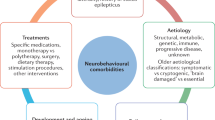
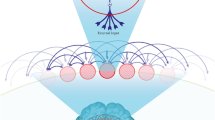
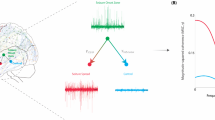
Abstract
Epilepsy is diagnosed when neural networks become capable of generating excessive or hypersynchronous activity patterns that result in observable seizures. In many cases, epilepsy is associated with cognitive comorbidities that persist between seizures and negatively impact quality of life. Dysregulation of the coordinated physiological network interactions that are required for cognitive function has been implicated in mediating these enduring symptoms, but the causal mechanisms are often elusive. Here, we provide an overview of neural network abnormalities with the potential to contribute to cognitive dysfunction in epilepsy. We examine these pathological interactions across spatial and temporal scales, additionally highlighting the dynamics that arise in response to the brain’s intrinsic capacity for plasticity. Understanding these processes will facilitate development of network-level interventions to address cognitive comorbidities that remain undertreated by currently available epilepsy therapeutics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Silberberg, G., Grillner, S., LeBeau, F. E. N., Maex, R. & Markram, H. Synaptic pathways in neural microcircuits. Trends Neurosci. 28, 541–551 (2005).
Iturria-Medina, Y. et al. Characterizing brain anatomical connections using diffusion weighted MRI and graph theory. NeuroImage 36, 645–660 (2007).
Song, S., Miller, K. D. & Abbott, L. F. Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat. Neurosci. 3, 919–926 (2000).
Salvador, R. et al. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb. Cortex 15, 1332–2342 (2005).
Bassett, D. S., Meyer-Lindenberg, A., Achard, S., Duke, T. & Bullmore, E. Adaptive reconfiguration of fractal small-world human brain functional networks. Proc. Natl Acad. Sci. USA 103, 19518–19523 (2006).
Yu, S., Huang, D., Singer, W. & Nikolić, D. A small world of neuronal synchrony. Cereb. Cortex 18, 2891–2901 (2008).
Fries, P. Rhythms for cognition: communication through coherence. Neuron 88, 220–235 (2015). This paper outlines the theory and evidence supporting oscillatory synchronization as a mechanism for effective neuronal communication.
Farrell, J. S., Nguyen, Q. A. & Soltesz, I. Resolving the micro–macro disconnect to address core features of seizure networks. Neuron 101, 1016–1028 (2019).
Spencer, S. S. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia 43, 219–227 (2002).
Engel, J. et al. Connectomics and epilepsy. Curr. Opin. Neurol. 26, 186–194 (2013).
Gil, F. et al. Beyond the epileptic focus: functional epileptic networks in focal epilepsy. Cereb. Cortex 30, 2338–2357 (2020).
Lehnertz, K., Bröhl, T. & von Wrede, R. Epileptic-network-based prediction and control of seizures in humans. Neurobiol. Dis. 181, 106098 (2023).
Jaggard, J. B., Wang, G. X. & Mourrain, P. Non-REM and REM/paradoxical sleep dynamics across phylogeny. Curr. Opin. Neurobiol. 71, 44–51 (2021).
Thompson, E. & Varela, F. J. Radical embodiment: neural dynamics and consciousness. Trends Cogn. Sci. 5, 418–425 (2001).
Von Stein, A., Chiang, C. & König, P. Top-down processing mediated by interareal synchronization. Proc. Natl Acad. Sci. USA 97, 14748–14753 (2000).
Gyorgy, B. & Andreas, D. Neuronal oscillations in cortical networks. Science 304, 1926–1929 (2004).
Fernández-Ruiz, A. & Herreras, O. Identifying the synaptic origin of ongoing neuronal oscillations through spatial discrimination of electric fields. Front. Comput. Neurosci. 7, 41565 (2013).
Buzsáki, G. Rhythms of the Brain https://doi.org/10.1093/ACPROF:OSO/9780195301069.001.0001 (Oxford Univ. Press, 2006).
Buzsáki, G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus 15, 827–840 (2005).
Colgin, L. L. et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 462, 353–357 (2009).
Jirsa, V. & Müller, V. Cross-frequency coupling in real and virtual brain networks. Front. Comput. Neurosci. https://doi.org/10.3389/fncom.2013.00078 (2013).
Buzsáki, G. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188 (2015). This paper describes mechanisms and function of a key population transient involved in cognition, the hippocampal sharp wave-ripple, and its epileptic counterpart, the pathological high-frequency oscillation.
Cossart, R. & Khazipov, R. How development sculpts hippocampal circuits and function. Physiol. Rev. 102, 343–378 (2022).
Seelke, A. M. H. & Blumberg, M. S. Developmental appearance and disappearance of cortical events and oscillations in infant rats. Brain Res. 1324, 34–42 (2010).
Khazipov, R. et al. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature 432, 758–761 (2004).
Domínguez, S. et al. A transient postnatal quiescent period precedes emergence of mature cortical dynamics. eLife 10, e69011 (2021).
Klausberger, T. & Somogyi, P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321, 53–57 (2008).
Kay, K. et al. Constant sub-second cycling between representations of possible futures in the hippocampus. Cell 180, 552–567.e25 (2020).
Dragoi, G., Harris, K. D. & Buzsáki, G. Place representation within hippocampal networks is modified by long-term potentiation. Neuron 39, 843–853 (2003).
Gupta, A. S., Van Der Meer, M. A. A., Touretzky, D. S. & Redish, A. D. Segmentation of spatial experience by hippocampal θ sequences. Nat. Neurosci. 15, 1032–1039 (2012).
Skaggs, W. E., McNaughton, B. L., Wilson, M. A. & Barnes, C. A. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6, 149–172 (1996).
Montgomery, S. M., Betancur, M. I. & Buzsáki, G. Behavior-dependent coordination of multiple theta dipoles in the hippocampus. J. Neurosci. 29, 1381 (2009).
Winson, J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science 201, 160–163 (1978).
Lega, B. C., Jacobs, J. & Kahana, M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus 22, 748–761 (2012).
Herweg, N. A., Solomon, E. A. & Kahana, M. J. Theta oscillations in human memory. Trends Cogn. Sci. 24, 208–227 (2020).
Dugladze, T. et al. Impaired hippocampal rhythmogenesis in a mouse model of mesial temporal lobe epilepsy. Proc. Natl Acad. Sci. USA 104, 17530–17535 (2007).
Chauvière, L. et al. Early deficits in spatial memory and theta rhythm in experimental temporal lobe epilepsy. J. Neurosci. 29, 5402–5410 (2009).
