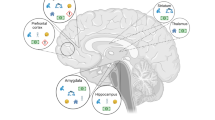
Abstract
Each year, millions of children around the world are exposed to a host of adverse experiences early in life. These include various forms of maltreatment, growing up in unsafe neighbourhoods, and witnessing intimate partner violence. These experiences exact a toll on the brain development and mental health of children. In this Review, we attempt to explain how brain architecture and circuitry are affected by exposure to such early adversity, which in turn increases susceptibility to mental health disorders later in life. We begin defining what we mean by early adversity and then summarize the experience-dependent nature of postnatal brain development. Within this context, we discuss times in development when the brain is particularly receptive to experience (critical periods) and, thus, is more vulnerable to adverse experiences. Drawing from studies with both rodent and non-human primate models and neuroimaging research with humans, we next discuss how the circuitry of the brain is affected by early-life adversity, with a focus on the subsequent effects upon neural network development. We then review the mental health consequences of adverse experiences in early life across mental health disorders and within specific dimensions of psychopathology. We conclude by offering a conceptual model of the pathway that links exposure to adversity early in life to these mental health outcomes later in life, and we provide suggestions for future research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
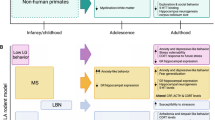
References
Berens, A. E., Jensen, S. K. G. & Nelson, C. A. 3rd Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med. 15, 135 (2017). A review that offers a conceptual model for how early childhood adversity may drive physiological changes that biologically embed experiences of adversity, resulting in a predisposition to common diseases across the life course.
Mueller, I. & Tronick, E. Early life exposure to violence: developmental consequences on brain and behavior. Front. Behav. Neurosci. 13, 156 (2019).
Galler, J. R. et al. Neurodevelopmental effects of childhood malnutrition: a neuroimaging perspective. Neuroimage 231, 117828 (2021).
Zundel, C. G. et al. Air pollution, depressive and anxiety disorders, and brain effects: a systematic review. Neurotoxicology 93, 272–300 (2022).
Hillis, S., Mercy, J., Amobi, A. & Kress, H. Global prevalence of past-year violence against children: a systematic review and minimum estimates. Pediatrics 137, e20154079 (2016).
Pinheiro, P. S. World Report on Violence Against Children (ATAR Roto Presse SA, 2006).
Hillis, S. D. et al. Global minimum estimates of children affected by COVID-19-associated orphanhood and deaths of caregivers: a modelling study. Lancet 398, 391–402 (2021).
Unwin, H. J. T. et al. Global, regional, and national minimum estimates of children affected by COVID-19-associated orphanhood and caregiver death, by age and family circumstance up to Oct 31, 2021: an updated modelling study. Lancet Child Adolesc. Health 6, 249–259 (2022).
Jones, C. M. et al. Estimated number of children who lost a parent to drug overdose in the US from 2011 to 2021. JAMA Psychiatry 81, 789–796 (2024).
Giano, Z., Wheeler, D. L. & Hubach, R. D. The frequencies and disparities of adverse childhood experiences in the US. BMC Public Health 20, 1–12 (2020).
Juwariah, T. et al. Childhood adversities and mental health problems: a systematic review. J. Public Health Res. 11, 22799036221106613 (2022).
Kim, B. & Royle, M. Annual research review: mapping the multifaceted approaches and impacts of adverse childhood experiences — an umbrella review of meta‐analyses. J. Child Psychol. Psychiatry 66, 399–416 (2024).
Wade, M., Wright, L. & Finegold, K. E. The effects of early life adversity on children’s mental health and cognitive functioning. Transl. Psychiatry 12, 244 (2022).
Carr, C. P., Martins, C. M., Stingel, A. M., Lemgruber, V. B. & Juruena, M. F. The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J. Nerv. Ment. Dis. 201, 1007–1020 (2013).
Hakamata, Y., Suzuki, Y., Kobashikawa, H. & Hori, H. Neurobiology of early life adversity: a systematic review of meta-analyses towards an integrative account of its neurobiological trajectories to mental disorders. Front. Neuroendocrinol. 65, 100994 (2022).
Kessler, R. C., Davis, C. G. & Kendler, K. S. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol. Med. 27, 1101–1119 (1997).
Luby, J. L., Barch, D., Whalen, D., Tillman, R. & Belden, A. Association between early life adversity and risk for poor emotional and physical health in adolescence: a putative mechanistic neurodevelopmental pathway. JAMA Pediatr. 171, 1168–1175 (2017).
