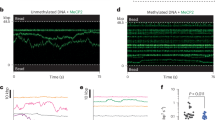
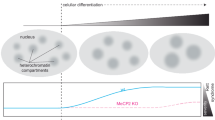
Abstract
Rett syndrome (RTT) is a neurodevelopmental disorder that is mainly caused by mutations in the methyl-DNA-binding protein MECP2. MECP2 is an important epigenetic regulator that plays a pivotal role in neuronal gene regulation, where it has been reported to function as both a repressor and an activator. Despite extensive efforts in mechanistic studies over the past two decades, a clear consensus on how MECP2 dysfunction impacts molecular mechanisms and contributes to disease progression has not been reached. Here, we review recent insights from epigenomic, transcriptomic and proteomic studies that advance our understanding of MECP2 as an interacting hub for DNA, RNA and transcription factors, orchestrating diverse processes that are crucial for neuronal function. By discussing findings from different model systems, we identify crucial epigenetic details and cofactor interactions, enriching our understanding of the multifaceted roles of MECP2 in transcriptional regulation and chromatin structure. These mechanistic insights offer potential avenues for rational therapeutic design for RTT.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Chahrour, M. & Zoghbi, H. Y. The story of Rett syndrome: from clinic to neurobiology. Neuron 56, 422–437 (2007).
Ip, J. P. K., Mellios, N. & Sur, M. Rett syndrome: insights into genetic, molecular and circuit mechanisms. Nat. Rev. Neurosci. 19, 368–382 (2018).
Leonard, H., Cobb, S. & Downs, J. Clinical and biological progress over 50 years in Rett syndrome. Nat. Rev. Neurol. 13, 37–51 (2017).
Lyst, M. J. & Bird, A. Rett syndrome: a complex disorder with simple roots. Nat. Rev. Genet. 16, 261–275 (2015).
Amir, R. E. et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 23, 185–188 (1999).
Trappe, R. et al. MECP2 mutations in sporadic cases of Rett syndrome are almost exclusively of paternal origin. Am. J. Hum. Genet. 68, 1093–1101 (2001).
Guy, J., Hendrich, B., Holmes, M., Martin, J. E. & Bird, A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 27, 322–326 (2001).
Chen, R. Z., Akbarian, S., Tudor, M. & Jaenisch, R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet. 27, 327–331 (2001).
Vashi, N. & Justice, M. J. Treating Rett syndrome: from mouse models to human therapies. Mamm. Genome 30, 90–110 (2019).
Giacometti, E., Luikenhuis, S., Beard, C. & Jaenisch, R. Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. Proc. Natl Acad. Sci. USA 104, 1931–1936 (2007).
Guy, J., Gan, J., Selfridge, J., Cobb, S. & Bird, A. Reversal of neurological defects in a mouse model of Rett syndrome. Science 315, 1143–1147 (2007).
Van Esch, H. MECP2 duplication syndrome. Mol. Syndromol. 2, 128–136 (2012).
Anguera, M. C. et al. Molecular signatures of human induced pluripotent stem cells highlight sex differences and cancer genes. Cell Stem Cell 11, 75–90 (2012).
Sharifi, O. & Yasui, D. H. The molecular functions of MeCP2 in Rett syndrome pathology. Front. Genet. 12, 624290 (2021).
Tillotson, R. & Bird, A. The molecular basis of MeCP2 function in the brain. J. Mol. Biol. 432, 1602–1623 (2020).
Horvath, P. M. & Monteggia, L. M. MeCP2 as an activator of gene expression. Trends Neurosci. 41, 72–74 (2018).
Meehan, R. R., Lewis, J. D. & Bird, A. P. Characterization of MeCP2, a vertebrate DNA binding protein with affinity for methylated DNA. Nucleic Acids Res. 20, 5085–5092 (1992).
Lewis, J. D. et al. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell 69, 905–914 (1992).
Free, A. et al. DNA recognition by the methyl-CpG binding domain of MeCP2. J. Biol. Chem. 276, 3353–3360 (2001).
Skene, P. J. et al. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol. Cell 37, 457–468 (2010). This study shows the global distribution of MECP2 and its correlation with mCpG density across the neuronal genome.
Guo, J. U. et al. Distribution, recognition and regulation of non-CpG methylation in the adult mammalian brain. Nat. Neurosci. 17, 215–222 (2014).
Xie, W. et al. Base-resolution analyses of sequence and parent-of-origin dependent DNA methylation in the mouse genome. Cell 148, 816–831 (2012).
Lister, R. et al. Global epigenomic reconfiguration during mammalian brain development. Science 341, 1237905 (2013).
Chen, L. et al. MeCP2 binds to non-CG methylated DNA as neurons mature, influencing transcription and the timing of onset for Rett syndrome. Proc. Natl Acad. Sci. USA 112, 5509–5514 (2015).
Gabel, H. W. et al. Disruption of DNA-methylation-dependent long gene repression in Rett syndrome. Nature 522, 89–93 (2015). The study shows association of gene length and non-CG DNA methylation with gene regulation by MECP2.
Lagger, S. et al. MeCP2 recognizes cytosine methylated tri-nucleotide and di-nucleotide sequences to tune transcription in the mammalian brain. PLoS Genet. 13, e1006793 (2017). This study shows a significant correlation of MECP2 binding to mCAC with transcriptional misregulation in mouse models of Rett syndrome.
Boxer, L. D. et al. MeCP2 represses the rate of transcriptional initiation of highly methylated long genes. Mol. Cell 77, 294–309.e299 (2020). This study describes a model of MECP2 acting at transcription start sites of highly methylated long genes to attenuate transcriptional initiation.
Kinde, B., Wu, D. Y., Greenberg, M. E. & Gabel, H. W. DNA methylation in the gene body influences MeCP2-mediated gene repression. Proc. Natl Acad. Sci. USA 113, 15114–15119 (2016).
Raman, A. T. et al. Apparent bias toward long gene misregulation in MeCP2 syndromes disappears after controlling for baseline variations. Nat. Commun. 9, 3225 (2018).
Clemens, A. W. et al. MeCP2 represses enhancers through chromosome topology-associated DNA methylation. Mol. Cell 77, 279–293.e278 (2020). Repressor model for MECP2 with binding to mCA domains to suppress intragenic enhancer activity and gene expression.
Nettles, S. A. et al. MeCP2 represses the activity of topoisomerase IIβ in long neuronal genes. Cell Rep. 42, 113538 (2023). This study describes a new mechanism by which MECP2 represses TOP2β activity to modulate the expression of neuronal long genes.
Tillotson, R. et al. Neuronal non-CG methylation is an essential target for MeCP2 function. Mol. Cell 81, 1260–1275 e1212 (2021). This research identifies MECP2 binding to mCAC as an essential contributor to gene dysregulation and neuronal phenotypes in Rett syndrome.
Nguyen, S., Meletis, K., Fu, D., Jhaveri, S. & Jaenisch, R. Ablation of de novo DNA methyltransferase Dnmt3a in the nervous system leads to neuromuscular defects and shortened lifespan. Dev. Dynam. 236, 1663–1676 (2007).
