Abstract
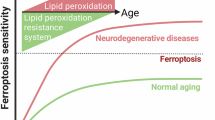
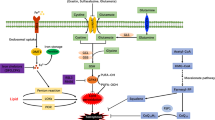
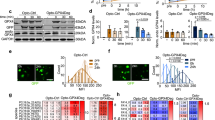
Ferroptosis is a type of cell death process defined by iron-dependent peroxidation of phospholipids leading to the destruction of cellular membranes and death of the cell. Ferroptosis occurs throughout the body, but a considerable research focus on ferroptosis in the brain — neuroferroptosis — has been driven by the rich lipid and iron content of the brain as well as its high oxygen consumption. Neurons also have an exceptionally large surface area and metabolic demand, which necessitates specific mechanisms (such as lipid antioxidants) to engage constantly to protect the plasma membrane against lipid peroxidation. Ferroptosis has been extensively linked to neurodegeneration and ischaemia and is increasingly implicated in physiological processes such as neuronal reprogramming. Astrocytes provide metabolic support to neurons, enabling them to defend against ferroptosis, yet ferroptotic signals in microglia can propagate damage to astrocytes and neurons, highlighting the complex intercellular (patho)physiology of neuroferroptosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wong, F. K. & Marin, O. Developmental cell death in the cerebral cortex. Annu. Rev. Cell Dev. Biol. 35, 523–542 (2019).
Kole, A. J., Annis, R. P. & Deshmukh, M. Mature neurons: equipped for survival. Cell Death Dis. 4, e689 (2013).
Ostrom, Q. T., Francis, S. S. & Barnholtz-Sloan, J. S. Epidemiology of brain and other CNS tumors. Curr. Neurol. Neurosci. Rep. 21, 68 (2021).
Herdy, J. R., Mertens, J. & Gage, F. H. Neuronal senescence may drive brain aging. Science 384, 1404–1406 (2024).
Wong, F. K. et al. Pyramidal cell regulation of interneuron survival sculpts cortical networks. Nature 557, 668–673 (2018).
Alves, F., Lane, D., Nguyen, T. P. M., Bush, A. I. & Ayton, S. In defence of ferroptosis. Signal. Transduct. Target. Ther. 10, 2 (2025).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012). This paper coins ferroptosis as a unique cell death pathway.
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Seiler, A. et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 8, 237–248 (2008). This early paper identifies loss of GPX4 as causing a unique cell death pathway.
Conrad, M. & Pratt, D. A. The chemical basis of ferroptosis. Nat. Chem. Biol. 15, 1137–1147 (2019).
Do, Q. & Xu, L. How do different lipid peroxidation mechanisms contribute to ferroptosis? Cell Rep. Phys. Sci. 4, 101683 (2023).
Ramos, P. et al. Iron levels in the human brain: a post-mortem study of anatomical region differences and age-related changes. J. Trace Elem. Med. Biol. 28, 13–17 (2014).
Levi, S. & Rovida, E. The role of iron in mitochondrial function. Biochim Biophys Acta 1790, 629–636 (2009).
Masini, A. et al. Dietary iron deficiency in the rat. II. Recovery from energy metabolism derangement of the hepatic tissue by iron therapy. Biochim Biophys Acta 1188, 53–57 (1994).
Halliwell, B. Oxidative stress and neurodegeneration: where are we now? J. Neurochem. 97, 1634–1658 (2006).
Zucca, F. A. et al. Neuromelanin and iron in human locus coeruleus and substantia nigra during aging: consequences for neuronal vulnerability. J. Neural Transm. 113, 757–767 (2006).
Devos, D. et al. Trial of deferiprone in Parkinson’s disease. N. Engl. J. Med. 387, 2045–2055 (2022).
Tian, R. et al. Genome-wide CRISPRi/a screens in human neurons link lysosomal failure to ferroptosis. Nat. Neurosci. 24, 1020–1034 (2021).
Reinert, A., Morawski, M., Seeger, J., Arendt, T. & Reinert, T. Iron concentrations in neurons and glial cells with estimates on ferritin concentrations. BMC Neurosci. 20, 25 (2019).