Najman, J. M. et al. Do adversities experienced over the early life course predict mental illness and substance use behaviour in adulthood: a birth cohort study. J. Psychiatr. Res. 155, 542–549 (2022).
Nakama, N., Usui, N., Doi, M. & Shimada, S. Early life stress impairs brain and mental development during childhood increasing the risk of developing psychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 126, 110783 (2023).
Gunnar, M. R. & Vazquez, D. in Developmental Psychopathology: Volume Two: Developmental Neuroscience 533–577 (Wiley, 2015).
Juruena, M. F., Eror, F., Cleare, A. J. & Young, A. H. The role of early life stress in HPA axis and anxiety. Adv. Exp. Med. Biol. 1191, 141–153 (2020).
Lupien, S. J., McEwen, B. S., Gunnar, M. R. & Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 10, 434–445 (2009).
Ishikawa, Y. & Furuyashiki, T. The impact of stress on immune systems and its relevance to mental illness. Neurosci. Res. 175, 16–24 (2022).
Miller, A. H., Haroon, E. & Felger, J. C. The immunology of behavior — exploring the role of the immune system in brain health and illness. Neuropsychopharmacology 42, 1–4 (2017).
Nelson, C. A. 3rd & Gabard-Durnam, L. J. Early adversity and critical periods: neurodevelopmental consequences of violating the expectable environment. Trends Neurosci. 43, 133–143 (2020).
Knudsen, E. I. Sensitive periods in the development of the brain and behavior. J. Cogn. Neurosci. 16, 1412–1425 (2004).
Black, J. E., Jones, T. A., Nelson, C. A. & Greenough, W. T. Neuronal plasticity and the developing brain. Handb. Child Adolesc. Psychiatry 6, 31–53 (1998).
Greenough, W. T., Black, J. E. & Wallace, C. S. Experience and brain development. Child Dev. 58, 539–559 (1987).
Gervain, J. et al. Valproate reopens critical-period learning of absolute pitch. Front. Syst. Neurosci. 7, 102 (2013).
Iwai, Y., Fagiolini, M., Obata, K. & Hensch, T. K. Rapid critical period induction by tonic inhibition in visual cortex. J. Neurosci. 23, 6695–6702 (2003).
Lee, H. H. C. et al. Genetic Otx2 mis-localization delays critical period plasticity across brain regions. Mol. Psychiatry 22, 680–688 (2017).
Takesian, A. E. & Hensch, T. K. Balancing plasticity/stability across brain development. Prog. Brain Res. 207, 3–34 (2013).
Lorenz, K. Z. The evolution of behavior. Sci. Am. 199, 67–74 (1958).
Nelson, C. A. III, Zeanah, C. H. & Fox, N. A. How early experience shapes human development: the case of psychosocial deprivation. Neural Plast. 2019, 1676285 (2019).
Choi, D., Black, A. K. & Werker, J. F. Cascading and multisensory influences on speech perception development. Mind Brain Educ. 12, 212–223 (2018).
Hensch, T. K. Critical period regulation. Annu. Rev. Neurosci. 27, 549–579 (2004).
Werker, J. F. & Hensch, T. K. Critical periods in speech perception: new directions. Annu. Rev. Psychol. 66, 173–196 (2015).
McLaughlin, K. A., Sheridan, M. A. & Nelson, C. A. Neglect as a violation of species-expectant experience: neurodevelopmental consequences. Biol. Psychiatry 82, 462–471 (2017).
Pinto, R. Q., Soares, I., Carvalho-Correia, E. & Mesquita, A. R. Gene-environment interactions in psychopathology throughout early childhood: a systematic review. Psychiatr. Genet. 25, 223–233 (2015).
Taylor, A. & Kim-Cohen, J. Meta-analysis of gene-environment interactions in developmental psychopathology. Dev. Psychopathol. 19, 1029–1037 (2007).
Thapar, A., Harold, G., Rice, F., Langley, K. & O’Donovan, M. The contribution of gene-environment interaction to psychopathology. Dev. Psychopathol. 19, 989–1004 (2007).
Schaefer, J. D., Cheng, T. W. & Dunn, E. C. Sensitive periods in development and risk for psychiatric disorders and related endpoints: a systematic review of child maltreatment findings. Lancet Psychiatry 9, 978–991 (2022).
Wang, Q., Timberlake, M. A. 2nd, Prall, K. & Dwivedi, Y. The recent progress in animal models of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 77, 99–109 (2017).