Inostroza, M., Brotons-Mas, J. R., Laurent, F., Cid, E. & de la Prida, L. M. Specific impairment of ‘what–where–when’ episodic-like memory in experimental models of temporal lobe epilepsy. J. Neurosci. 33, 17749–17762 (2013).
Lenck-Santini, P. P. & Holmes, G. L. Altered phase precession and compression of temporal sequences by place cells in epileptic rats. J. Neurosci. 28, 5053–5062 (2008). This study demonstrates derangement of oscillatory activity, spatial coding and concomitant memory impairment in an animal model of epilepsy.
Arski, O. N. et al. Epilepsy disrupts hippocampal phase precision and impairs working memory. Epilepsia 63, 2583–2596 (2022).
Shuman, T. et al. Breakdown of spatial coding and interneuron synchronization in epileptic mice. Nat. Neurosci. 23, 229–238 (2020).
Lopez-Pigozzi, D. et al. Altered oscillatory dynamics of CA1 parvalbumin basket cells during theta–gamma rhythmopathies of temporal lobe epilepsy. eNeuro 3, 1–20 (2016).
Laurent, F. et al. Proximodistal structure of theta coordination in the dorsal hippocampus of epileptic rats. J. Neurosci. 35, 4760–4775 (2015).
Garrido Sanabria, E. R. et al. Septal GABAergic neurons are selectively vulnerable to pilocarpine-induced status epilepticus and chronic spontaneous seizures. Neuroscience 142, 871–883 (2006).
Barry, J. M. et al. Temporal coordination of hippocampal neurons reflects cognitive outcome post-febrile status epilepticus. eBioMedicine 7, 175–190 (2016).
Richard, G. R. et al. Speed modulation of hippocampal theta frequency correlates with spatial memory performance. Hippocampus 23, 1269–1279 (2013).
Solomon, E. A. et al. Dynamic theta networks in the human medial temporal lobe support episodic memory. Curr. Biol. 29, 1100–1111.e4 (2019).
Chen, D. et al. Theta oscillations coordinate grid-like representations between ventromedial prefrontal and entorhinal cortex. Sci. Adv. 7, eabj0200 (2021).
Igarashi, K. M., Lu, L., Colgin, L. L., Moser, M. B. & Moser, E. I. Coordination of entorhinal–hippocampal ensemble activity during associative learning. Nature 510, 143–147 (2014).
Fujisawa, S. & Buzsáki, G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron 72, 153–165 (2011).
Benchenane, K. et al. Coherent theta oscillations and reorganization of spike timing in the hippocampal– prefrontal network upon learning. Neuron 66, 921–936 (2010).
Malkov, A., Shevkova, L., Latyshkova, A. & Kitchigina, V. Theta and gamma hippocampal–neocortical oscillations during the episodic-like memory test: impairment in epileptogenic rats. Exp. Neurol. 354, 114110 (2022).
Froriep, U. P. et al. Altered θ coupling between medial entorhinal cortex and dentate gyrus in temporal lobe epilepsy. Epilepsia 53, 1937–1947 (2012).
Lee, D. J. et al. Stimulation of the medial septum improves performance in spatial learning following pilocarpine-induced status epilepticus. Epilepsy Res. 130, 53–63 (2017).
Cantero, J. L. et al. Sleep-dependent θ oscillations in the human hippocampus and neocortex. J. Neurosci. 23, 10897–10903 (2003).
Boyce, R., Glasgow, S. D., Williams, S. & Adamantidis, A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 352, 812–816 (2016).
Popa, D., Duvarci, S., Popescu, A. T., Léna, C. & Paré, D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc. Natl Acad. Sci. USA 107, 6516–6519 (2010).
Li, W., Ma, L., Yang, G. & Gan, W. B. REM sleep selectively prunes and maintains new synapses in development and learning. Nat. Neurosci. 20, 427–437 (2017).
Ruggiero, R. N. et al. Dysfunctional hippocampal–prefrontal network underlies a multidimensional neuropsychiatric phenotype following early-life seizure. eLife 12, RP90997 (2024).
Mendes, R. A. V. et al. Hijacking of hippocampal–cortical oscillatory coupling during sleep in temporal lobe epilepsy. Epilepsy Behav. 121, 106608 (2021).
Bieri, K. W., Bobbitt, K. N. & Colgin, L. L. Slow and fast γ rhythms coordinate different spatial coding modes in hippocampal place cells. Neuron 82, 670–681 (2014).
Bragin, A. et al. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J. Neurosci. 15, 47–60 (1995).
Harris, K. D., Csicsvari, J., Hirase, H., Dragoi, G. & Buzsáki, G. Organization of cell assemblies in the hippocampus. Nature 424, 552–556 (2003).
Gray, C. M. & Singer, W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc. Natl Acad. Sci. USA 86, 1698–1702 (1989).
Buzsáki, G. & Wang, X.-J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 35, 203–225 (2012).
Lasztóczi, B. & Klausberger, T. Layer-specific GABAergic control of distinct gamma oscillations in the CA1 hippocampus. Neuron 81, 1126–1139 (2014).
Fernández-Ruiz, A. et al. Gamma rhythm communication between entorhinal cortex and dentate gyrus neuronal assemblies. Science 372, eabf3119 (2021).
Colgin, L. L. Theta–gamma coupling in the entorhinal–hippocampal system. Curr. Opin. Neurobiol. 31, 45–50 (2015).
Hermes, D., Kasteleijn-Nolst Trenité, D. G. A. & Winawer, J. Gamma oscillations and photosensitive epilepsy. Curr. Biol. 27, R336–R338 (2017).
Sanchez-Carpintero, R. et al. Abnormal brain gamma oscillations in response to auditory stimulation in Dravet syndrome. Eur. J. Paediatr. Neurol. 24, 134–141 (2020).
Lega, B., Dionisio, S., Bingaman, W., Najm, I. & Gonzalez-Martinez, J. The gamma band effect for episodic memory encoding is absent in epileptogenic hippocampi. Clin. Neurophysiol. 126, 866–872 (2015).
Den Bakker, H. et al. Abnormal coherence and sleep composition in children with Angelman syndrome: a retrospective EEG study. Mol. Autism 9, 32 (2018).
Doesburg, S. M. et al. Altered Rolandic gamma-band activation associated with motor impairment and ictal network desynchronization in childhood epilepsy. PLoS ONE 8, e54943 (2013).
Kleen, J. K., Wu, E. X., Holmes, G. L., Scott, R. C. & Lenck-Santini, P. P. Enhanced oscillatory activity in the hippocampal–prefrontal network is related to short-term memory function after early-life seizures. J. Neurosci. 31, 15397–15406 (2011).
Thut, G., Nietzel, A., Brandt, S. A. & Pascual-Leone, A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J. Neurosci. 26, 9494–9502 (2006).