Liddell, J. R. et al. Microglial ferroptotic stress causes non-cell autonomous neuronal death. Mol. Neurodegener. 19, 14 (2024). This study demonstrates how ferroptosis in microglia can impact on astrocytes and neurons.
Faux, N. G. et al. An anemia of Alzheimer’s disease. Mol. Psychiatry 19, 1227–1234 (2014).
Hogarth, P. Neurodegeneration with brain iron accumulation: diagnosis and management. J. Mov. Disord. 8, 1–13 (2015).
O’Brien, J. S. & Sampson, E. L. Fatty acid and fatty aldehyde composition of the major brain lipids in normal human gray matter, white matter, and myelin. J. Lipid Res. 6, 545–551 (1965).
Osetrova, M. et al. Lipidome atlas of the adult human brain. Nat. Commun. 15, 4455 (2024).
Bazinet, R. P. & Laye, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 15, 771–785 (2014).
Liang, D. et al. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell 186, 2748–2764.e22 (2023).
Reed, A. et al. LPCAT3 inhibitors remodel the polyunsaturated phospholipid content of human cells and protect from ferroptosis. ACS Chem. Biol. 17, 1607–1618 (2022).
Magtanong, L. et al. Exogenous monounsaturated fatty acids promote a ferroptosis-resistant cell state. Cell Chem. Biol. https://doi.org/10.1016/j.chembiol.2018.11.016 (2018).
Doll, S. et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13, 91–98 (2017).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Jakaria, M., Belaidi, A. A., Bush, A. I. & Ayton, S. Vitamin A metabolites inhibit ferroptosis. Biomed. Pharmacother. 164, 114930 (2023).
Mishima, E. et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 608, 778–783 (2022).
Doll, S. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698 (2019).
Kraft, V. A. N. et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent. Sci. 6, 41–53 (2020).
Seibt, T. M., Proneth, B. & Conrad, M. Role of GPX4 in ferroptosis and its pharmacological implication. Free. Radic. Biol. Med. 133, 144–152 (2019).
Smith, A. C. et al. Mutations in the enzyme glutathione peroxidase 4 cause sedaghatian-type spondylometaphyseal dysplasia. J. Med. Genet. 51, 470–474 (2014).
Ingold, I. et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell https://doi.org/10.1016/j.cell.2017.11.048 (2017). This study demonstrates how efficient GPX4 activity is required to prevent neuronal death by ferroptosis.
Chiu-Ugalde, J. et al. Mutation of megalin leads to urinary loss of selenoprotein P and selenium deficiency in serum, liver, kidneys and brain. Biochem. J. 431, 103–111 (2010).
Solovyev, N., Drobyshev, E., Blume, B. & Michalke, B. Selenium at the neural barriers: a review. Front. Neurosci. 15, 630016 (2021).
Burk, R. F. et al. Deletion of apolipoprotein E receptor-2 in mice lowers brain selenium and causes severe neurological dysfunction and death when a low-selenium diet is fed. J. Neurosci. 27, 6207–6211 (2007).
Valentine, W. M., Abel, T. W., Hill, K. E., Austin, L. M. & Burk, R. F. Neurodegeneration in mice resulting from loss of functional selenoprotein P or its receptor apolipoprotein E receptor 2. J. Neuropathol. Exp. Neurol. 67, 68–77 (2008).
Leiter, O. et al. Lrp8 knockout mice fed a selenium-replete diet display subtle deficits in their spatial learning and memory function. Behav. Neurosci. 138, 125–141 (2024).
Hill, K. E., Zhou, J., McMahan, W. J., Motley, A. K. & Burk, R. F. Neurological dysfunction occurs in mice with targeted deletion of the selenoprotein P gene. J. Nutr. 134, 157–161 (2004).
Peters, M. M., Hill, K. E., Burk, R. F. & Weeber, E. J. Altered hippocampus synaptic function in selenoprotein P deficient mice. Mol. Neurodegener. 1, 12 (2006).
Leiter, O. et al. Selenium mediates exercise-induced adult neurogenesis and reverses learning deficits induced by hippocampal injury and aging. Cell Metab. 34, 408–423.e8 (2022).