Lavery, L. A. et al. Losing Dnmt3a dependent methylation in inhibitory neurons impairs neural function by a mechanism impacting Rett syndrome. eLife 9, e52981 (2020).
Stroud, H. et al. Early-life gene expression in neurons modulates lasting epigenetic states. Cell 171, 1151–1164.e1116 (2017).
Ghosh, R. P. et al. Unique physical properties and interactions of the domains of methylated DNA binding protein 2. Biochemistry 49, 4395–4410 (2010).
Klose, R. J. et al. DNA binding selectivity of MeCP2 due to a requirement for A/T sequences adjacent to methyl-CpG. Mol. Cell 19, 667–678 (2005).
Piccolo, F. M. et al. MeCP2 nuclear dynamics in live neurons results from low and high affinity chromatin interactions. eLife 8, e51449 (2019).
Mellén, M., Ayata, P., Dewell, S., Kriaucionis, S. & Heintz, N. MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system. Cell 151, 1417–1430 (2012).
Valinluck, V. et al. Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2). Nucleic Acids Res. 32, 4100–4108 (2004).
Tahiliani, M. et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324, 930–935 (2009).
Ito, S. et al. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 466, 1129–1133 (2010).
Kriaucionis, S. & Heintz, N. The nuclear DNA base 5-hydroxymethylcytosine is present in purkinje neurons and the brain. Science 324, 929–930 (2009).
Globisch, D. et al. Tissue distribution of 5-hydroxymethylcytosine and search for active demethylation intermediates. PLoS One 5, e15367 (2010).
Song, C. X. et al. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat. Biotechnol. 29, 68–72 (2011).
Münzel, M. et al. Quantification of the sixth DNA base hydroxymethylcytosine in the brain. Angew. Chem. 49, 5375–5377 (2010).
Khare, T. et al. 5-hmC in the brain is abundant in synaptic genes and shows differences at the exon-intron boundary. Nat. Struct. Mol. Biol. 19, 1037–1043 (2012).
Fasolino, M. & Zhou, Z. The crucial role of DNA methylation and MeCP2 in neuronal function. Genes 8, 141 (2017).
Hashimoto, H. et al. Recognition and potential mechanisms for replication and erasure of cytosine hydroxymethylation. Nucleic Acids Res. 40, 4841–4849 (2012).
Khrapunov, S. et al. Unusual characteristics of the DNA binding domain of epigenetic regulatory protein MeCP2 determine its binding specificity. Biochemistry 53, 3379–3391 (2014).
Mellén, M., Ayata, P. & Heintz, N. 5-hydroxymethylcytosine accumulation in postmitotic neurons results in functional demethylation of expressed genes. Proc. Natl Acad. Sci. USA 114, E7812–e7821 (2017).
Ibrahim, A. et al. MeCP2 is a microsatellite binding protein that protects CA repeats from nucleosome invasion. Science 372, eabd5581 (2021).
Chhatbar, K., Connelly, J., Webb, S., Kriaucionis, S. & Bird, A. A critique of the hypothesis that CA repeats are primary targets of neuronal MeCP2. Life Sci. Alliance 5, e202201522 (2022).
Lentini, A. et al. A reassessment of DNA-immunoprecipitation-based genomic profiling. Nat. Methods 15, 499–504 (2018).
Yasui, D. H. et al. Integrated epigenomic analyses of neuronal MeCP2 reveal a role for long-range interaction with active genes. Proc. Natl Acad. Sci. USA 104, 19416–19421 (2007).
Chahrour, M. et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320, 1224–1229 (2008).
Weitzel, J. M., Buhrmester, H. & Strätling, W. H. Chicken MAR-binding protein ARBP is homologous to rat methyl-CpG-binding protein MeCP2. Mol. Cell. Biol. 17, 5656–5666 (1997).
von Kries, J. P., Buhrmester, H. & Strätling, W. H. A matrix/scaffold attachment region binding protein: identification, purification, and mode of binding. Cell 64, 123–135 (1991).
Buhrmester, H., von Kries, J. P. & Strätling, W. H. Nuclear matrix protein ARBP recognizes a novel DNA sequence motif with high affinity. Biochemistry 34, 4108–4117 (1995).
Lei, M., Tempel, W., Chen, S., Liu, K. & Min, J. Plasticity at the DNA recognition site of the MeCP2 mCG-binding domain. Biochim. biophys. Acta Gene Regul. Mech. 1862, 194409 (2019).
Weirauch, M. T. et al. Evaluation of methods for modeling transcription factor sequence specificity. Nat. Biotechnol. 31, 126–134 (2013).
Connelly, J. C. et al. Absence of MeCP2 binding to non-methylated GT-rich sequences in vivo. Nucleic Acids Res. 48, 3542–3552 (2020).
Rube, H. T. et al. Sequence features accurately predict genome-wide MeCP2 binding in vivo. Nat. Commun. 7, 11025 (2016).
Baubec, T., Ivánek, R., Lienert, F. & Schübeler, D. Methylation-dependent and -independent genomic targeting principles of the MBD protein family. Cell 153, 480–492 (2013).
Liu, K. et al. Structural basis for the ability of MBD domains to bind methyl-CG and TG sites in DNA. J. Biol. Chem. 293, 7344–7354 (2018).
Lee, W., Kim, J., Yun, J. M., Ohn, T. & Gong, Q. MeCP2 regulates gene expression through recognition of H3K27me3. Nat. Commun. 11, 3140 (2020).
Ortega-Alarcon, D. et al. Extending MeCP2 interactome: canonical nucleosomal histones interact with MeCP2. Nucleic Acids Res. 52, 3636–3653 (2024).
Nan, X., Campoy, F. J. & Bird, A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell 88, 471–481 (1997).
Nikitina, T. et al. MeCP2-chromatin interactions include the formation of chromatosome-like structures and are altered in mutations causing Rett syndrome. J. Biol. Chem. 282, 28237–28245 (2007).
Ghosh, R. P., Horowitz-Scherer, R. A., Nikitina, T., Shlyakhtenko, L. S. & Woodcock, C. L. MeCP2 binds cooperatively to its substrate and competes with histone H1 for chromatin binding sites. Mol. Cell. Biol. 30, 4656–4670 (2010).
Ito-Ishida, A. et al. Genome-wide distribution of linker histone H1.0 is independent of MeCP2. Nat. Neurosci. 21, 794–798 (2018).
Chua, G. N. L. et al. Differential dynamics specify MeCP2 function at nucleosomes and methylated DNA. Nat. Struct. Mol. Biol. 31, 1789–1797 (2024). This work presents direct observation of the in vitro molecular behaviour of MECP2 on chromatin using a single-molecule approach.
Yang, C., van der Woerd, M. J., Muthurajan, U. M., Hansen, J. C. & Luger, K. Biophysical analysis and small-angle X-ray scattering-derived structures of MeCP2–nucleosome complexes. Nucleic Acids Res. 39, 4122–4135 (2011).
Bartke, T. et al. Nucleosome-interacting proteins regulated by DNA and histone methylation. Cell 143, 470–484 (2010).