Bonapersona, V. et al. The behavioral phenotype of early life adversity: a 3-level meta-analysis of rodent studies. Neurosci. Biobehav. Rev. 102, 299–307 (2019).
Packard, K. & Opendak, M. Rodent models of early adversity: impacts on developing social behavior circuitry and clinical implications. Front. Behav. Neurosci. 16, 918862 (2022).
Raineki, C. et al. During infant maltreatment, stress targets hippocampus, but stress with mother present targets amygdala and social behavior. Proc. Natl Acad. Sci. USA 116, 22821–22832 (2019).
Walker, C. D. et al. Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress 20, 421–448 (2017).
Zhao, M. et al. Effects of traumatic stress in adolescence on PTSD-like behaviors, dendrite development, and H3K9me2/BDNF expression in the amygdala of male rats. J. Affect. Disord. 296, 388–399 (2022).
Opendak, M. et al. Bidirectional control of infant rat social behavior via dopaminergic innervation of the basolateral amygdala. Neuron 109, 4018–4035.e7 (2021).
Nishi, M. Effects of early-life stress on the brain and behaviors: implications of early maternal separation in rodents. Int. J. Mol. Sci. 21, 7212 (2020).
Orso, R. et al. How early life stress impact maternal care: a systematic review of rodent studies. Front. Behav. Neurosci. 13, 197 (2019).
Barr, G. A., Opendak, M., Perry, R. E., Sarro, E. & Sullivan, R. M. Infant pain vs. pain with parental suppression: immediate and enduring impact on brain, pain and affect. PLoS ONE 18, e0290871 (2023).
Shupe, E. A. & Clinton, S. M. Neonatal resource scarcity alters maternal care and impacts offspring core temperature and growth in rats. Dev. Psychobiol. 63, e22144 (2021).
Rincon-Cortes, M. & Grace, A. A. Postpartum scarcity-adversity disrupts maternal behavior and induces a hypodopaminergic state in the rat dam and adult female offspring. Neuropsychopharmacology 47, 488–496 (2022).
Rosenbaum, S. & Kuzawa, C. W. The promise of great apes as model organisms for understanding the downstream consequences of early life experiences. Neurosci. Biobehav. Rev. 152, 105240 (2023).
Sanchez, M. M., Ladd, C. O. & Plotsky, P. M. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev. Psychopathol. 13, 419–449 (2001).
Harlow, H. F. The nature of love. Am. Psychol. 13, 673 (1958).
Harlow, H. F., Harlow, M. K., Dodsworth, R. O. & Arling, G. Maternal behavior of rhesus monkeys deprived of mothering and peer associations in infancy. Proc. Am. Philos. Soc. 110, 58–66 (1966).
Suomi, S. J., Harlow, H. F. & Kimball, S. D. Behavioral effects of prolonged partial social isolation in the rhesus monkey. Psychol. Rep. 29, 1171–1177 (1971).
McCormack, K. M. et al. The developmental consequences of early adverse care on infant macaques: a cross-fostering study. Psychoneuroendocrinology 146, 105947 (2022).
Tromp, D. P. M. et al. Early life adversity in primates: behavioral, endocrine, and neural effects. Psychoneuroendocrinology 162, 106953 (2024).
Sabatini, M. J. et al. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. J. Neurosci. 27, 3295–3304 (2007).
Maestripieri, D. & Carroll, K. A. Child abuse and neglect: usefulness of the animal data. Psychol. Bull. 123, 211–223 (1998).
Morin, E. L. et al. Developmental outcomes of early adverse care on amygdala functional connectivity in nonhuman primates. Dev. Psychopathol. 32, 1579–1596 (2020).
Lyons, D. M., Parker, K. J., Katz, M. & Schatzberg, A. F. Developmental cascades linking stress inoculation, arousal regulation, and resilience. Front. Behav. Neurosci. 3, 32 (2009).
Gabard-Durnam, L. J. & McLaughlin, K. A. Do sensitive periods exist for exposure to adversity? Biol. Psychiatry 85, 789–791 (2019).
Ho, T. C. & King, L. S. Mechanisms of neuroplasticity linking early adversity to depression: developmental considerations. Transl. Psychiatry 11, 517 (2021).
Smith, K. E. & Pollak, S. D. Rethinking concepts and categories for understanding the neurodevelopmental effects of childhood adversity. Perspect. Psychol. Sci. 16, 67–93 (2021).
Sripada, R. K., Swain, J. E., Evans, G. W., Welsh, R. C. & Liberzon, I. Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology 39, 2244–2251 (2014).