Klimesch, W. α-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 16, 606–617 (2012).
Sauseng, P. et al. EEG alpha synchronization and functional coupling during top-down processing in a working memory task. Hum. Brain Mapp. 26, 148–155 (2005).
VanRullen, R., Reddy, L. & Koch, C. Attention-driven discrete sampling of motion perception. Proc. Natl Acad. Sci. USA 102, 5291–5296 (2005).
Chapeton, J. I., Haque, R., Wittig, J. H., Inati, S. K. & Zaghloul, K. A. Large-scale communication in the human brain is rhythmically modulated through alpha coherence. Curr. Biol. 29, 2801–2811.e5 (2019).
Palva, S. & Palva, J. M. New vistas for alpha-frequency band oscillations. Trends Neurosci. 30, 150–158 (2007).
Sadaghiani, S. et al. Intrinsic connectivity networks, alpha oscillations, and tonic alertness: a simultaneous electroencephalography/functional magnetic resonance imaging study. J. Neurosci. 30, 10243–10250 (2010).
Qin, Y. et al. How alpha rhythm spatiotemporally acts upon the thalamus-default mode circuit in idiopathic generalized epilepsy. IEEE Trans. Biomed. Eng. 68, 1282–1292 (2021).
Vaudano, A. E. et al. Photosensitive epilepsy is associated with reduced inhibition of alpha rhythm generating networks. Brain 140, 981–997 (2017).
Rotondi, F., Franceschetti, S., Avanzini, G. & Panzica, F. Altered EEG resting-state effective connectivity in drug-naïve childhood absence epilepsy. Clin. Neurophysiol. 127, 1130–1137 (2016).
Wessel, J. R. β-bursts reveal the trial-to-trial dynamics of movement initiation and cancellation. J. Neurosci. 40, 411–423 (2020).
Lundqvist, M. et al. Gamma and beta bursts underlie working memory. Neuron 90, 152–164 (2016).
Jayachandran, M. et al. Nucleus reuniens transiently synchronizes memory networks at beta frequencies. Nat. Commun. 14, 4326 (2023).
Cagnan, H. et al. Temporal evolution of beta bursts in the parkinsonian cortical and basal ganglia network. Proc. Natl Acad. Sci. USA 116, 16095–16104 (2019).
Song, D. Y. et al. Beta oscillations in the sensorimotor cortex correlate with disease and remission in benign epilepsy with centrotemporal spikes. Brain Behav. 9, e01237 (2019).
Varotto, G. et al. Enhanced frontocentral EEG connectivity in photosensitive generalized epilepsies: a partial directed coherence study. Epilepsia 53, 359–367 (2012).
Fernandez, L. M. J. & Lüthi, A. Sleep spindles: mechanisms and functions. Physiol. Rev. 100, 805–868 (2020).
Klinzing, J. G., Niethard, N. & Born, J. Mechanisms of systems memory consolidation during sleep. Nat. Neurosci. 22, 1598–1610 (2019).
Hennies, N., Ralph, M. A. L., Kempkes, M., Cousins, J. N. & Lewis, P. A. Sleep spindle density predicts the effect of prior knowledge on memory consolidation. J. Neurosci. 36, 3799–3810 (2016).
Boutin, A. & Doyon, J. A sleep spindle framework for motor memory consolidation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 375, 20190232 (2020).
Johnson, L. A., Euston, D. R., Tatsuno, M. & McNaughton, B. L. Stored-trace reactivation in rat prefrontal cortex is correlated with down-to-up state fluctuation density. J. Neurosci. 30, 2650–2651 (2010).
Latchoumane, C. F. V., Ngo, H. V. V., Born, J. & Shin, H. S. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron 95, 424–435.e6 (2017).
Nir, Y. et al. Regional slow waves and spindles in human sleep. Neuron 70, 153–169 (2011).
Mofrad, M. H. et al. Waveform detection by deep learning reveals multi-area spindles that are selectively modulated by memory load. eLife 11, e75769 (2022).
Muller, L. et al. Rotating waves during human sleep spindles organize global patterns of activity that repeat precisely through the night. eLife 5, e17267 (2016).
Bender, A. C. et al. Altered sleep microarchitecture and cognitive impairment in patients with temporal lobe epilepsy. Neurology 101, E2376–E2387 (2023).
Zhang, W., Xin, M., Song, G. & Liang, J. Childhood absence epilepsy patients with cognitive impairment have decreased sleep spindle density. Sleep Med. 103, 89–97 (2023).
Huang, Y. et al. Differences in the topographical distribution of sleep spindles among adult epilepsy with cognitive impairment. Epilepsia Open 8, 980–990 (2023).
Kramer, M. A. et al. Focal sleep spindle deficits reveal focal thalamocortical dysfunction and predict cognitive deficits in sleep activated developmental epilepsy. J. Neurosci. 41, 1816–1829 (2021).
Schiller, K. et al. Focal epilepsy disrupts spindle structure and function. Sci. Rep. 12, 11137 (2022).
Dahal, P. et al. Interictal epileptiform discharges shape large-scale intercortical communication. Brain 142, 3502–3513 (2019).
Kwon, H. et al. Association of sleep spindle rate with memory consolidation in children with rolandic epilepsy. Neurology 104, e210232 (2025). This study demonstrates the correlation of decreased occurrence of a key memory-related oscillation (sleep spindle) with impaired memory consolidation in a paediatric epilepsy syndrome.
Staresina, B. P., Niediek, J., Borger, V., Surges, R. & Mormann, F. How coupled slow oscillations, spindles and ripples coordinate neuronal processing and communication during human sleep. Nat. Neurosci. 26, 1429–1437 (2023).
Frauscher, B. et al. Facilitation of epileptic activity during sleep is mediated by high amplitude slow waves. Brain 138, 1629–1641 (2015).
Sheybani, L. et al. Asymmetry of sleep electrophysiological markers in patients with focal epilepsy. Brain Commun. 5, fcad161 (2023).
Panet-Raymond, D. & Gotman, J. Asymmetry in delta activity in patients with focal epilepsy. Electroencephalogr. Clin. Neurophysiol. 75, 474–481 (1990).
Boly, M. et al. Altered sleep homeostasis correlates with cognitive impairment in patients with focal epilepsy. Brain 140, 1026–1040 (2017).
Henin, S. et al. Closed-loop acoustic stimulation enhances sleep oscillations but not memory performance. eNeuro 6, ENEURO.0306-19.2019 (2019).
Marshall, L., Helgadóttir, H., Mölle, M. & Born, J. Boosting slow oscillations during sleep potentiates memory. Nature 444, 610–613 (2006).
Ngo, H. V. V., Martinetz, T., Born, J. & Mölle, M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron 78, 545–553 (2013).