Georgel, P. T. et al. Chromatin compaction by human MeCP2. Assembly of novel secondary chromatin structures in the absence of DNA methylation. J. Biol. Chem. 278, 32181–32188 (2003).
Nikitina, T. et al. Multiple modes of interaction between the methylated DNA binding protein MeCP2 and chromatin. Mol. Cell. Biol. 27, 864–877 (2007).
Thambirajah, A. A. et al. MeCP2 binds to nucleosome free (linker DNA) regions and to H3K9/H3K27 methylated nucleosomes in the brain. Nucleic Acids Res. 40, 2884–2897 (2011).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
ENCODE Project Consortium. A user’s guide to the encyclopedia of DNA elements (ENCODE). PLoS Biol. 9, e1001046 (2011).
ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012).
Cholewa-Waclaw, J. et al. Quantitative modelling predicts the impact of DNA methylation on RNA polymerase II traffic. Proc. Natl Acad. Sci. USA 116, 14995–15000 (2019).
Kaya-Okur, H. S. et al. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun. 10, 1930 (2019).
Kaya-Okur, H. S., Janssens, D. H., Henikoff, J. G., Ahmad, K. & Henikoff, S. Efficient low-cost chromatin profiling with CUT&Tag. Nat. Protoc. 15, 3264–3283 (2020).
Henikoff, S., Henikoff, J. G., Kaya-Okur, H. S. & Ahmad, K. Efficient chromatin accessibility mapping in situ by nucleosome-tethered tagmentation. eLife 9, e63274 (2020).
Skene, P. J. & Henikoff, S. An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. eLife 6, e21856 (2017).
Skene, P. J., Henikoff, J. G. & Henikoff, S. Targeted in situ genome-wide profiling with high efficiency for low cell numbers. Nat. Protoc. 13, 1006–1019 (2018).
Liu, Y. et al. MECP2 directly interacts with RNA polymerase II to modulate transcription in human neurons. Neuron 112, 1943–1958.e1910 (2024). This study describes a new class of MECP2 binding to unmethylated promoter regions as a cofactor for RNA Pol II transcription in human neurons.
Mishra, G. P. et al. Interaction of methyl-CpG-binding protein 2 (MeCP2) with distinct enhancers in the mouse cortex. Nat. Neurosci. 28, 62–71 (2025). This research describes a methylation-independent mechanism by which MECP2 binds and modulates enhancer activity to repress gene expression.
Martin, S. et al. Embryonic stem cell-derived neurons as a model system for epigenome maturation during development. Genes 14, 957 (2023).
Zhang, Y. et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 78, 785–798 (2013).
Liu, S. et al. Cell type-specific 3D-genome organization and transcription regulation in the brain. Sci. Adv. 11, eadv2067 (2025). This study presents an advanced spatial imaging technique to reveal cell-type-dependent regulation by MECP2 of chromatin organization in mouse brains.
Luo, C. et al. Single-cell methylomes identify neuronal subtypes and regulatory elements in mammalian cortex. Science 357, 600–604 (2017).
He, Y. et al. Spatiotemporal DNA methylome dynamics of the developing mouse fetus. Nature 583, 752–759 (2020).
Johnson, B. S. et al. Biotin tagging of MeCP2 in mice reveals contextual insights into the Rett syndrome transcriptome. Nat. Med. 23, 1203–1214 (2017). An engineered Rett syndrome mouse model that selectively tags MECP2 to enable the cell-type-specific analysis of Rett-causing mutations on gene expression.
Bartosovic, M., Kabbe, M. & Castelo-Branco, G. Single-cell CUT&Tag profiles histone modifications and transcription factors in complex tissues. Nat. Biotechnol. 39, 825–835 (2021).
Bartosovic, M. & Castelo-Branco, G. Multimodal chromatin profiling using nanobody-based single-cell CUT&Tag. Nat. Biotechnol. 41, 794–805 (2023).
Carter, B. et al. Mapping histone modifications in low cell number and single cells using antibody-guided chromatin tagmentation (ACT-seq). Nat. Commun. 10, 3747 (2019).
Xie, Y. et al. Droplet-based single-cell joint profiling of histone modifications and transcriptomes. Nat. Struct. Mol. Biol. 30, 1428–1433 (2023).
Deng, Y. et al. Spatial-CUT&Tag: spatially resolved chromatin modification profiling at the cellular level. Science 375, 681–686 (2022).
Jones, P. L. et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 19, 187–191 (1998).
Kudo, S. et al. Heterogeneity in residual function of MeCP2 carrying missense mutations in the methyl CpG binding domain. J. Med. Genet. 40, 487–493 (2003).
Yusufzai, T. M. & Wolffe, A. P. Functional consequences of Rett syndrome mutations on human MeCP2. Nucleic Acids Res. 28, 4172–4179 (2000).
Lyst, M. J. et al. Rett syndrome mutations abolish the interaction of MeCP2 with the NCoR/SMRT co-repressor. Nat. Neurosci. 16, 898–902 (2013). This study reveals the mechanism of MECP2 as a repressor through its interaction with the NCoR/SMRT co-repressor complex.
Kruusvee, V. et al. Structure of the MeCP2–TBLR1 complex reveals a molecular basis for Rett syndrome and related disorders. Proc. Natl Acad. Sci. USA 114, E3243–e3250 (2017).
Sugino, K. et al. Cell-type-specific repression by methyl-CpG-binding protein 2 is biased toward long genes. J. Neurosci. 34, 12877–12883 (2014).
Renthal, W. et al. Characterization of human mosaic Rett syndrome brain tissue by single-nucleus RNA sequencing. Nat. Neurosci. 21, 1670–1679 (2018).
Donati, B., Lorenzini, E. & Ciarrocchi, A. BRD4 and cancer: going beyond transcriptional regulation. Mol. Cancer 17, 164 (2018).
Xiang, Y. et al. Dysregulation of BRD4 function underlies the functional abnormalities of MeCP2 mutant neurons. Mol. Cell 79, 84–98.e89 (2020).
Sharifi, O. et al. Sex-specific single cell-level transcriptomic signatures of Rett syndrome disease progression. Commun. Biol. 7, 1292 (2024). This work describes longitudinal study of sex-specific, cell-type-dependent and disease stage-associated transcriptional dysregulation in mouse models of Rett syndrome.
Neul, J. L. et al. Developmental delay in Rett syndrome: data from the natural history study. J. Neurodev. Disord. 6, 20 (2014).
Bu, Q. et al. CREB signaling is involved in Rett syndrome pathogenesis. J. Neurosci. 37, 3671–3685 (2017).
Li, C. H. et al. MeCP2 links heterochromatin condensates and neurodevelopmental disease. Nature 586, 440–444 (2020). This study proposes a model of MECP2-driven phase separation that facilitates heterochromatin formation.
Li, Y. et al. Global transcriptional and translational repression in human-embryonic-stem-cell-derived Rett syndrome neurons. Cell Stem Cell 13, 446–458 (2013). This study presents an application of RNA spike-in normalization for gene expression analysis to uncover global transcriptional downregulation in MECP2 knockout human neurons.