De Miguel, Z. et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature 600, 494–499 (2021).
Biglari, S. et al. Ataxia with vitamin E deficiency: case series, vitamin E therapy response, founder effect, and in silico analysis. Clin Genet. https://doi.org/10.1111/cge.14658 (2024).
Ulatowski, L. et al. Vitamin E is essential for Purkinje neuron integrity. Neuroscience 260, 120–129 (2014).
Hambright, W. S., Fonseca, R. S., Chen, L., Na, R. & Ran, Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 12, 8–17 (2017).
Olson, C. R. & Mello, C. V. Significance of vitamin A to brain function, behavior and learning. Mol. Nutr. Food Res. 54, 489–495 (2010).
Tschuck, J. et al. Suppression of ferroptosis by vitamin A or radical-trapping antioxidants is essential for neuronal development. Nat. Commun. 15, 7611 (2024).
Couto, N., Wood, J. & Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free. Radic. Biol. Med. 95, 27–42 (2016).
Barclay, L. R. The cooperative antioxidant role of glutathione with a lipid-soluble and a water-soluble antioxidant during peroxidation of liposomes initiated in the aqueous phase and in the lipid phase. J. Biol. Chem. 263, 16138–16142 (1988).
Crabtree, M. J., Tatham, A. L., Hale, A. B., Alp, N. J. & Channon, K. M. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways. J. Biol. Chem. 284, 28128–28136 (2009).
Shimada, K., Hayano, M., Pagano, N. C. & Stockwell, B. R. Cell-line selectivity improves the predictive power of pharmacogenomic analyses and helps identify NADPH as biomarker for ferroptosis sensitivity. Cell Chem. Biol. 23, 225–235 (2016).
TeSlaa, T., Ralser, M., Fan, J. & Rabinowitz, J. D. The pentose phosphate pathway in health and disease. Nat. Metab. 5, 1275–1289 (2023).
Herrero-Mendez, A. et al. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat. Cell Biol. 11, 747–752 (2009).
Jimenez-Blasco, D. et al. Weak neuronal glycolysis sustains cognition and organismal fitness. Nat. Metab. 6, 1253–1267 (2024).
Suzuki, A. et al. Astrocyte–neuron lactate transport is required for long-term memory formation. Cell 144, 810–823 (2011).
Morgello, S., Uson, R. R., Schwartz, E. J. & Haber, R. S. The human blood–brain barrier glucose transporter (GLUT1) is a glucose transporter of gray matter astrocytes. Glia 14, 43–54 (1995).
Pierre, K. & Pellerin, L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J. Neurochem. 94, 1–14 (2005).
Sagara, J. I., Miura, K. & Bannai, S. Maintenance of neuronal glutathione by glial cells. J. Neurochem. 61, 1672–1676 (1993).
Kobayashi, S. et al. Carnosine dipeptidase II (CNDP2) protects cells under cysteine insufficiency by hydrolyzing glutathione-related peptides. Free. Radic. Biol. Med. 174, 12–27 (2021).
Ryan, S. K. et al. Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration. Nat. Neurosci. 26, 12–26 (2023). This study demonstrates how ferroptosis in microglia can impact neurodegeneration in Parkinson disease.
Southwell, D. G. et al. Intrinsically determined cell death of developing cortical interneurons. Nature 491, 109–113 (2012).
Burek, M. J. & Oppenheim, R. W. Programmed cell death in the developing nervous system. Brain Pathol. 6, 427–446 (1996).
Tafti, M. & Ghyselinck, N. B. Functional implication of the vitamin A signaling pathway in the brain. Arch. Neurol. 64, 1706–1711 (2007).
Sierra, A. et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7, 483–495 (2010).
Yu, S. W. et al. Autophagic death of adult hippocampal neural stem cells following insulin withdrawal. Stem Cell 26, 2602–2610 (2008).
Gascon, S. et al. Identification and successful negotiation of a metabolic checkpoint in direct neuronal reprogramming. Cell Stem Cell 18, 396–409 (2016).
