.
- Nulling
-
The process of eliminating the magnetic signal coming from a particular tissue.
- Partial volume effects
-
Effects that occur when a voxel contains signals from two or more tissues. The resultant signal is therefore an average of the signal arising from these tissues. Qualitatively, the image appears blurred and is quantitatively biased. Partial volume effects are particularly prominent in small regions or along borders of regions.
- Proton density
-
The concentration of protons in each voxel, indicated by the voxel intensity.
- Quantitative susceptibility mapping
-
Uses both magnitude and phase as a combined process to highlight the presence of compounds that could be diamagnetic (for example, calcification), paramagnetic (for example, deoxyhaemoglobin, due to fewer red blood cells causing anaemia) or ferromagnetic (for example, high iron content, making it behave as a magnet on its own).
- Relaxation
-
The spin from hydrogen atoms aligns with the scanner’s main magnetic field (B0) in the longitudinal plane. When a radiofrequency pulse is applied, the spins absorb energy and their magnetization flips from the longitudinal into the transverse plane. When removed, the spins undergo relaxation to align again with the main B0 magnetization.
- Relaxation times
-
The time taken by the spins from the hydrogen atoms to lose their energy. Transverse and longitudinal relaxation times are labelled T1 and T2, respectively.
- Resting-state fMRI
-
(rs-fMRI). Refers to measuring the fluctuations occurring in the brain when not subject to a specific task. Studies can be hypothesis driven, directly looking at the synchronicity between regions in either static (time invariant) or dynamic (observing switching or transitions) models, at a whole-brain level, through independent component analysis or graph theory methods.
- Susceptibility
-
Each hydrogen atom has a local magnetic field associated to it. The human body also contains other compounds (for example, calcium) that have magnetic properties, hence distorting the local magnetic field. This leads to a change in the phase of local tissue and hence to a change in the signal measured.
- Susceptibility-weighted imaging
-
Shows the presence (through magnitude only) of tissue susceptibility by weighting the resulting MRI images.
- T1-weighted (T1w) imaging
-
Imaging in which the contrast between tissues is due to the differences in their T1 relaxation times. The longer the T1 relaxation time, the darker the signal (for example, cerebrospinal fluid has much longer T1 relaxation times than white or grey matter and is dark in T1w-MRI).
- T2-weighted imaging
-
Imaging in which the contrast between tissues is due to the differences in their T2 relaxation times.
- T2*-weighted imaging
-
Imaging in which the contrast between tissues is due to the differences in their T2* relaxation times. This method takes into account magnetic field inhomogeneities in addition to T2 relaxation and is therefore faster than T2.
- Tractography
-
Modelling of the pathway of white matter tracts using scalar and vector measurements from diffusion tensor imaging.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Segobin, S., Haast, R.A.M., Kumar, V.J. et al. A roadmap towards standardized neuroimaging approaches for human thalamic nuclei. Nat. Rev. Neurosci. 25, 792–808 (2024). https://doi.org/10.1038/s41583-024-00867-1
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41583-024-00867-1