Zhou, J. et al. Disruption of MeCP2-TCF20 complex underlies distinct neurodevelopmental disorders. Proc. Natl Acad. Sci. USA 119, e2119078119 (2022).
Sonn, J. Y. et al. MeCP2 interacts with the super elongation complex to regulate transcription. Preprint at BioRxiv https://doi.org/10.1101/2024.06.30.601446 (2024).
Seczynska, M., Bloor, S., Cuesta, S. M. & Lehner, P. J. Genome surveillance by HUSH-mediated silencing of intronless mobile elements. Nature 601, 440–445 (2022).
Castello, A. et al. Comprehensive identification of RNA-binding domains in human cells. Mol. Cell 63, 696–710 (2016).
Soto, L. F. et al. Compendium of human transcription factor effector domains. Mol. Cell 82, 514–526 (2022).
DelRosso, N. et al. Large-scale mapping and mutagenesis of human transcriptional effector domains. Nature 616, 365–372 (2023).
Bajikar, S. S. et al. Acute MeCP2 loss in adult mice reveals transcriptional and chromatin changes that precede neurological dysfunction and inform pathogenesis. Neuron 113, 380–395.e8 (2024). In this study, a controlled perturbation system in adult mice is used to assess the immediate effects of MECP2 loss and the subsequent pathogenic cascade.
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Dillies, M. A. et al. A comprehensive evaluation of normalization methods for Illumina high-throughput RNA sequencing data analysis. Brief. Bioinforma. 14, 671–683 (2013).
Evans, C., Hardin, J. & Stoebel, D. M. Selecting between-sample RNA-seq normalization methods from the perspective of their assumptions. Brief. Bioinforma. 19, 776–792 (2018).
Chen, K. et al. The overlooked fact: fundamental need for spike-in control for virtually all genome-wide analyses. Mol. Cell. Biol. 36, 662–667 (2015).
Yazdani, M. et al. Disease modeling using embryonic stem cells: MeCP2 regulates nuclear size and RNA synthesis in neurons. Stem Cell 30, 2128–2139 (2012).
Lovén, J. et al. Revisiting global gene expression analysis. Cell 151, 476–482 (2012).
Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, R25 (2010).
Risso, D., Ngai, J., Speed, T. P. & Dudoit, S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat. Biotechnol. 32, 896–902 (2014).
SEQC/MAQC-III Consortium. A comprehensive assessment of RNA-seq accuracy, reproducibility and information content by the sequencing quality control consortium. Nat. Biotechnol. 32, 903–914 (2014).
Agarwal, N. et al. MeCP2 interacts with HP1 and modulates its heterochromatin association during myogenic differentiation. Nucleic Acids Res. 35, 5402–5408 (2007).
Nan, X. et al. Interaction between chromatin proteins MECP2 and ATRX is disrupted by mutations that cause inherited mental retardation. Proc. Natl Acad. Sci. USA 104, 2709–2714 (2007).
Ito-Ishida, A. et al. MeCP2 levels regulate the 3D structure of heterochromatic foci in mouse neurons. J. Neurosci. 40, 8746–8766 (2020).
Wang, L. et al. Rett syndrome-causing mutations compromise MeCP2-mediated liquid–liquid phase separation of chromatin. Cell Res. 30, 393–407 (2020).
Lesire, S. et al. LEDGF interacts with the NID domain of MeCP2 and modulates MeCP2 condensates. Structure 33, 78–90.e6 (2024).
Pantier, R. et al. MeCP2 binds to methylated DNA independently of phase separation and heterochromatin organisation. Nat. Commun. 15, 3880 (2024).
Horike, S., Cai, S., Miyano, M., Cheng, J. F. & Kohwi-Shigematsu, T. Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome. Nat. Genet. 37, 31–40 (2005).
Kernohan, K. D., Vernimmen, D., Gloor, G. B. & Bérubé, N. G. Analysis of neonatal brain lacking ATRX or MeCP2 reveals changes in nucleosome density, CTCF binding and chromatin looping. Nucleic Acids Res. 42, 8356–8368 (2014).
Kernohan, K. D. et al. ATRX partners with cohesin and MeCP2 and contributes to developmental silencing of imprinted genes in the brain. Dev. Cell 18, 191–202 (2010).
Brito, D. V. C., Gulmez Karaca, K., Kupke, J., Frank, L. & Oliveira, A. M. M. MeCP2 gates spatial learning-induced alternative splicing events in the mouse hippocampus. Mol. Brain 13, 156 (2020).
Jiang, Y. et al. Rett syndrome linked to defects in forming the MeCP2/Rbfox/LASR complex in mouse models. Nat. Commun. 12, 5767 (2021). This study presents mechanistic insights into how MECP2 regulates RNA splicing via the RBFOX/LASR complex.
Chhatbar, K., Cholewa-Waclaw, J., Shah, R., Bird, A. & Sanguinetti, G. Quantitative analysis questions the role of MeCP2 as a global regulator of alternative splicing. PLoS Genet. 16, e1009087 (2020).
Enikanolaiye, A. et al. Suppressor mutations in Mecp2-null mice implicate the DNA damage response in Rett syndrome pathology. Genome Res. 30, 540–552 (2020).
Aguilera, P. & López-Contreras, A. J. ATRX, a guardian of chromatin. Trends Genet. 39, 505–519 (2023).
Marchena-Cruz, E. et al. DDX47, MeCP2, and other functionally heterogeneous factors protect cells from harmful R loops. Cell Rep. 42, 112148 (2023).
Rodrigues, D. C. et al. Buffering of transcription rate by mRNA half-life is a conserved feature of Rett syndrome models. Nat. Commun. 14, 1896 (2023). This study describes a conserved transcriptional buffering characteristic in humans with Rett syndrome and mouse Rett syndrome models.
Albizzati, E. et al. Mecp2 knock-out astrocytes affect synaptogenesis by interleukin 6 dependent mechanisms. iScience 27, 109296 (2024).
Sun, J. et al. Mutations in the transcriptional regulator MeCP2 severely impact key cellular and molecular signatures of human astrocytes during maturation. Cell Rep. 42, 111942 (2023).
Dong, Q., Kim, J., Nguyen, L., Bu, Q. & Chang, Q. An astrocytic influence on impaired tonic inhibition in hippocampal CA1 pyramidal neurons in a mouse model of Rett syndrome. J. Neurosci. 40, 6250–6261 (2020).
Tomasello, D. L. et al. Mitochondrial dysfunction and increased reactive oxygen species production in MECP2 mutant astrocytes and their impact on neurons. Sci. Rep. 14, 20565 (2024).
Cao, Z. et al. RIPK1 activation in Mecp2-deficient microglia promotes inflammation and glutamate release in RTT. Proc. Natl Acad. Sci. USA 121, e2320383121 (2024).
Mifflin, L., Ofengeim, D. & Yuan, J. Receptor-interacting protein kinase 1 (RIPK1) as a therapeutic target. Nat. Rev. Drug. Discov. 19, 553–571 (2020).
Khoury, E. S. et al. Dendrimer-conjugated glutaminase inhibitor selectively targets microglial glutaminase in a mouse model of Rett syndrome. Theranostics 10, 5736–5748 (2020).