Tang, C. F. et al. Gastrodin attenuates high fructose-induced sweet taste preference decrease by inhibiting hippocampal neural stem cell ferroptosis. J. Adv. Res. https://doi.org/10.1016/j.jare.2024.09.025 (2024).
Jiang, P. et al. Puerarin attenuates valproate-induced features of ASD in male mice via regulating Slc7a11-dependent ferroptosis. Neuropsychopharma 49, 497–507 (2024).
Song, R., Wang, R., Shen, Z. & Chu, H. Sevoflurane diminishes neurogenesis and promotes ferroptosis in embryonic prefrontal cortex via inhibiting nuclear factor-erythroid 2-related factor 2 expression. Neuroreport 33, 252–258 (2022).
Nobuta, H. et al. Oligodendrocyte death in Pelizaeus–Merzbacher disease is rescued by iron chelation. Cell Stem Cell 25, 531–541.e6 (2019).
Hoshino, T., Yamakado, H., Takahashi, R. & Matsuzawa, S. I. Susceptibility to erastin-induced ferroptosis decreases during maturation in a human oligodendrocyte cell line. FEBS Open. Bio 10, 1758–1764 (2020).
Fan, B. Y. et al. Liproxstatin-1 is an effective inhibitor of oligodendrocyte ferroptosis induced by inhibition of glutathione peroxidase 4. Neural Regen. Res. 16, 561–566 (2021).
Shen, D. et al. Ferroptosis in oligodendrocyte progenitor cells mediates white matter injury after hemorrhagic stroke. Cell Death Dis. 13, 259 (2022).
Jhelum, P. et al. Ferroptosis mediates cuprizone-induced loss of oligodendrocytes and demyelination. J. Neurosci. 40, 9327–9341 (2020).
Yang, J. et al. Perilipin-2 mediates ferroptosis in oligodendrocyte progenitor cells and myelin injury after ischemic stroke. Neural Regen. Res. 20, 2015–2028 (2025).
Shen, W. et al. Celastrol inhibits oligodendrocyte and neuron ferroptosis to promote spinal cord injury recovery. Phytomedicine 128, 155380 (2024).
Zhao, J., Zang, F., Huo, X. & Zheng, S. Novel approaches targeting ferroptosis in treatment of glioma. Front. Neurol. 14, 1292160 (2023).
Yant, L. J. et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free. Radic. Biol. Med. 34, 496–502 (2003).
Ran, Q. et al. Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. J. Gerontol. Ser. A, Biol. Sci. Med. Sci. 62, 932–942 (2007).
Berus, E. I., Gabuda, S. P., Ershov Iu, A. & Artamonov, V. B. Molecular mobility of soluble collagen [Russian]. Biofizika 32, 226–228 (1987).
Lei, G. et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 30, 146–162 (2020).
Upadhyayula, P. S. et al. Dietary restriction of cysteine and methionine sensitizes gliomas to ferroptosis and induces alterations in energetic metabolism. Nat. Commun. 14, 1187 (2023).
Li, S. et al. RSL3 drives ferroptosis through NF-κB pathway activation and GPX4 depletion in glioblastoma. Oxid. Med. Cell. Longev. 2021, 2915019 (2021).
Patel, D. et al. Novel analogs of sulfasalazine as system \({{\rm{x}}}_{{\rm{c}}}^{-}\) antiporter inhibitors: insights from the molecular modeling studies. Drug. Dev. Res. 80, 758–777 (2019).
Chen, T. C. et al. AR ubiquitination induced by the curcumin analog suppresses growth of temozolomide-resistant glioblastoma through disrupting GPX4-mediated redox homeostasis. Redox Biol. 30, 101413 (2020).
Chen, H. & Wen, J. Iron oxide nanoparticles loaded with paclitaxel inhibits glioblastoma by enhancing autophagy-dependent ferroptosis pathway. Eur. J. Pharmacol. 921, 174860 (2022).
Luo, Y. et al. Ferroptosis and its potential role in glioma: from molecular mechanisms to therapeutic opportunities. Antioxidants 11, 2123 (2022).
Bussian, T. J. et al. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562, 578–582 (2018).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Masaldan, S. et al. Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol. 14, 100–115 (2018).