Weigenand, A., Mölle, M., Werner, F., Martinetz, T. & Marshall, L. Timing matters: open-loop stimulation does not improve overnight consolidation of word pairs in humans. Eur. J. Neurosci. 44, 2357–2368 (2016).
Rector, D. M., Schei, J. L., Van Dongen, H. P. A., Belenky, G. & Krueger, J. M. Physiological markers of local sleep. Eur. J. Neurosci. 29, 1771–1778 (2009).
Sachdev, R. N. S. et al. Delta rhythm in wakefulness: evidence from intracranial recordings in human beings. J. Neurophysiol. 114, 1248–1254 (2015).
Crochet, S. & Petersen, C. C. H. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat. Neurosci. 9, 608–610 (2006).
Vyazovskiy, V. V. et al. Local sleep in awake rats. Nature 472, 443–447 (2011).
Tao, J. X. et al. Interictal regional delta slowing is an EEG marker of epileptic network in temporal lobe epilepsy. Epilepsia 52, 467–476 (2011).
Englot, D. J. et al. The sensitivity and significance of lateralized interictal slow activity on magnetoencephalography in focal epilepsy. Epilepsy Res. 121, 21–28 (2016).
Nita, D. A., Cissé, Y., Timofeev, I. & Steriade, M. Waking–sleep modulation of paroxysmal activities induced by partial cortical deafferentation. Cereb. Cortex 17, 272–283 (2007).
Avramescu, S. & Timofeev, I. Synaptic strength modulation after cortical trauma: a role in epileptogenesis. J. Neurosci. 28, 6760–6772 (2008).
Pasquetti, M. V. et al. Hippocampal CA1 and cortical interictal oscillations in the pilocarpine model of epilepsy. Brain Res. 1722, 146351 (2019).
Sheybani, L. et al. Wake slow waves in focal human epilepsy impact network activity and cognition. Nat. Commun. 14, 7397 (2023). This study characterizes pathological slow waves in epileptic networks and outlines a hypothesis that these oscillations may prevent epileptiform activity at the expense of impaired information processing.
Ranasinghe, K. G. et al. Neuronal synchrony abnormalities associated with subclinical epileptiform activity in early-onset Alzheimer’s disease. Brain 145, 744–753 (2022).
Annika Melissa, S. et al. Lateralization of delta band power in magnetoencephalography (MEG) in patients with unilateral focal epilepsy and its relation to verbal fluency. Brain Behav. 13, e3257 (2023).
Fristen, K. J., Frith, C. D., Fletcher, P., Liddle, P. F. & Frackowiak, R. S. J. Functional topography: multidimensional scaling and functional connectivity in the brain. Cereb. Cortex 6, 156–164 (1996).
Büchel, C. & Friston, K. Assessing interactions among neuronal systems using functional neuroimaging. Neural Netw. 13, 871–882 (2000).
Wendling, F., Ansari-Asl, K., Bartolomei, F. & Senhadji, L. From EEG signals to brain connectivity: a model-based evaluation of interdependence measures. J. Neurosci. Methods 183, 9–18 (2009).
Kam, J. W. Y. et al. Default network and frontoparietal control network theta connectivity supports internal attention. Nat. Hum. Behav. 3, 1263–1270 (2019).
Sarnthein, J., Petsche, H., Rappelsberger, P., Shaw, G. L. & Von Stein, A. Synchronization between prefrontal and posterior association cortex during human working memory. Proc. Natl Acad. Sci. USA 95, 7092–7096 (1998).
Zhang, X., Pan, W. J. & Keilholz, S. D. The relationship between BOLD and neural activity arises from temporally sparse events. NeuroImage 207, 116390 (2020).
Liu, X. & Duyn, J. H. Time-varying functional network information extracted from brief instances of spontaneous brain activity. Proc. Natl Acad. Sci. USA 110, 4392–4397 (2013).
Nunez-Elizalde, A. O. et al. Neural correlates of blood flow measured by ultrasound. Neuron 110, 1631–1640.e4 (2022).
Bergel, A. et al. Adaptive modulation of brain hemodynamics across stereotyped running episodes. Nat. Commun. 11, 6193 (2020).
Lee, K. et al. Disruption, emergence and lateralization of brain network hubs in mesial temporal lobe epilepsy. NeuroImage Clin. 20, 71–84 (2018).
Bettus, G. et al. Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res. 81, 58–68 (2008).
Warren, C. P. et al. Synchrony in normal and focal epileptic brain: the seizure onset zone is functionally disconnected. J. Neurophysiol. 104, 3530 (2010).
Braakman, H. M. H. et al. Frontal lobe connectivity and cognitive impairment in pediatric frontal lobe epilepsy. Epilepsia 54, 446–454 (2013).
Doucet, G., Osipowicz, K., Sharan, A., Sperling, M. R. & Tracy, J. I. Extratemporal functional connectivity impairments at rest are related to memory performance in mesial temporal epilepsy. Hum. Brain Mapp. 34, 2202–2216 (2013).
Ren, Y. et al. Theta oscillation and functional connectivity alterations related to executive control in temporal lobe epilepsy with comorbid depression. Clin. Neurophysiol. 131, 1599–1609 (2020).
McCormick, C., Quraan, M., Cohn, M., Valiante, T. A. & McAndrews, M. P. Default mode network connectivity indicates episodic memory capacity in mesial temporal lobe epilepsy. Epilepsia 54, 809–818 (2013).
Zhang, Z. et al. Altered spontaneous neuronal activity of the default-mode network in mesial temporal lobe epilepsy. Brain Res. 1323, 152–160 (2010).
Van Kerkoerle, T. et al. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc. Natl Acad. Sci. USA 111, 14332–14341 (2014).
Bastos, A. M., Lundqvist, M., Waite, A. S., Kopell, N. & Miller, E. K. Layer and rhythm specificity for predictive routing. Proc. Natl Acad. Sci. USA 117, 31459–31469 (2020).
Lundqvist, M. et al. Working memory control dynamics follow principles of spatial computing. Nat. Commun. 14, 1429 (2023).
Llinás, R. R., Ribary, U., Jeanmonod, D., Kronberg, E. & Mitra, P. P. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl Acad. Sci. USA 96, 15222 (1999).
Martire, D. J. et al. Thalamocortical dysrhythmia in intraoperative recordings of focal epilepsy. J. Neurophysiol. 121, 2020–2027 (2019).
van Ede, F., Quinn, A. J., Woolrich, M. W. & Nobre, A. C. Neural oscillations: sustained rhythms or transient burst-events? Trends Neurosci. 41, 415–417 (2018).
Buzsáki, G., Horváth, Z., Urioste, R., Hetke, J. & Wise, K. High-frequency network oscillation in the hippocampus. Science 256, 1025–1027 (1992).