Hanson, J. L. et al. Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Dev. 84, 1566–1578 (2013).
Rakesh, D., Whittle, S., Sheridan, M. A. & McLaughlin, K. A. Childhood socioeconomic status and the pace of structural neurodevelopment: accelerated, delayed, or simply different? Trends Cogn. Sci. 27, 833–851 (2023).
Mehta, M. A. et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J. Child Psychol. Psychiatry 50, 943–951 (2009).
Beck, D. et al. Dimensions of early-life adversity are differentially associated with patterns of delayed and accelerated brain maturation. Biol. Psychiatry 97, 64–72 (2025).
Gee, D. G. et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl Acad. Sci. USA 110, 15638–15643 (2013). A resting-state fMRI study that finds evidence for accelerated maturation of connections between the amygdala and medial prefrontal cortex in previously institutionalized youth, conferring some emotion regulation benefits (decreasing their anxiety levels) despite overall higher levels of anxiety in this group than in children raised in foster care placements.
Sheridan, M. A. et al. Early deprivation alters structural brain development from middle childhood to adolescence. Sci. Adv. 8, eabn4316 (2022).
Debnath, R., Tang, A., Zeanah, C. H., Nelson, C. A. & Fox, N. A. The long-term effects of institutional rearing, foster care intervention and disruptions in care on brain electrical activity in adolescence. Dev. Sci. 23, e12872 (2020).
Marshall, P. J., Fox, N. A. & Bucharest Early Intervention Project Core Group. A comparison of the electroencephalogram between institutionalized and community children in Romania. J. Cogn. Neurosci. 16, 1327–1338 (2004).
Vanderwert, R. E., Marshall, P. J., Nelson, C. A. 3rd, Zeanah, C. H. & Fox, N. A. Timing of intervention affects brain electrical activity in children exposed to severe psychosocial neglect. PLoS ONE 5, e11415 (2010). EEG findings from the BEIP demonstrating that children removed from institutional care before 2 years of age show a more typical EEG profile than those fostered after 2 years of age, highlighting that there may be a sensitive period to ameliorate the effects of psychosocial deprivation on the brain.
Tottenham, N. et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev. Sci. 13, 46–61 (2010).
Joseph, J. et al. Greater maltreatment severity is associated with smaller brain volume with implication for intellectual ability in young children. Neurobiol. Stress 27, 100576 (2023).
Teicher, M. H., Samson, J. A., Anderson, C. M. & Ohashi, K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 17, 652–666 (2016).
McLaughlin, K. A., Weissman, D. & Bitran, D. Childhood adversity and neural development: a systematic review. Annu. Rev. Dev. Psychol. 1, 277–312 (2019).
Gerin, M. I., Viding, E., Herringa, R. J., Russell, J. D. & McCrory, E. J. A systematic review of childhood maltreatment and resting state functional connectivity. Dev. Cogn. Neurosci. 64, 101322 (2023).
Teicher, M. H. & Samson, J. A. Annual research review: enduring neurobiological effects of childhood abuse and neglect. J. Child Psychol. Psychiatry 57, 241–266 (2016).
Holz, N. E. et al. A stable and replicable neural signature of lifespan adversity in the adult brain. Nat. Neurosci. 26, 1603–1612 (2023).
Gehred, M. Z. et al. Long-term neural embedding of childhood adversity in a population-representative birth cohort followed for 5 decades. Biol. Psychiatry 90, 182–193 (2021).
McLaughlin, K. A. & Sheridan, M. A. Beyond cumulative risk: a dimensional approach to childhood adversity. Curr. Dir. Psychol. Sci. 25, 239–245 (2016). A study that provides a framework for the conceptualization of adversity in early life, proposing a dimensional approach along the axis of threat versus deprivation and highlighting how different forms of adversity might differentially influence learning mechanisms.
Schafer, J. L. et al. Threat and deprivation are associated with distinct aspects of cognition, emotional processing, and psychopathology in children and adolescents. Dev. Sci. 26, e13267 (2023).
Usacheva, M., Choe, D., Liu, S., Timmer, S. & Belsky, J. Testing the empirical integration of threat-deprivation and harshness-unpredictability dimensional models of adversity. Dev. Psychopathol. 34, 513–526 (2022).
Ellis, B. J., Sheridan, M. A., Belsky, J. & McLaughlin, K. A. Why and how does early adversity influence development? Toward an integrated model of dimensions of environmental experience. Dev. Psychopathol. 34, 447–471 (2022).