Feng, Y. et al. Iron retardation in lysosomes protects senescent cells from ferroptosis. Aging 16, 7683–7703 (2024).
Go, S. et al. The senolytic drug JQ1 removes senescent cells via ferroptosis. Tissue Eng. Regen. Med. 18, 841–850 (2021).
Kordower, J. H. et al. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 136, 2419–2431 (2013).
Fearnley, J. M. & Lees, A. J. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114, 2283–2301 (1991).
Rossler, M., Zarski, R., Bohl, J. & Ohm, T. G. Stage-dependent and sector-specific neuronal loss in hippocampus during Alzheimer’s disease. Acta Neuropathol. 103, 363–369 (2002).
Price, J. L. et al. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch. Neurol. 58, 1395–1402 (2001).
Gabitto, M. I. et al. Integrated multimodal cell atlas of Alzheimer’s disease. Nat. Neurosci. https://doi.org/10.1038/s41593-024-01774-5 (2024).
Belaidi, A. A. et al. Marked age-related changes in brain iron homeostasis in amyloid protein precursor knockout mice. Neurotherapeutics 15, 1055–1062 (2018).
Fazlollahi, A. et al. Restricted effect of cerebral microbleeds on regional magnetic susceptibility. J. Alzheimers Dis. 76, 571–577 (2020).
Chen, X. et al. The molecular mechanisms of ferroptosis and its role in blood–brain barrier dysfunction. Front. Cell. Neurosci. 16, 889765 (2022).
Ayton, S. et al. Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease. Ann. Neurol. 73, 554–559 (2013).
Agarwal, P. et al. Brain copper may protect from cognitive decline and Alzheimer’s disease pathology: a community-based study. Mol. Psychiatry 27, 4307–4313 (2022).
Ayton, S. et al. Deferiprone in Alzheimer disease: a randomized clinical trial. JAMA Neurol. 82, 11–18 (2025).
Ayton, S. et al. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol. Psychiatry 25, 2932–2941 (2020).
Ayton, S. et al. Regional brain iron associated with deterioration in Alzheimer’s disease: a large cohort study and theoretical significance. Alzheimers Dement. 17, 1244–1256 (2021). This study demonstrates the clear relationship between iron and disease progression in a large post-mortem study of Alzheimer disease.
Majernikova, N. et al. The link between amyloid β and ferroptosis pathway in Alzheimer’s disease progression. Cell Death Dis. 15, 782 (2024).
Ansari, M. A. & Scheff, S. W. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J. Neuropathol. Exp. Neurol. 69, 155–167 (2010).
Ashraf, A., Jeandriens, J., Parkes, H. G. & So, P. W. Iron dyshomeostasis, lipid peroxidation and perturbed expression of cystine/glutamate antiporter in Alzheimer’s disease: evidence of ferroptosis. Redox Biol. 32, 101494 (2020).
Ayton, S., Faux, N. G., Bush, A. I. & Alzheimer’s Disease Neuroimaging Iniitiative. Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat. Commun. 6, 6760 (2015).
Ayton, S., Faux, N. G. & Bush, A. I. Association of cerebrospinal fluid ferritin level with preclinical cognitive decline in APOE-ε4 carriers. JAMA Neurol. 74, 122–125 (2017).
Duce, J. A. et al. Iron-export ferroxidase activity of β-amyloid precursor protein is inhibited by zinc in Alzheimer’s disease. Cell 142, 857–867 (2010).
Lei, P. et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat. Med. 18, 291–295 (2012).
Lei, P. et al. Lithium suppression of tau induces brain iron accumulation and neurodegeneration. Mol. Psychiatry 22, 396–406 (2017).
Bao, W. D. et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 28, 1548–1562 (2021).
Greenough, M. A. et al. Selective ferroptosis vulnerability due to familial Alzheimer’s disease presenilin mutations. Cell Death Differ. 29, 2123–2136 (2022). This study demonstrates how presenilin, a major gene involved in Alzheimer disease, is important for GPX4 in neurons and the defence against ferroptosis.