Stark, E. et al. Pyramidal cell–interneuron interactions underlie hippocampal ripple oscillations. Neuron 83, 467–480 (2014).
Nádasdy, Z., Hirase, H., Czurkó, A., Csicsvari, J. & Buzsáki, G. Replay and time compression of recurring spike sequences in the hippocampus. J. Neurosci. 19, 9497–9507 (1999).
Skaggs, W. E. & McNaughton, B. L. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271, 1870–1873 (1996).
Wilson, M. A. & McNaughton, B. L. Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679 (1994).
Girardeau, G., Benchenane, K., Wiener, S. I., Buzsáki, G. & Zugaro, M. B. Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci. 12, 1222–1223 (2009).
Jadhav, S. P., Kemere, C., German, P. W. & Frank, L. M. Awake hippocampal sharp-wave ripples support spatial memory. Science 336, 1454–1458 (2012).
Buzsáki, G. Two-stage model of memory trace formation: a role for ‘noisy’ brain states. Neuroscience 31, 551–570 (1989).
Gelinas, J. N., Khodagholy, D., Thesen, T., Devinsky, O. & Buzsáki, G. Interictal epileptiform discharges induce hippocampal–cortical coupling in temporal lobe epilepsy. Nat. Med. 22, 641–648 (2016). This study demonstrates that hippocampal interictal epileptiform discharges temporally couple with cortical sleep spindles in a rodent model of epilepsy and in patients with epilepsy, hijacking physiological modes of network communication and disrupting memory consolidation.
Henin, S. et al. Spatiotemporal dynamics between interictal epileptiform discharges and ripples during associative memory processing. Brain 144, 1590–1602 (2021).
Cheah, C. S., Lundstrom, B. N., Catteral, W. A. & Oakley, J. C. Impairment of sharp-wave ripples in a murine model of Dravet syndrome. J. Neurosci. 39, 9251–9260 (2019).
Karlócai, M. R. et al. Physiological sharp wave-ripples and interictal events in vitro: what’s the difference? Brain 137, 463–485 (2014).
Foffani, G., Uzcategui, Y. G., Gal, B. & Menendez de la Prida, L. Reduced spike-timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron 55, 930–941 (2007).
Valero, M. et al. Mechanisms for selective single-cell reactivation during offline sharp-wave ripples and their distortion by fast ripples. Neuron 94, 1234–1247.e7 (2017).
Ewell, L. A., Fischer, K. B., Leibold, C., Leutgeb, S. & Leutgeb, J. K. The impact of pathological high-frequency oscillations on hippocampal network activity in rats with chronic epilepsy. eLife 8, e42148 (2019).
Fink, C. G., Gliske, S., Catoni, N. & Stacey, W. C. Network mechanisms generating abnormal and normal hippocampal high-frequency oscillations: a computational analysis. eNeuro https://doi.org/10.1523/ENEURO.0024-15.2015 (2015).
Buzsáki, G., Ponomareff, G. L., Bayardo, F., Ruiz, R. & Gage, F. H. Neuronal activity in the subcortically denervated hippocampus: a chronic model for epilepsy. Neuroscience 28, 527–538 (1989).
Buzsáki, G., Bayardo, F., Miles, R., Wong, R. K. S. & Gage, F. H. The grafted hippocampus: an epileptic focus. Exp. Neurol. 105, 10–22 (1989).
Suzuki, S. S. & Smith, G. K. Spontaneous EEG spikes in the normal hippocampus. V. Effects of ether, urethane, pentobarbital, atropine, diazepam and bicuculline. Electroencephalogr. Clin. Neurophysiol. 70, 84–95 (1988).
Liotta, A. et al. Partial disinhibition is required for transition of stimulus-induced sharp wave-ripple complexes into recurrent epileptiform discharges in rat hippocampal slices. J. Neurophysiol. 105, 172–187 (2011).
Norman, Y. et al. Hippocampal sharp-wave ripples linked to visual episodic recollection in humans. Science 365, eaax1030 (2019).
Vaz, A. P., Inati, S. K., Brunel, N. & Zaghloul, K. A. Coupled ripple oscillations between the medial temporal lobe and neocortex retrieve human memory. Science 363, 975–978 (2019).
Khodagholy, D., Gelinas, J. N. & Buzsáki, G. Learning-enhanced coupling between ripple oscillations in association cortices and hippocampus. Science 358, 369–372 (2017).
Hu, H., Hostetler, R. E. & Agmon, A. Ultrafast (400 Hz) network oscillations induced in mouse barrel cortex by optogenetic activation of thalamocortical axons. eLife 12, e82412 (2023).
Jones, M. S., MacDonald, K. D., Choi, B., Dudek, F. E. & Barth, D. S. Intracellular correlates of fast (>200 Hz) electrical oscillations in rat somatosensory cortex. J. Neurophysiol. 84, 1505–1518 (2000).
Nitzan, N. et al. Propagation of hippocampal ripples to the neocortex by way of a subiculum–retrosplenial pathway. Nat. Commun. 11, 1947 (2020).
Wilber, A. A., Skelin, I., Wu, W. & McNaughton, B. L. Laminar organization of encoding and memory reactivation in the parietal cortex. Neuron 95, 1406–1419.e5 (2017).
Zijlmans, M. et al. High-frequency oscillations as a new biomarker in epilepsy. Ann. Neurol. 71, 169–178 (2012).
Waldman, Z. J. et al. Ripple oscillations in the left temporal neocortex are associated with impaired verbal episodic memory encoding. Epilepsy Behav. 88, 33–40 (2018).
Prince, D. A. The depolarization shift in ‘epileptic’ neurons. Exp. Neurol. 21, 467–485 (1968).
Prince, D. A. Cortical cellular activities during cyclically occurring inter-ictal epileptiform discharges. Electroencephalogr. Clin. Neurophysiol. 31, 469–484 (1971).
Wadman, W. J., Lopes Da Silva, F. H. & Leung, L. W. S. Two types of interictal transients of reversed polarity in rat hippocampus during kindling. Electroencephalogr. Clin. Neurophysiol. 55, 314–319 (1983).
Fabó, D. et al. Properties of in vivo interictal spike generation in the human subiculum. Brain 131, 485–499 (2008).
Keller, C. J. et al. Heterogeneous neuronal firing patterns during interictal epileptiform discharges in the human cortex. Brain 133, 1668–1681 (2010). This study characterizes the heterogeneous single-unit activity underpinning interictal epileptiform discharges in patients with epilepsy and highlights the interplay of diverse cell types within complex networks in generation of these activity patterns.