Mesci, P. et al. Human microglial cells as a therapeutic target in a neurodevelopmental disease model. Stem Cell Rep. 19, 1074–1091 (2024).
Osaki, T. et al. miR126-mediated impaired vascular integrity in Rett syndrome. Preprint at BioRxiv https://doi.org/10.1101/2024.10.11.617929 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05740761 (2023).
Sinnamon, J. R. et al. Targeted RNA editing in brainstem alleviates respiratory dysfunction in a mouse model of Rett syndrome. Proc. Natl Acad. Sci. USA 119, e2206053119 (2022).
Reautschnig, P. et al. Precise in vivo RNA base editing with a wobble-enhanced circular CLUSTER guide RNA. Nat. Biotechnol. 43, 545–557 (2024).
Manjunath, A. et al. Nucleoside analogs in ADAR guide strands enable editing at 5′-GA sites. Biomolecules 14, 1229 (2024).
Jacobsen, C. S. et al. Library screening reveals sequence motifs that enable ADAR2 editing at recalcitrant sites. ACS Chem. Biol. 18, 2188–2199 (2023).
Doherty, E. E. et al. ADAR activation by inducing a syn conformation at guanosine adjacent to an editing site. Nucleic Acids Res. 50, 10857–10868 (2022).
Sinnamon, J. R. et al. In vivo repair of a protein underlying a neurological disorder by programmable RNA editing. Cell Rep. 32, 107878 (2020).
Qian, J. et al. Multiplex epigenome editing of MECP2 to rescue Rett syndrome neurons. Sci. Transl. Med. 15, eadd46666 (2023).
Aguilar, R. et al. Targeting Xist with compounds that disrupt RNA structure and X inactivation. Nature 604, 160–166 (2022).
Przanowski, P. et al. Pharmacological reactivation of inactive X-linked Mecp2 in cerebral cortical neurons of living mice. Proc. Natl Acad. Sci. 115, 7991–7996 (2018).
Lee, H. M. et al. A small-molecule screen reveals novel modulators of MeCP2 and X-chromosome inactivation maintenance. J. Neurodev. Disord. 12, 29 (2020).
Sripathy, S. et al. Screen for reactivation of MeCP2 on the inactive X chromosome identifies the BMP/TGF-β superfamily as a regulator of XIST expression. Proc. Natl Acad. Sci. USA 114, 1619–1624 (2017).
Palmieri, M., Pozzer, D. & Landsberger, N. Advanced genetic therapies for the treatment of Rett syndrome: state of the art and future perspectives. Front. Neurosci. 17, 1172805 (2023).
Sadhu, C. et al. The efficacy of a human-ready miniMECP2 gene therapy in a pre-clinical model of Rett syndrome. Genes 15, 31 (2023).
Yang, L., Kirby, J. E., Sunwoo, H. & Lee, J. T. Female mice lacking Xist RNA show partial dosage compensation and survive to term. Genes Dev. 30, 1747–1760 (2016).
Bhatnagar, S. et al. Genetic and pharmacological reactivation of the mammalian inactive X chromosome. Proc. Natl Acad. Sci. USA 111, 12591–12598 (2014).
Merritt, J. K., Collins, B. E., Erickson, K. R., Dong, H. & Neul, J. L. Pharmacological read-through of R294X Mecp2 in a novel mouse model of Rett syndrome. Hum. Mol. Genet. 29, 2461–2470 (2020).
Wong, K. M. et al. Evaluation of novel enhancer compounds in gentamicin-mediated readthrough of nonsense mutations in Rett syndrome. Int. J. Mol. Sci. 24, 11665 (2023).
May, D. et al. Characterizing the journey of Rett syndrome among females in the United States: a real-world evidence study using the Rett syndrome natural history study database. J. Neurodev. Disord. 16, 42 (2024).
Krajnc, N. Management of epilepsy in patients with Rett syndrome: perspectives and considerations. Ther. Clin. Risk Manag. 11, 925–932 (2015).
Kennedy, M. et al. Development of trofinetide for the treatment of Rett syndrome: from bench to bedside. Front. Pharmacol. 14, 1341746 (2023).
Arjunan, A., Sah, D. K., Woo, M. & Song, J. Identification of the molecular mechanism of insulin-like growth factor-1 (IGF-1): a promising therapeutic target for neurodegenerative diseases associated with metabolic syndrome. Cell Biosci. 13, 16 (2023).
Conti, V. et al. MeCP2 affects skeletal muscle growth and morphology through non cell-autonomous mechanisms. PLoS One 10, e0130183 (2015).
Singh, A., Balasundaram, M. K. & Gupta, D. Trofinetide in Rett syndrome: a brief review of safety and efficacy. Intractable Rare Dis. Res. 12, 262–266 (2023).
Baker, A. M. et al. Central penetration and stability of N-terminal tripeptide of insulin-like growth factor-I, glycine-proline-glutamate in adult rat. Neuropeptides 39, 81–87 (2005).
Guan, J. et al. Neuroprotective effects of the N-terminal tripeptide of insulin-like growth factor-1, glycine-proline-glutamate (GPE) following intravenous infusion in hypoxic-ischemic adult rats. Neuropharmacology 47, 892–903 (2004).
Bickerdike, M. J. et al. NNZ-2566: a Gly–Pro–Glu analogue with neuroprotective efficacy in a rat model of acute focal stroke. J. Neurol. Sci. 278, 85–90 (2009).
Neul, J. L. et al. Trofinetide for the treatment of Rett syndrome: a randomized phase 3 study. Nat. Med. 29, 1468–1475 (2023). This research outlines the clinical importance of trofinetide in treating Rett syndrome.
Lopes, A. G., Loganathan, S. K. & Caliaperumal, J. Rett syndrome and the role of MECP2: signaling to clinical trials. Brain Sci. 14, 120 (2024).
Sun, X. et al. Deep single-cell-type proteome profiling of mouse brain by nonsurgical AAV-mediated proximity labeling. Anal. Chem. 94, 5325–5334 (2022).
Zhou, J. et al. A novel pathogenic mutation of MeCP2 impairs chromatin association independent of protein levels. Genes Dev. 37, 883–900 (2023).
Kouzarides, T. Chromatin modifications and their function. Cell 128, 693–705 (2007).
Young, J. I. et al. Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proc. Natl Acad. Sci. USA 102, 17551–17558 (2005).
Oksuz, O. et al. Transcription factors interact with RNA to regulate genes. Mol. Cell 83, 2449–2463.e2413 (2023).
Lee, Y., Okita, T. W. & Szymanski, D. B. A co-fractionation mass spectrometry-based prediction of protein complex assemblies in the developing rice aleurone-subaleurone. Plant. Cell 33, 2965–2980 (2021).
Rhee, H. W. et al. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science 339, 1328–1331 (2013).
Dumrongprechachan, V. et al. Cell-type and subcellular compartment-specific APEX2 proximity labeling reveals activity-dependent nuclear proteome dynamics in the striatum. Nat. Commun. 12, 4855 (2021).