Belaidi, A. A. et al. Apolipoprotein E potently inhibits ferroptosis by blocking ferritinophagy. Mol. Psychiatry 29, 211–220 (2024). This study demonstrates how APOE, a major gene implicated in Alzheimer disease, impacts on ferroptosis signalling.
Ayton, S. & Lei, P. Nigral iron elevation is an invariable feature of Parkinson’s disease and is a sufficient cause of neurodegeneration. BioMed. Res. Int. 2014, 581256 (2014).
Ayton, S. et al. Iron accumulation confers neurotoxicity to a vulnerable population of nigral neurons: implications for Parkinson’s disease. Mol. Neurodegener. 9, 27 (2014).
Hare, D. J. et al. An iron–dopamine index predicts risk of parkinsonian neurodegeneration in the substantia nigra pars compacta. Chem. Sci. 5, 2160–2169 (2014).
Tang, F. et al. Inhibition of ACSL4 alleviates parkinsonism phenotypes by reduction of lipid reactive oxygen species. Neurotherapeutics 20, 1154–1166 (2023).
Do Van, B. et al. Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol. Dis. 94, 169–178 (2016).
Brauer, R. et al. Glitazone treatment and incidence of Parkinson’s disease among people with diabetes: a retrospective cohort study. PLoS Med. 12, e1001854 (2015).
Sun, J. et al. Midbrain dopamine oxidation links ubiquitination of glutathione peroxidase 4 to ferroptosis of dopaminergic neurons. J. Clin. Investig. 133, e165228 (2023).
Schriever, S. C. et al. Alterations in neuronal control of body weight and anxiety behavior by glutathione peroxidase 4 deficiency. Neuroscience 357, 241–254 (2017).
Vallerga, C. L. et al. Analysis of DNA methylation associates the cystine-glutamate antiporter SLC7A11 with risk of Parkinson’s disease. Nat. Commun. 11, 1238 (2020).
Cao, J. et al. DJ-1 suppresses ferroptosis through preserving the activity of S-adenosyl homocysteine hydrolase. Nat. Commun. 11, 1251 (2020).
Guiney, S. J., Adlard, P. A., Bush, A. I., Finkelstein, D. I. & Ayton, S. Ferroptosis and cell death mechanisms in Parkinson’s disease. Neurochem. Int. 104, 34–48 (2017).
Angelova, P. R. et al. α-Synuclein aggregation drives ferroptosis: an interplay of iron, calcium and lipid peroxidation. Cell Death Differ. 27, 2781–2796 (2020).
Mahoney-Sanchez, L. et al. α-Synuclein determines ferroptosis sensitivity in dopaminergic neurons via modulation of ether–phospholipid membrane composition. Cell Rep. 40, 111231 (2022).
Sun, W. Y. et al. Phospholipase iPLA2β averts ferroptosis by eliminating a redox lipid death signal. Nat. Chem. Biol. 17, 465–476 (2021).
Devos, D. et al. A ferroptosis-based panel of prognostic biomarkers for amyotrophic lateral sclerosis. Sci. Rep. 9, 2918 (2019).
Wang, T. et al. Ferroptosis mediates selective motor neuron death in amyotrophic lateral sclerosis. Cell Death Differ. 29, 1187–1198 (2022). This study demonstrates how GPX4 protects against a mouse model of ALS.
Wang, L. Q. et al. Amyloid fibril structures and ferroptosis activation induced by ALS-causing SOD1 mutations. Sci. Adv. 10, eado8499 (2024).
Southon, A. et al. CuII (atsm) inhibits ferroptosis: Implications for treatment of neurodegenerative disease. Br. J. Pharmacol. 177, 656–667 (2020).
Wang, D. et al. SPY1 inhibits neuronal ferroptosis in amyotrophic lateral sclerosis by reducing lipid peroxidation through regulation of GCH1 and TFR1. Cell Death Differ. 30, 369–382 (2023).
Ceron-Codorniu, M. et al. TDP-43 dysfunction leads to bioenergetic failure and lipid metabolic rewiring in human cells. Redox Biol. 75, 103301 (2024).