Neumann, A. R. et al. Involvement of fast-spiking cells in ictal sequences during spontaneous seizures in rats with chronic temporal lobe epilepsy. Brain 140, 2355–2369 (2017).
Despouy, E. et al. Neuronal spiking activity highlights a gradient of epileptogenicity in human tuberous sclerosis lesions. Clin. Neurophysiol. 130, 537–547 (2019).
Alvarado-Rojas, C. et al. Single-unit activities during epileptic discharges in the human hippocampal formation. Front. Comput. Neurosci. 7, 140 (2013).
Schevon, C. A., Goodman, R. R., McKhann, G. & Emerson, R. G. Propagation of epileptiform activity on a submillimeter scale. J. Clin. Neurophysiol. 27, 406–411 (2010).
Altafullah, I., Halgren, E., Stapleton, J. M. & Crandall, P. H. Interictal spike-wave complexes in the human medial temporal lobe: typical topography and comparisons with cognitive potentials. Electroencephalogr. Clin. Neurophysiol. 63, 503–516 (1986).
Muldoon, S. F. et al. GABAergic inhibition shapes interictal dynamics in awake epileptic mice. Brain 138, 2875–2890 (2015).
Dranias, M. R., Westover, M. B., Cash, S. & Vandongen, A. M. J. Stimulus information stored in lasting active and hidden network states is destroyed by network bursts. Front. Integr. Neurosci. 9, 1–17 (2015).
Binnie, C. D. Cognitive impairment during epileptiform discharges: is it ever justifiable to treat the EEG? Lancet Neurol. 2, 725–730 (2003).
Kleen, J. K. et al. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology 81, 18–24 (2013). This study demonstrates the disruptive effects of hippocampal interictal epileptiform discharges on human memory.
Kleen, J. K., Scott, R. C., Holmes, G. L. & Lenck-Santini, P. P. Hippocampal interictal spikes disrupt cognition in rats. Ann. Neurol. 67, 250–257 (2010).
Krauss, G. L., Summerfield, M., Brandt, J., Breiter, S. & Ruchkin, D. Mesial temporal spikes interfere with working memory. Neurology 49, 975–980 (1997).
Quon, R. J. et al. Features of intracranial interictal epileptiform discharges associated with memory encoding. Epilepsia 62, 2615–2626 (2021).
Ung, H. et al. Interictal epileptiform activity outside the seizure onset zone impacts cognition. Brain 140, 2157–2168 (2017).
Devulder, A. et al. Epileptic activity on foramen ovale electrodes is associated with sleep and tau pathology in Alzheimer’s disease. Brain 148, 506–520 (2025).
Silvestri, R. et al. Ictal and interictal EEG abnormalities in ADHD children recorded over night by video-polysomnography. Epilepsy Res. 75, 130–137 (2007).
Soula, M. et al. Interictal epileptiform discharges affect memory in an Alzheimer’s disease mouse model. Proc. Natl Acad. Sci. USA 120, e2302676120 (2023).
Wierzynski, C. M., Lubenov, E. V., Gu, M. & Siapas, A. G. State-dependent spike–timing relationships between hippocampal and prefrontal circuits during sleep. Neuron 61, 587–596 (2009).
Logothetis, N. K. et al. Hippocampal–cortical interaction during periods of subcortical silence. Nature 491, 547–553 (2012).
Chrobak, J. J. & Buzsáki, G. Selective activation of deep layer (V–VI) retrohippocampal cortical neurons during hippocampal sharp waves in the behaving rat. J. Neurosci. 14, 6160–6170 (1994).
Siapas, A. G. & Wilson, M. A. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 21, 1123–1128 (1998).
Isomura, Y. et al. Integration and segregation of activity in entorhinal–hippocampal subregions by neocortical slow oscillations. Neuron 52, 871–882 (2006).
Sirota, A., Csicsvari, J., Buhl, D. & Buzsáki, G. Communication between neocortex and hippocampus during sleep in rodents. Proc. Natl Acad. Sci. USA 100, 2065–2069 (2003).
Mölle, M., Yeshenko, O., Marshall, L., Sara, S. J. & Born, J. Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. J. Neurophysiol. 96, 62–70 (2006).
Ibrahim, G. M. et al. Resilience of developing brain networks to interictal epileptiform discharges is associated with cognitive outcome. Brain 137, 2690–2702 (2014).
Weiss, S. A. et al. Delta oscillation coupled propagating fast ripples precede epileptiform discharges in patients with focal epilepsy. Neurobiol. Dis. 175, 105928 (2022).
Curot, J. et al. Local neuronal excitation and global inhibition during epileptic fast ripples in humans. Brain 146, 561–575 (2023).
Yu, H. et al. Interaction of interictal epileptiform activity with sleep spindles is associated with cognitive deficits and adverse surgical outcome in pediatric focal epilepsy. Epilepsia 65, 190–203 (2024).
Sákovics, A. et al. Prolongation of cortical sleep spindles during hippocampal interictal epileptiform discharges in epilepsy patients. Epilepsia 63, 2256–2268 (2022).
Ferrero, J. J. et al. Closed-loop electrical stimulation to prevent focal epilepsy progression and long-term memory impairment. Preprint at bioRxiv https://doi.org/10.1101/2024.02.09.579660 (2024).
Madar, A. D. et al. Deficits in behavioral and neuronal pattern separation in temporal lobe epilepsy. J. Neurosci. 41, 9669–9686 (2021).
Bui, A. D. et al. Dentate gyrus mossy cells control spontaneous convulsive seizures and spatial memory. Science 359, 787–790 (2018). This study demonstrates that induction of a cell-type-specific impairment can recapitulate cognitive deficits observed in a rodent model of epilepsy.
Cid, E. et al. Sublayer- and cell-type-specific neurodegenerative transcriptional trajectories in hippocampal sclerosis. Cell Rep. 35, 109229 (2021).
Douw, L. et al. Cellular substrates of functional network integration and memory in temporal lobe epilepsy. Cereb. Cortex 32, 2424–2436 (2022).
Pfisterer, U. et al. Identification of epilepsy-associated neuronal subtypes and gene expression underlying epileptogenesis. Nat. Commun. 11, 5038 (2020).
Chen, L., Li, X., Tjia, M. & Thapliyal, S. Homeostatic plasticity and excitation–inhibition balance: the good, the bad, and the ugly. Curr. Opin. Neurobiol. 75, 102553 (2022).
Abraham, W. C. & Bear, M. F. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 19, 126–130 (1996).
Martin, S. J., Grimwood, P. D. & Morris, R. G. M. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 23, 649–711 (2000).
Kolb, B., Harker, A. & Gibb, R. Principles of plasticity in the developing brain. Dev. Med. Child Neurol. 59, 1218–1223 (2017).