Brown, K. et al. The molecular basis of variable phenotypic severity among common missense mutations causing Rett syndrome. Hum. Mol. Genet. 25, 558–570 (2016).
Fabio, R. A. et al. Recent insights into genotype-phenotype relationships in patients with Rett syndrome using a fine grain scale. Res. Dev. Disabil. 35, 2976–2986 (2014).
Gonzales, M. L. & LaSalle, J. M. The role of MeCP2 in brain development and neurodevelopmental disorders. Curr. Psychiatry Rep. 12, 127–134 (2010).
Achilly, N. P., Wang, W. & Zoghbi, H. Y. Presymptomatic training mitigates functional deficits in a mouse model of Rett syndrome. Nature 592, 596–600 (2021).
Downs, J. et al. Environmental enrichment intervention for Rett syndrome: an individually randomised stepped wedge trial. Orphanet J. Rare Dis. 13, 3 (2018).
Schmidt, A., Zhang, H. & Cardoso, M. C. MeCP2 and chromatin compartmentalization. Cells 9, 878 (2020).
Good, K., Kalani, L., Vincent, J. & Ausió, J. Multifaceted roles of MeCP2 in cellular regulation and phase separation: implications for neurodevelopmental disorders, depression, and oxidative stress. Biochem. Cell Biol. 103, 1–12 (2025).
Good, K. V., Vincent, J. B. & Ausió, J. MeCP2: the genetic driver of Rett syndrome epigenetics. Front. Genet. 12, 620859 (2021).
Lavery, L. A. & Zoghbi, H. Y. The distinct methylation landscape of maturing neurons and its role in Rett syndrome pathogenesis. Curr. Opin. Neurobiol. 59, 180–188 (2019).
Sonn, J. Y. & Zoghbi, H. Y. MeCP2 goes into unmethylated territories. Nat. Neurosci. 28, 4–5 (2025).
Baker, S. A. et al. An AT-hook domain in MeCP2 determines the clinical course of Rett syndrome and related disorders. Cell 152, 984–996 (2013).
Nan, X. et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393, 386–389 (1998).
Dominguez, G., Wu, Y. & Zhou, J. Epigenetic regulation and neurodevelopmental disorders: from MeCP2 to the TCF20/PHF14 complex. Genes 15, 1653 (2024).
Wu, H. et al. Genome-wide analysis reveals methyl-CpG-binding protein 2-dependent regulation of microRNAs in a mouse model of Rett syndrome. Proc. Natl Acad. Sci. USA 107, 18161–18166 (2010).
Nomura, T. et al. MeCP2-dependent repression of an imprinted miR-184 released by depolarization. Hum. Mol. Genet. 17, 1192–1199 (2008).
Gao, Y. et al. Inhibition of miR-15a promotes BDNF expression and rescues dendritic maturation deficits in MeCP2-deficient neurons. Stem Cell 33, 1618–1629 (2015).
Szulwach, K. E. et al. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J. Cell Biol. 189, 127–141 (2010).
Chen, Y., Shin, B. C., Thamotharan, S. & Devaskar, S. U. Differential methylation of the micro-RNA 7b gene targets postnatal maturation of murine neuronal Mecp2 gene expression. Dev. Neurobiol. 74, 407–425 (2014).
Mellios, N. et al. MeCP2-regulated miRNAs control early human neurogenesis through differential effects on ERK and AKT signaling. Mol. Psychiatry 23, 1051–1065 (2018).
Cheng, T. L. et al. MeCP2 suppresses nuclear microRNA processing and dendritic growth by regulating the DGCR8/Drosha complex. Dev. Cell 28, 547–560 (2014).
Mnatzakanian, G. N. et al. A previously unidentified MECP2 open reading frame defines a new protein isoform relevant to Rett syndrome. Nat. Genet. 36, 339–341 (2004).
Kriaucionis, S. & Bird, A. The major form of MeCP2 has a novel N-terminus generated by alternative splicing. Nucleic Acids Res. 32, 1818–1823 (2004).
Olson, C. O., Zachariah, R. M., Ezeonwuka, C. D., Liyanage, V. R. & Rastegar, M. Brain region-specific expression of MeCP2 isoforms correlates with DNA methylation within Mecp2 regulatory elements. PLoS One 9, e90645 (2014).
Zachariah, R. M., Olson, C. O., Ezeonwuka, C. & Rastegar, M. Novel MeCP2 isoform-specific antibody reveals the endogenous MeCP2E1 expression in murine brain, primary neurons and astrocytes. PLoS One 7, e49763 (2012).
Liyanage, V. R., Zachariah, R. M. & Rastegar, M. Decitabine alters the expression of Mecp2 isoforms via dynamic DNA methylation at the Mecp2 regulatory elements in neural stem cells. Mol. Autism 4, 46 (2013).
Nagarajan, R. P., Hogart, A. R., Gwye, Y., Martin, M. R. & LaSalle, J. M. Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics 1, e1–e11 (2006).
Franklin, T. B. et al. Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry 68, 408–415 (2010).
Lockman, S. et al. Transcriptional inhibition of the Mecp2 promoter by MeCP2E1 and MeCP2E2 isoforms suggests negative auto-regulatory feedback that can be moderated by metformin. J. Mol. Neurosci. 74, 14 (2024).
Buist, M. et al. Differential sensitivity of the protein translation initiation machinery and mTOR signaling to MECP2 gain- and loss-of-function involves MeCP2 isoform-specific homeostasis in the brain. Cells 11, 1442 (2022).
Fichou, Y. et al. The first missense mutation causing Rett syndrome specifically affecting the MeCP2_e1 isoform. Neurogenetics 10, 127–133 (2009).
Saunders, C. J., Minassian, B. E., Chow, E. W., Zhao, W. & Vincent, J. B. Novel exon 1 mutations in MECP2 implicate isoform MeCP2_e1 in classical Rett syndrome. Am. J. Med. Genet. A 149A, 1019–1023 (2009).
Gianakopoulos, P. J. et al. Mutations in MECP2 exon 1 in classical Rett patients disrupt MECP2_e1 transcription, but not transcription of MECP2_e2. Am. J. Med. Genet. B Neuropsychiatr. Genet. 159B, 210–216 (2012).
Yasui, D. H. et al. Mice with an isoform-ablating Mecp2 exon 1 mutation recapitulate the neurologic deficits of Rett syndrome. Hum. Mol. Genet. 23, 2447–2458 (2014).
Vogel Ciernia, A. et al. MeCP2 isoform e1 mutant mice recapitulate motor and metabolic phenotypes of Rett syndrome. Hum. Mol. Genet. 27, 4077–4093 (2018).
Djuric, U. et al. MECP2e1 isoform mutation affects the form and function of neurons derived from Rett syndrome patient iPS cells. Neurobiol. Dis. 76, 37–45 (2015).
Itoh, M. et al. Methyl CpG-binding protein isoform MeCP2_e2 is dispensable for Rett syndrome phenotypes but essential for embryo viability and placenta development. J. Biol. Chem. 287, 13859–13867 (2012).
Kerr, B. et al. Transgenic complementation of MeCP2 deficiency: phenotypic rescue of Mecp2-null mice by isoform-specific transgenes. Eur. J. Hum. Genet. 20, 69–76 (2012).