Ismail, M., Grossmann, D. & Hermann, A. Increased vulnerability to ferroptosis in FUS-ALS. Biology 13, 215 (2024).
Davalos, A. et al. Body iron stores and early neurologic deterioration in acute cerebral infarction. Neurology 54, 1568–1574 (2000).
Tuo, Q. Z. et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol. Psychiatry 22, 1520–1530 (2017).
Tuo, Q. Z. et al. Thrombin induces ACSL4-dependent ferroptosis during cerebral ischemia/reperfusion. Signal. Transduct. Target. Ther. 7, 59 (2022). This study demonstrates how thrombin signalling in ferroptosis contributes to ferroptosis in stroke.
Nakamura, A. et al. PLA2G2E-mediated lipid metabolism triggers brain-autonomous neural repair after ischemic stroke. Neuron 111, 2995–3010.e9 (2023).
Alim, I. et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 177, 1262–1279.e25 (2019).
Tuo, Q. Z. et al. Characterization of selenium compounds for anti-ferroptotic activity in neuronal cells and after cerebral ischemia–reperfusion injury. Neurotherapeutics 18, 2682–2691 (2021).
Qin, C. et al. The foam cell-derived exosomal miRNA Novel-3 drives neuroinflammation and ferroptosis during ischemic stroke. Nat Aging https://doi.org/10.1038/s43587-024-00727-8 (2024).
Hu, C. L. et al. Reduced expression of the ferroptosis inhibitor glutathione peroxidase-4 in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neurochem. 148, 426–439 (2019).
Luoqian, J. et al. Ferroptosis promotes T-cell activation-induced neurodegeneration in multiple sclerosis. Cell Mol. Immunol. 19, 913–924 (2022).
Woo, M. S. et al. STING orchestrates the neuronal inflammatory stress response in multiple sclerosis. Cell 187, 4043–4060.e30 (2024). This study demonstrates how neuroinflammation can contribute to ferroptosis in multiple sclerosis.
Rothammer, N. et al. G9a dictates neuronal vulnerability to inflammatory stress via transcriptional control of ferroptosis. Sci. Adv. 8, eabm5500 (2022).
Van San, E. et al. Ferroptosis contributes to multiple sclerosis and its pharmacological targeting suppresses experimental disease progression. Cell Death Differ. 30, 2092–2103 (2023).
Tan, S., Schubert, D. & Maher, P. Oxytosis: a novel form of programmed cell death. Curr. Top. Med. Chem. 1, 497–506 (2001).
Murphy, T. H., Miyamoto, M., Sastre, A., Schnaar, R. L. & Coyle, J. T. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 2, 1547–1558 (1989).
Guo, S. et al. Sulfiredoxin-1 accelerates erastin-induced ferroptosis in HT-22 hippocampal neurons by driving heme oxygenase-1 activation. Free. Radic. Biol. Med. 223, 430–442 (2024).
Song, S. et al. ALOX5-mediated ferroptosis acts as a distinct cell death pathway upon oxidative stress in Huntington’s disease. Genes. Dev. 37, 204–217 (2023).
Li, K. et al. ALOX5 inhibition protects against dopaminergic neurons undergoing ferroptosis. Pharmacol. Res. 193, 106779 (2023).
Lu, R. et al. A Shortage of FTH induces ROS and sensitizes RAS-proficient neuroblastoma N2A cells to ferroptosis. Int. J. Mol. Sci. 22, 8898 (2021).
Tang, S., Fuss, A., Fattahi, Z. & Culmsee, C. Drp1 depletion protects against ferroptotic cell death by preserving mitochondrial integrity and redox homeostasis. Cell Death Dis. 15, 626 (2024).
Jiao, L. et al. Iron metabolism mediates microglia susceptibility in ferroptosis. Front. Cell. Neurosci. 16, 995084 (2022).
Martinez, A. M., Kim, A., Flores, C. A., Rahman, D. F. & Yang, W. S. Mouse embryonic stem cell-derived motor neurons are susceptible to ferroptosis. FEBS Open. Bio 13, 419–433 (2023).
Renner, N. et al. Modeling ferroptosis in human dopaminergic neurons: pitfalls and opportunities for neurodegeneration research. Redox Biol. 73, 103165 (2024).