Malenka, R. C. The long-term potential of LTP. Nat. Rev. Neurosci. 4, 923–926 (2003).
Stasheff, S. F., Anderson, W. W., Clark, S. & Wilson, W. A. NMDA antagonists differentiate epileptogenesis from seizure expression in an in vitro model. Science 245, 648–651 (1989).
Abegg, M. H., Savic, N., Ehrengruber, M. U., McKinney, R. A. & Gähwiler, B. H. Epileptiform activity in rat hippocampus strengthens excitatory synapses. J. Physiol. 554, 439–448 (2004).
Loup, F., Picard, F., Yonekawa, Y., Wieser, H. G. & Fritschy, J. M. Selective changes in GABAA receptor subtypes in white matter neurons of patients with focal epilepsy. Brain 132, 2449–2463 (2009).
Katona, I. & Freund, T. F. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 14, 923–930 (2008).
Wetherington, J., Serrano, G. & Dingledine, R. Astrocytes in the epileptic brain. Neuron 58, 168–178 (2008).
Nasarudeen, R., Singh, A., Rana, Z. S. & Punnakkal, P. Epileptiform activity induced metaplasticity impairs bidirectional plasticity in the hippocampal CA1 synapses via GluN2B NMDA receptors. Exp. Brain Res. 240, 3339–3349 (2022).
Schubert, M., Siegmund, H., Pape, H. C. & Albrecht, D. Kindling-induced changes in plasticity of the rat amygdala and hippocampus. Learn. Mem. 12, 520–526 (2005).
Rehberg, M. et al. Functional metaplasticity of hippocampal Schaffer collateral-CA1 synapses is reversed in chronically epileptic rats. Neural Plast. 2017, 8087401 (2017).
Swann, J. W., Al-Noori, S., Jiang, M. & Lee, C. L. Spine loss and other dendritic abnormalities in epilepsy. Hippocampus 10, 617–625 (2000).
Sheehan, J. J., Benedetti, B. L. & Barth, A. L. Anticonvulsant effects of the BK-channel antagonist paxilline. Epilepsia 50, 711–720 (2009).
Brewster, A. L. et al. Rapamycin reverses status epilepticus-induced memory deficits and dendritic damage. PLoS ONE 8, e57808 (2013).
Houser, C. et al. in Jasper’s Basic Mechanisms of the Epilepsies (National Center for Biotechnology Information, 2012).
Kloc, M. L. et al. Spatial learning impairments and discoordination of entorhinal–hippocampal circuit coding following prolonged febrile seizures. Hippocampus 33, 970–992 (2023).
Hernan, A. E. et al. Focal epileptiform activity in the prefrontal cortex is associated with long-term attention and sociability deficits. Neurobiol. Dis. 63, 25–34 (2014).
Karnam, H. B. et al. Early life seizures cause long-standing impairment of the hippocampal map. Exp. Neurol. 217, 378–387 (2009).
Jiang, M., Lee, C. L., Smith, K. L. & Swann, J. W. Spine loss and other persistent alterations of hippocampal pyramidal cell dendrites in a model of early-onset epilepsy. J. Neurosci. 18, 8356–8368 (1998).
Nishimura, M., Gu, X. & Swann, J. W. Seizures in early life suppress hippocampal dendrite growth while impairing spatial learning. Neurobiol. Dis. 44, 205–214 (2011).
Sun, H. et al. Early seizures prematurely unsilence auditory synapses to disrupt thalamocortical critical period plasticity. Cell Rep. 23, 2533–2540 (2018).
Tuchman, R., Cuccaro, M. & Alessandri, M. Autism and epilepsy: historical perspective. Brain Dev. 32, 709–718 (2010).
Swann, J. W. & Rho, J. M. How is homeostatic plasticity important in epilepsy? Adv. Exp. Med. Biol. 813, 123–131 (2014).
Bullmore, E. & Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 13, 336–349 (2012).
Stam, C. J. Modern network science of neurological disorders. Nat. Rev. Neurosci. 15, 683–695 (2014).
Muldoon, S. F., Soltesz, I. & Cossart, R. Spatially clustered neuronal assemblies comprise the microstructure of synchrony in chronically epileptic networks. Proc. Natl Acad. Sci. USA 110, 3567–3572 (2013).
Bui, A., Kim, H. K., Maroso, M. & Soltesz, I. Microcircuits in epilepsy: heterogeneity and hub cells in network synchronization. Cold Spring Harb. Perspect. Med. 5, a022855 (2015).
Royer, J. et al. Epilepsy and brain network hubs. Epilepsia 63, 537–550 (2022).
Mula, M., Coleman, H. & Wilson, S. J. Neuropsychiatric and cognitive comorbidities in epilepsy. Continuum 28, 457–482 (2022).
Hill, S. F. & Meisler, M. H. Antisense oligonucleotide therapy for neurodevelopmental disorders. Dev. Neurosci. 43, 247–252 (2021).
Shore, A. N. et al. Reduced GABAergic neuron excitability, altered synaptic connectivity, and seizures in a KCNT1 gain-of-function mouse model of childhood epilepsy. Cell Rep. 33, 108303 (2020).
Xu, D. et al. Precision therapy with quinidine of KCNT1-related epileptic disorders: a systematic review. Br. J. Clin. Pharmacol. 88, 5096–5112 (2022).
Soh, H. et al. Deletion of KCNQ2/3 potassium channels from PV+ interneurons leads to homeostatic potentiation of excitatory transmission. eLife 7, e38617 (2018).
Warsi, N. M. et al. Which is more deleterious to cognitive performance? Interictal epileptiform discharges vs anti-seizure medication. Epilepsia 64, e75–e81 (2023).
Ortinski, P. & Meador, K. J. Cognitive side effects of antiepileptic drugs. Epilepsy Behav. 5, 60–65 (2004).
Park, S. P. & Kwon, S. H. Cognitive effects of antiepileptic drugs. J. Clin. Neurol. 4, 99–106 (2008).
Chapman, K. E. et al. Unmet needs in epileptic encephalopathy with spike-and-wave activation in sleep: a systematic review. Epilepsy Res. 199, 107278 (2024).
Wang, Z. et al. Prognostic value of complete resection of the high-frequency oscillation area in intracranial EEG: a systematic review and meta-analysis. Neurology 102, e209216 (2024).
Gong, R. et al. Quantifying hubness to predict surgical outcomes in epilepsy: assessing resection-hub alignment in interictal intracranial EEG networks. Epilepsia 65, 3362–3375 (2024).
Habibabadi, J. M., Zare, M. & Tabrizi, N. The role of interictal epileptiform discharges in epilepsy surgery outcome. Int. J. Prev. Med. 10, 101 (2019).