Martinez de Paz, A. et al. MeCP2-E1 isoform is a dynamically expressed, weakly DNA-bound protein with different protein and DNA interactions compared to MeCP2-E2. Epigenetics Chromatin 12, 63 (2019).
Ariani, F. et al. FOXG1 is responsible for the congenital variant of Rett syndrome. Am. J. Hum. Genet. 83, 89–93 (2008).
Dastidar, S. G. et al. Isoform-specific toxicity of Mecp2 in postmitotic neurons: suppression of neurotoxicity by FoxG1. J. Neurosci. 32, 2846–2855 (2012).
Li, R. et al. Misregulation of alternative splicing in a mouse model of Rett syndrome. PLoS Genet. 12, e1006129 (2016).
Maxwell, S. S., Pelka, G. J., Tam, P. P. & El-Osta, A. Chromatin context and ncRNA highlight targets of MeCP2 in brain. RNA Biol. 10, 1741–1757 (2013).
Long, S. W., Ooi, J. Y., Yau, P. M. & Jones, P. L. A brain-derived MeCP2 complex supports a role for MeCP2 in RNA processing. Biosci. Rep. 31, 333–343 (2011).
Khan, A. W. et al. MeCP2 interacts with chromosomal microRNAs in brain. Epigenetics 12, 1028–1037 (2017).
Fioriniello, S. et al. MeCP2 and major satellite forward RNA cooperate for Pericentric heterochromatin organization. Stem Cell Rep. 15, 1317–1332 (2020).
Dyson, H. J. Roles of intrinsic disorder in protein-nucleic acid interactions. Mol. Biosyst. 8, 97–104 (2012).
Cermakova, K. & Hodges, H. C. Interaction modules that impart specificity to disordered protein. Trends Biochem. Sci. 48, 477–490 (2023).
Ahmed, R. & Forman-Kay, J. D. NMR insights into dynamic, multivalent interactions of intrinsically disordered regions: from discrete complexes to condensates. Essays Biochem. 66, 863–873 (2022).
Yugandhar, K., Gupta, S. & Yu, H. Inferring protein–protein interaction networks from mass spectrometry-based proteomic approaches: a mini-review. Comput. Struct. Biotechnol. J. 17, 805–811 (2019).
Pfeiffer, C. T., Paulo, J. A., Gygi, S. P. & Rockman, H. A. Proximity labeling for investigating protein-protein interactions. Methods Cell Biol. 169, 237–266 (2022).
Branon, T. C. et al. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 36, 880–887 (2018).
Kido, K. et al. AirID, a novel proximity biotinylation enzyme, for analysis of protein–protein interactions. eLife 9, e54983 (2020).
Lam, S. S. et al. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat. Methods 12, 51–54 (2015).
Qiu, Z. Deciphering MECP2-associated disorders: disrupted circuits and the hope for repair. Curr. Opin. Neurobiol. 48, 30–36 (2018).
Samaco, R. C. et al. Female Mecp2+/− mice display robust behavioral deficits on two different genetic backgrounds providing a framework for pre-clinical studies. Hum. Mol. Genet. 22, 96–109 (2013).
Lau, B. Y. B., Krishnan, K., Huang, Z. J. & Shea, S. D. Maternal experience-dependent cortical plasticity in mice is circuit- and stimulus-specific and requires MECP2. J. Neurosci. 40, 1514–1526 (2020).
Lau, B. Y. B. et al. Lateralized expression of cortical perineuronal nets during maternal experience is dependent on MECP2. eNeuro 7, eneuro.0500-19.2020 (2020).
Xu, P. et al. Pattern decorrelation in the mouse medial prefrontal cortex enables social preference and requires MeCP2. Nat. Commun. 13, 3899 (2022).
Boyle, N. et al. MeCP2 deficiency alters the response selectivity of prefrontal cortical neurons to different social stimuli. eNeuro 11, eneuro.0003-24.2024 (2024).
Yue, Y. et al. MeCP2 deficiency impairs motor cortical circuit flexibility associated with motor learning. Mol. Brain 15, 76 (2022).
Yue, Y. et al. Motor training improves coordination and anxiety in symptomatic Mecp2-null mice despite impaired functional connectivity within the motor circuit. Sci. Adv. 7, eabf7467 (2021).
Ward, C. S. et al. Loss of MeCP2 function across several neuronal populations impairs breathing response to acute hypoxia. Front. Neurol. 11, 593554 (2020).
Chao, H. T. et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 468, 263–269 (2010).
He, L. J. et al. Conditional deletion of Mecp2 in parvalbumin-expressing GABAergic cells results in the absence of critical period plasticity. Nat. Commun. 5, 5036 (2014).
Goffin, D., Brodkin, E. S., Blendy, J. A., Siegel, S. J. & Zhou, Z. Cellular origins of auditory event-related potential deficits in Rett syndrome. Nat. Neurosci. 17, 804–806 (2014).
Krishnan, K. et al. MeCP2 regulates the timing of critical period plasticity that shapes functional connectivity in primary visual cortex. Proc. Natl Acad. Sci. USA 112, E4782–E4791 (2015).
Durand, S. et al. NMDA receptor regulation prevents regression of visual cortical function in the absence of Mecp2. Neuron 76, 1078–1090 (2012).
Patrizi, A. et al. Accelerated hyper-maturation of parvalbumin circuits in the absence of MeCP2. Cereb. Cortex 30, 256–268 (2020).
Li, J., Kells, P. A., Osgood, A. C., Gautam, S. H. & Shew, W. L. Collapse of complexity of brain and body activity due to excessive inhibition and MeCP2 disruption. Proc. Natl Acad. Sci. USA 118, e2106378118 (2021).
Ballinger, E. C. et al. Mecp2 deletion from cholinergic neurons selectively impairs recognition memory and disrupts cholinergic modulation of the perirhinal cortex. eNeuro 6, eneuro.0134-19.2019 (2019).
Rakela, B., Brehm, P. & Mandel, G. Astrocytic modulation of excitatory synaptic signaling in a mouse model of Rett syndrome. eLife 7, e31629 (2018).
Trujillo, C. A. et al. Pharmacological reversal of synaptic and network pathology in human MECP2-KO neurons and cortical organoids. EMBO Mol. Med. 13, e12523 (2021).
Chen, X. et al. Graded and pan-neural disease phenotypes of Rett syndrome linked with dosage of functional MeCP2. Protein Cell 12, 639–652 (2021).
Mok, R. S. F. et al. Wide spectrum of neuronal and network phenotypes in human stem cell-derived excitatory neurons with Rett syndrome-associated MECP2 mutations. Transl. Psychiatry 12, 450 (2022).
Osaki, T. et al. Early differential impact of MeCP2 mutations on functional networks in Rett syndrome patient-derived human cerebral organoids. Preprint at BioRxiv https://doi.org/10.1101/2024.08.10.607464 (2024).
Gomes, A. R., Fernandes, T. G., Cabral, J. M. S. & Diogo, M. M. Modeling Rett syndrome with human pluripotent stem cells: mechanistic outcomes and future clinical perspectives. Int. J. Mol. Sci. 22, 3751 (2021).