Davis, E. P. et al. Early life exposure to unpredictable parental sensory signals shapes cognitive development across three species. Front. Behav. Neurosci. 16, 960262 (2022).
Pollak, S. D. & Smith, K. E. Thinking clearly about biology and childhood adversity: next steps for continued progress. Perspect. Psychol. Sci. 16, 1473–1477 (2021).
Gee, D. G. Early adversity and development: parsing heterogeneity and identifying pathways of risk and resilience. Am. J. Psychiatry 178, 998–1013 (2021).
Durham, E. L. et al. Association of gray matter volumes with general and specific dimensions of psychopathology in children. Neuropsychopharmacology 46, 1333–1339 (2021).
Neumann, A. et al. White matter microstructure and the general psychopathology factor in children. J. Am. Acad. Child Adolesc. Psychiatry 59, 1285–1296 (2020).
Qiu, A. & Liu, C. Pathways link environmental and genetic factors with structural brain networks and psychopathology in youth. Neuropsychopharmacology 48, 1042–1051 (2023).
Vanes, L. D. & Dolan, R. J. Transdiagnostic neuroimaging markers of psychiatric risk: a narrative review. Neuroimage Clin. 30, 102634 (2021).
Vanes, L. D. et al. White matter tract myelin maturation and its association with general psychopathology in adolescence and early adulthood. Hum. Brain Mapp. 41, 827–839 (2020).
Woodward, N. D. & Cascio, C. J. Resting-state functional connectivity in psychiatric disorders. JAMA Psychiatry 72, 743–744 (2015).
Xia, C. H. et al. Linked dimensions of psychopathology and connectivity in functional brain networks. Nat. Commun. 9, 3003 (2018).
Sha, Z., Wager, T. D., Mechelli, A. & He, Y. Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol. Psychiatry 85, 379–388 (2019).
Goodkind, M. et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 72, 305–315 (2015).
Hamilton, J. P. et al. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am. J. Psychiatry 169, 693–703 (2012).
Chang, X. et al. Altered default mode and fronto-parietal network subsystems in patients with schizophrenia and their unaffected siblings. Brain Res. 1562, 87–99 (2014).
Menon, V., Palaniyappan, L. & Supekar, K. Integrative brain network and salience models of psychopathology and cognitive dysfunction in schizophrenia. Biol. Psychiatry 94, 108–120 (2023).
Sylvester, C. M. et al. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 35, 527–535 (2012).
Konrad, K. & Eickhoff, S. B. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum. Brain Mapp. 31, 904–916 (2010).
Castellanos, F. X. et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry 63, 332–337 (2008).
Casey, B. J., Tottenham, N., Liston, C. & Durston, S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn. Sci. 9, 104–110 (2005).
Menon, V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 15, 483–506 (2011).
Casey, B. J., Getz, S. & Galvan, A. The adolescent brain. Dev. Rev. 28, 62–77 (2008).
Crone, E. A. & Dahl, R. E. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat. Rev. Neurosci. 13, 636–650 (2012).
Somerville, L. H. & Casey, B. J. Developmental neurobiology of cognitive control and motivational systems. Curr. Opin. Neurobiol. 20, 236–241 (2010).
Kaiser, R. H., Andrews-Hanna, J. R., Wager, T. D. & Pizzagalli, D. A. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72, 603–611 (2015).
Eaton, S., Cornwell, H., Hamilton-Giachritsis, C. & Fairchild, G. Resilience and young people’s brain structure, function and connectivity: a systematic review. Neurosci. Biobehav. Rev. 132, 936–956 (2022).
Bolsinger, J., Seifritz, E., Kleim, B. & Manoliu, A. Neuroimaging correlates of resilience to traumatic events — a comprehensive review. Front. Psychiatry 9, 693 (2018).
Sheridan, M. A., Fox, N. A., Zeanah, C. H., McLaughlin, K. A. & Nelson, C. A. 3rd Variation in neural development as a result of exposure to institutionalization early in childhood. Proc. Natl Acad. Sci. USA 109, 12927–12932 (2012).
Bick, J. et al. Effect of early institutionalization and foster care on long-term white matter development: a randomized clinical trial. JAMA Pediatr. 169, 211–219 (2015).
Marshall, P. J., Reeb, B. C., Fox, N. A., Nelson, C. A. 3rd & Zeanah, C. H. Effects of early intervention on EEG power and coherence in previously institutionalized children in Romania. Dev. Psychopathol. 20, 861–880 (2008).