Benifla, M. et al. Neurosurgical management of intractable rolandic epilepsy in children: role of resection in eloquent cortex. Clinical article. J. Neurosurg. Pediatr. 4, 199–216 (2009).
Maingret, N., Girardeau, G., Todorova, R., Goutierre, M. & Zugaro, M. Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat. Neurosci. 19, 959–964 (2016).
Fernández-Ruiz, A. et al. Long-duration hippocampal sharp wave ripples improve memory. Science 364, 1082–1086 (2019). This study demonstrates that artificial prolongation of an oscillatory pattern using optogenetic stimulation can enhance naturally initiated spike sequences and improve memory.
Sierra, R. O. et al. Closed-loop brain stimulation augments fear extinction in male rats. Nat. Commun. 14, 3972 (2023).
Quirk, C. R. et al. Precisely timed theta oscillations are selectively required during the encoding phase of memory. Nat. Neurosci. 24, 1614–1627 (2021).
Mouchati, P. R., Kloc, M. L., Holmes, G. L., White, S. L. & Barry, J. M. Optogenetic ‘low-theta’ pacing of the septohippocampal circuit is sufficient for spatial goal finding and is influenced by behavioral state and cognitive demand. Hippocampus 30, 1167–1193 (2020).
Scangos, K. W. et al. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat. Med. 27, 1696–1700 (2021).
Geva-Sagiv, M. et al. Augmenting hippocampal-prefrontal neuronal synchrony during sleep enhances memory consolidation in humans. Nat. Neurosci. 26, 1100–1110 (2023). This study demonstrates that closed-loop deep brain stimulation in human subjects that enhances physiological network communication can improve memory.
Suthana, N. et al. Memory enhancement and deep-brain stimulation of the entorhinal area. N. Engl. J. Med. 366, 502–510 (2012).
Mankin, E. A. et al. Stimulation of the right entorhinal white matter enhances visual memory encoding in humans. Brain Stimul. 14, 131–140 (2021).
Jacobs, J. et al. Direct electrical stimulation of the human entorhinal region and hippocampus impairs memory. Neuron 92, 983–990 (2016).
Basu, I. et al. Closed-loop enhancement and neural decoding of cognitive control in humans. Nat. Biomed. Eng. 4, 576–588 (2023).
Ledri, M., Madsen, M. G., Nikitidou, L., Kirik, D. & Kokaia, M. Global optogenetic activation of inhibitory interneurons during epileptiform activity. J. Neurosci. 34, 3364–3377 (2014).
Călin, A., Ilie, A. S. & Akerman, C. J. Disrupting epileptiform activity by preventing parvalbumin interneuron depolarization block. J. Neurosci. 41, 9452–9465 (2021).
Krook-Magnuson, E., Armstrong, C., Oijala, M. & Soltesz, I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat. Commun. 4, 1376 (2013).
Sorokin, J. M. et al. Bidirectional control of generalized epilepsy networks via rapid real-time switching of firing mode. Neuron 93, 194–210 (2017).
Vlachos, I., Kugiumtzis, D., Tsalikakis, D. G. & Kimiskidis, V. K. TMS-induced brain connectivity modulation in genetic generalized epilepsy. Clin. Neurophysiol. 133, 83–93 (2022).
Klinzing, J. G. et al. Auditory stimulation during sleep suppresses spike activity in benign epilepsy with centrotemporal spikes. Cell Rep. Med. 2, 100432 (2021).
Vyazovskiy, V. V., Faraguna, U., Cirelli, C. & Tononi, G. Triggering slow waves during NREM sleep in the rat by intracortical electrical stimulation: effects of sleep/wake history and background activity. J. Neurophysiol. 101, 1921–1931 (2009).
Zhao, Z., Cea, C., Gelinas, J. N. & Khodagholy, D. Responsive manipulation of neural circuit pathology by fully implantable, front-end multiplexed embedded neuroelectronics. Proc. Natl Acad. Sci. USA 118, e2022659118 (2021).
Babu, H. et al. First-in-human trial of NRTX-1001 GABAergic interneuron cell therapy for treatment of focal epilepsy — emerging clinical trial results (S19.002). Neurology https://doi.org/10.1212/WNL.0000000000206002 (2024).
Anderson, D. N. et al. Closed-loop stimulation in periods with less epileptiform activity drives improved epilepsy outcomes. Brain 147, 521–531 (2024).
Kloc, M. L., Daglian, J. M., Holmes, G. L., Baram, T. Z. & Barry, J. M. Recurrent febrile seizures alter intrahippocampal temporal coordination but do not cause spatial learning impairments. Epilepsia 62, 3117–3130 (2021).
Takeuchi, Y. & Berényi, A. Oscillotherapeutics — time-targeted interventions in epilepsy and beyond. Neurosci. Res. 152, 87–107 (2020). This paper reviews theory and experimental evidence for modulation of oscillatory patterns to address symptoms of neuropsychiatric disorders.
Cea, C. et al. Enhancement-mode ion-based transistor as a comprehensive interface and real-time processing unit for in vivo electrophysiology. Nat. Mater. 19, 679–686 (2020).
Khodagholy, D. et al. NeuroGrid: recording action potentials from the surface of the brain. Nat. Neurosci. 18, 310–315 (2015).
Formento, E., D’Anna, E., Gribi, S., Lacour, S. P. & Micera, S. A biomimetic electrical stimulation strategy to induce asynchronous stochastic neural activity. J. Neural Eng. 17, 046019 (2020).
Fuller, E. J. et al. Parallel programming of an ionic floating-gate memory array for scalable neuromorphic computing. Science 364, 570–574 (2019).
Park, J., Kim, G. & Jung, S. D. A 128-channel FPGA-based real-time spike-sorting bidirectional closed-loop neural interface system. IEEE Trans. Neural Syst. Rehabil. Eng. 25, 2227–2238 (2017).
Khodagholy, D., Ferrero, J. J., Park, J., Zhao, Z. & Gelinas, J. N. Large-scale, closed-loop interrogation of neural circuits underlying cognition. Trends Neurosci. 45, 968–983 (2022). This study details conceptual and technical advances required to implement spatiotemporally targeted manipulation of neural networks involved in cognitive processes.
Acknowledgements
This work was supported by the National Institutes of Health grant no. R01NS118091 (J.N.G.). The authors thank all Khodagholy and Gelinas laboratory members for their support.
Author information
Authors and Affiliations
Contributions
J.N.G. and D.K. performed the literature review, discussed article content, and wrote and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks Jeremy M. Barry, George Ibrahim and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gelinas, J.N., Khodagholy, D. Interictal network dysfunction and cognitive impairment in epilepsy. Nat. Rev. Neurosci. 26, 399–414 (2025). https://doi.org/10.1038/s41583-025-00924-3
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41583-025-00924-3