Alborzinia, H. et al. LRP8-mediated selenocysteine uptake is a targetable vulnerability in MYCN-amplified neuroblastoma. EMBO Mol. Med. 15, e18014 (2023).
Chen, L., Hambright, W. S., Na, R. & Ran, Q. Ablation of the ferroptosis inhibitor glutathione peroxidase 4 in neurons results in rapid motor neuron degeneration and paralysis. J. Biol. Chem. 290, 28097–28106 (2015). This study demonstrates how loss of GPX4 in neurons causes marked ferroptotic cell death.
Wirth, E. K. et al. Neuronal selenoprotein expression is required for interneuron development and prevents seizures and neurodegeneration. FASEB J. 24, 844–852 (2010).
Cui, S. et al. Identification of hyperoxidized PRDX3 as a ferroptosis marker reveals ferroptotic damage in chronic liver diseases. Mol. Cell 83, 3931–3939.e5 (2023).
Iwashita, H., Tokunaga, R. & Shioji, K. Visualization of ferroptosis-induced lipid peroxidation using plasma membrane-specific fluorescent probe LipoxPM. Chemistry 31, e202404323 (2025).
Ayton, S. et al. Cerebral quantitative susceptibility mapping predicts amyloid-β-related cognitive decline. Brain 140, 2112–2119 (2017).
Witzel, S. et al. Safety and effectiveness of long-term intravenous administration of edaravone for treatment of patients with amyotrophic lateral sclerosis. JAMA Neurol. 79, 121–130 (2022).
Dysken, M. W. et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 311, 33–44 (2014).
Graf, M. et al. High dose vitamin E therapy in amyotrophic lateral sclerosis as add-on therapy to riluzole: results of a placebo-controlled double-blind study. J. Neural Transm. 112, 649–660 (2005).
Parkinson Study Group, Q. E. I. et al. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol. 71, 543–552 (2014).
Kaufmann, P. et al. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann. Neurol. 66, 235–244 (2009).
Crapper McLachlan, D. R. et al. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 337, 1304–1308 (1991).
Devos, D. et al. Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid. Redox Signal. 21, 195–210 (2014).
Pereira, M. E. et al. Effects of selenium supplementation in patients with mild cognitive impairment or Alzheimer’s disease: a systematic review and meta-analysis. Nutrients 14, 3205 (2022).
Deepmala et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci. Biobehav. Rev. 55, 294–321 (2015).
Acknowledgements
This research was funded in whole or part by the Noncommunicable Chronic Diseases — National Science and Technology Major Project (2023ZD0507200) and the National Health and Medical Research Council (GNT2008359). The Florey Institute of Neuroscience and Mental Health acknowledges support from the Victorian Government, in particular funding from the Operational Infrastructure Support Grant. For the purposes of open access, the authors have applied a CC BY public copyright licence to any author accepted manuscript version arising from this submission.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Peer review
Peer review information
Nature Reviews Neuroscience thanks the anonymous reviewers for their contribution to the peer review of this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lei, P., Walker, T. & Ayton, S. Neuroferroptosis in health and diseases. Nat. Rev. Neurosci. 26, 497–511 (2025). https://doi.org/10.1038/s41583-025-00930-5
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41583-025-00930-5
This article is cited by
-
Ferroptosis in neurological diseases: moving towards therapeutic intervention
Molecular Psychiatry (2026)
-
ACSL4 Inhibition by AS-252424 Protects Visual Function by Suppressing RGC Ferroptosis After Retinal Ischemia Reperfusion Injury
Molecular Neurobiology (2026)
-
VKORC1L1 Downregulation Induced Vitamin K Cycle Disorder Exacerbates Neuronal Ferroptosis After Spinal Cord Injury
Molecular Neurobiology (2026)
-
Abnormal brain iron deposition in patients with new daily persistent headache: a prospective quantitative susceptibility mapping study
The Journal of Headache and Pain (2025)
-
O-GlcNAcylation in novel regulated cell death: ferroptosis, pyroptosis, and necroptosis
Cell Death Discovery (2025)