Haase, F. D. et al. Pre-clinical investigation of Rett syndrome using human stem cell-based disease models. Front. Neurosci. 15, 698812 (2021).
Pejhan, S. & Rastegar, M. Role of DNA methyl-CpG-binding protein MeCP2 in Rett syndrome pathobiology and mechanism of disease. Biomolecules 11, 75 (2021).
Zhou, Z. et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron 52, 255–269 (2006).
Li, W. & Pozzo-Miller, L. BDNF deregulation in Rett syndrome. Neuropharmacology 76 Pt C, 737–746 (2014).
Chen, W. G. et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 302, 885–889 (2003).
Vo, N. et al. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc. Natl Acad. Sci. USA 102, 16426–16431 (2005).
Klein, M. E. et al. Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat. Neurosci. 10, 1513–1514 (2007).
Remenyi, J. et al. Regulation of the miR-212/132 locus by MSK1 and CREB in response to neurotrophins. Biochem. J. 428, 281–291 (2010).
Hansen, K. F., Sakamoto, K., Wayman, G. A., Impey, S. & Obrietan, K. Transgenic miR132 alters neuronal spine density and impairs novel object recognition memory. PLoS One 5, e154977 (2010).
Han, K. et al. Human-specific regulation of MeCP2 levels in fetal brains by microRNA miR-483-5p. Genes Dev. 27, 485–490 (2013).
Su, M., Hong, J., Zhao, Y., Liu, S. & Xue, X. MeCP2 controls hippocampal brain-derived neurotrophic factor expression via homeostatic interactions with microRNA-132 in rats with depression. Mol. Med. Rep. 12, 5399–5406 (2015).
Kawashima, H. et al. Glucocorticoid attenuates brain-derived neurotrophic factor-dependent upregulation of glutamate receptors via the suppression of microRNA-132 expression. Neuroscience 165, 1301–1311 (2010).
Pejhan, S., Del Bigio, M. R. & Rastegar, M. The MeCP2E1/E2-BDNF-miR132 homeostasis regulatory network is region-dependent in the human brain and is impaired in Rett syndrome patients. Front. Cell Dev. Biol. 8, 763 (2020).
Zhang, J., Li, H. & Niswander, L. A. m5C methylated lncRncr3-MeCP2 interaction restricts miR124a-initiated neurogenesis. Nat. Commun. 15, 5136 (2024).
Im, H. I., Hollander, J. A., Bali, P. & Kenny, P. J. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat. Neurosci. 13, 1120–1127 (2010).
Yan, B. et al. MiR-218 targets MeCP2 and inhibits heroin seeking behavior. Sci. Rep. 7, 40413 (2017).
Xu, W. et al. Role of nucleus accumbens microRNA-181a and MeCP2 in incubation of heroin craving in male rats. Psychopharmacology 238, 2313–2324 (2021).
Rodrigues, D. C. et al. MECP2 is post-transcriptionally regulated during human neurodevelopment by combinatorial action of RNA-binding proteins and miRNAs. Cell Rep. 17, 720–734 (2016).
Acknowledgements
The authors thank L. Laurent and M. Oulmou for their valuable insights into the role of MECP2 in non-neuronal cells, DNA damage, RNA splicing and post-transcriptional regulation. All figures were created using BioRender. This work was supported by NIH grant 5R01MH104610 (R.J.) and International Rett Syndrome Foundation Research Independence Award (Y.L.). This research was supported by a generous gift from The Owens Family Foundation (Y.L. and R.J.).
Author information
Authors and Affiliations
Contributions
Y.L., R.G., T.W.W. and G.W.B. researched data for the article. Y.L., R.G., R.A.Y. and R.J. contributed substantially to discussion of the content. Y.L., T.W.W., G.W.B., R.G. and A.F. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
R.J. is an adviser and co-founder of Fate Therapeutics and Fulcrum Therapeutics. A.F. is a co-founder and shareholder of StemAxon. R.A.Y. is a founder and shareholder of Syros Pharmaceuticals, Camp4 Therapeutics, Omega Therapeutics, Dewpoint Therapeutics and Paratus Sciences. All other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks Janine LaSalle, Mojgan Rastegar and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- AT hooks
-
DNA-binding motifs targeting the minor groove of AT-rich DNA.
- Chromatin
-
A mixture of DNA and proteins, primarily histones, that forms the chromosomes within the nucleus of a eukaryotic cell.
- Chromatin loops
-
Structures formed by the folding of chromatin fibres and anchored by specific proteins such as CTCF and cohesin complexes, these bring distinct regions on the same chromosome closer to each other.
- Condensates
-
Membraneless compartments formed by phase separation of biomolecules including proteins and nucleic acids.
- DNA damage repair
-
A process by which cells identify and correct damages to DNA molecules, thereby restoring genomic integrity.
- Enhancer
-
A short regulatory DNA sequence that is in close spatial proximity to its target genes and can be bound by transcription factors to increase the transcription of its targets.
- Gene body
-
The region of the gene that starts at the transcription start site and extends to the transcription termination site, including both exons and introns.
- Heterochromatin
-
A type of chromatin that is densely packed and transcriptionally inactive.
- Long genes
-
Genes that are longer than 100 kb. These genes are crucial for the development and function of neurons, which contain longer transcripts in the transcriptome than non-neuronal cells.
- MicroRNAs
-
Non-coding RNAs that regulate gene expression by binding to and influencing mRNA targets in diverse biological processes.
- Microsatellites
-
Short, repetitive DNA sequences consisting of 1–6 bp motifs, which are scattered throughout the genome.
- Microtubule
-
A hollow polymeric tube composed of tubulin that helps support the shape of a cell.
- Nucleosome
-
The basic repeating subunit of chromatin consisting of a section of DNA wrapping around a histone octamer.
- Post-transcriptional regulation
-
Processes that control gene expression at the RNA level, between transcription and translation, primarily including capping, splicing, polyadenylation and nuclear export.
- Promoter
-
A regulatory DNA sequence that is located upstream of its target gene and can be bound by RNA polymerase II and transcription factors to initiate the transcription of its targets.
- RNA splicing
-
A biological process in which a newly transcribed precursor mRNA is modified by removing introns and rejoining exons to produce a mature mRNA that can be translated into a protein.
- Topologically associating domains
-
Fundamental units of 3D nuclear DNA organization in which cis-regulatory elements and their targets can frequently interact.
- Transcription start sites
-
Locations on a DNA molecule at which the first nucleotide is transcribed into RNA.
- X chromosome inactivation
-
A dosage compensation mechanism in female mammals, where one of the two X chromosomes in each cell is randomly silenced to equalize the expression of X-linked genes between males and females.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Whitfield, T.W., Bell, G.W. et al. Exploring the complexity of MECP2 function in Rett syndrome. Nat. Rev. Neurosci. 26, 379–398 (2025). https://doi.org/10.1038/s41583-025-00926-1
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41583-025-00926-1
This article is cited by
-
Non-CG DNA methylation in animal genomes
Nature Genetics (2025)