Humphreys, K. et al. Effects of early deprivation on psychopathology at age 12 years: follow-up of a randomized controlled trial. Lancet Psychiatry 2, 625–634 (2015).
Nelson, C. A. III et al. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science 318, 1937–1940 (2007).
Wade, M., Fox, N. A., Zeanah, C. H. & Nelson, C. A. 3rd Long-term effects of institutional rearing, foster care, and brain activity on memory and executive functioning. Proc. Natl Acad. Sci. USA 116, 1808–1813 (2019).
Zeanah, C. H. et al. Institutional rearing and psychiatric disorders in Romanian preschool children. Am. J. Psychiatry 166, 777–785 (2009).
Drury, S. S. et al. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Mol. Psychiatry 17, 719–727 (2012).
Humphreys, K. L. et al. Accelerated telomere shortening: tracking the lasting impact of early institutional care at the cellular level. Psychiatry Res. 246, 95–100 (2016).
Mandela, N. R. Speech by President Nelson Mandela at the launch of the Nelson Mandela Children’s Fund (1995-05-08). The Nelson Mandela Foundation Archive https://archive.nelsonmandela.org/index.php/za-com-mr-s-250 (2018).
Ellis, B. J. et al. Hidden talents in harsh environments. Dev. Psychopathol. 34, 95–113 (2022).
Frankenhuis, W. E. & Gopnik, A. Early adversity and the development of explore-exploit tradeoffs. Trends Cogn. Sci. 27, 616–630 (2023).
Pollak, S. D. & Sinha, P. Effects of early experience on children’s recognition of facial displays of emotion. Dev. Psychol. 38, 784–791 (2002).
Gleason, M. M. et al. Validity of evidence-derived criteria for reactive attachment disorder: indiscriminately social/disinhibited and emotionally withdrawn/inhibited types. J. Am. Acad. Child Adolesc. Psychiatry 50, 216–231.e3 (2011).
Masten, A. S., Best, K. M. & Garmezy, N. Resilience and development: contributions from the study of children who overcome adversity. Dev. Psychopathol. 2, 425–444 (1990).
Acknowledgements
The authors thank the members of Charles Nelson’s Harvard College first year student seminar who graciously offered comments on an earlier version of this paper (A. Ashaye, A. Holtey, J. Lane, M. Morrow, M. Unger and C. Wu). The authors also extend their gratitude to S. Odabashian for proofing and editing the manuscript. The writing of this paper was made possible by the Jacobs Foundation (Klaus J. Jacobs award) and the National Institutes of Health (1U24 DA055325 and MH091363 to C.A.N. and T32MH112510 to E.F.S.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurosciences thanks Maya Opendak, Christine Heim and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Adolescent Brain and Child Development Study: https://abcdstudy.org/
Bucharest Early Intervention Project: https://bucharestearlyinterventionproject.org/
Glossary
- Experience-expectant development
-
Situations in which a species-typical experience (one common and typical for all members of the species) has a requisite role in the development and ultimate organization of the nervous system. For instance, the visual cortex relies on exposure to patterned light for typical visual development, and deviations from these expected inputs can lead to atypical developmental trajectories.
- Gene × environment interactions
-
The effects of the genetic background of individuals on their developmental outcomes can differ depending on the modulating influences of their environment and experiences (experience ‘writes’ against the genetic background of an individual).
- Mental health disorder
-
Conditions that influence the emotional, psychological and/or behavioural well-being of an individual, in which the symptoms must lead to marked distress or impairment in daily functioning; commonly classified according to standardized diagnostic criteria in the Diagnostic and Statistical Manual of Mental Disorders (DSM) and International Classification of Diseases (ICD).
- Neural network dysconnectivity
-
Abnormal or disrupted communication patterns within or between neural networks, which can be characterized by decreased and/or heightened structural or functional connectivity.
- Neurobiological embedding
-
The processes by which early experiences, especially adversity and stressors, affect brain development and can result in lasting changes to brain structure and function via mechanisms such as neural plasticity.
- Protective factors
-
Experiences or environmental features that can mitigate the effects of risk factors (such as high-quality caregiving in the face of adversity) and may contribute to resilience.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nelson, C.A., Sullivan, E.F. & Valdes, V. Early adversity alters brain architecture and increases susceptibility to mental health disorders. Nat. Rev. Neurosci. 26, 642–656 (2025). https://doi.org/10.1038/s41583-025-00948-9
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41583-025-00948-9