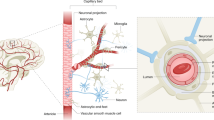
Abstract
There is increasing evidence to suggest that vascular dysfunction can contribute to cognitive decline in ageing and dementia. This dysfunction can take the form of a reduction of cerebral blood flow (CBF), a loss of blood–brain barrier (BBB) function or a combination of the two. Indeed, CBF and BBB changes may be causally linked, although this possible causality and its directionality are understudied. Appreciation of the role of vascular dysfunction in initiating cognitive decline in ageing and dementia, as well as the mechanisms involved, is important because it opens up new avenues for the development of much-needed therapies for these conditions, which are becoming major causes of death. Here we assess the evidence for the importance of vascular contributions to dementia, draw parallels with changes that occur in normal ageing and discuss the initiating cells and signalling mechanisms involved. We suggest that attempting to maintain or restore CBF should be a central aim of therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Attwell, D. & Laughlin, S. B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow. Metab. 21, 1133–1145 (2001).
Smith, M. A., Riby, L. M., van Eekelen, J. A. M. & Foster, J. K. Glucose enhancement of human memory: a comprehensive research review of the glucose memory facilitation effect. Neurosci. Biobehav. Rev. 35, 770–783 (2011).
Fruehwald-Schultes, B., Born, J., Pateres, A. & Fehm, H. L. Adaptation of cognitive function to hypoglycemia in healthy men. Diabetes Care 23, 1059–1066 (2000).
Allen, K. V. et al. Effects of acute hypoglycemia on working memory and language processing in adults with and without type 1 diabetes. Diabetes Care 38, 1108–1115 (2015).
Zhou, Z. et al. Deeper cerebral hypoperfusion leads to spatial cognitive impairment in mice. Stroke Vasc. Neurol. 7, e001594 (2022).
Hainsworth, R. Pathophysiology of syncope. Clin. Auton. Res. 14, 18–24 (2004).
Asllani, I. et al. Multivariate and univariate analysis of continuous arterial spin labeling perfusion MRI in Alzheimer’s disease. J. Cereb. Blood Flow. Metab. 28, 725–736 (2008). Quantifies the reduction in CBF in different brain regions in AD, highlighting CBF decreases as a therapeutic target.
Dash, S., Agarwal, Y., Jain, S., Sharma, A. & Chaudhry, N. Perfusion CT imaging as a diagnostic and prognostic tool for dementia: prospective case-control study. Postgrad. Med. J. 99, 318–325 (2023).
Hu, J. et al. Regional changes in cerebral perfusion with age when accounting for changes in gray-matter volume. Mag. Res. Med. 93, 1807–1820 (2025).
Nortley, R. et al. Amyloid β oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science 365, eaav9518 (2019). This study discovered that pericyte-mediated capillary constriction causes the CBF reduction in human AD, identifying pericyte dysfunction as a possible new therapeutic target.
Montagne, A. et al. APOE4 accelerates advanced-stage vascular and neurodegenerative disorder in old Alzheimer’s mice via cyclophilin A independently of amyloid-β. Nat. Aging 1, 506–520 (2021).
Wardlaw, J. M. et al. Isosorbide mononitrate and cilostazol treatment in patients with symptomatic cerebral small vessel disease: the lacunar intervention trial-2 (LACI-2) randomized clinical trial. JAMA Neurol. 80, 682–692 (2023). This phase 2 clinical trial repurposed two existing and widely available medications (a nitric oxide donor and an antiplatelet drug) to successfully reduce the development of dementia after SVD-induced stroke.
Korte, N. et al. Inhibiting Ca2+ channels in Alzheimer’s disease model mice relaxes pericytes, improves cerebral blood flow and reduces immune cell stalling and hypoxia. Nat. Neurosci. 27, 2086–2100 (2024).The L-type Ca2+ channel blocker, nimodipine, reversed capillary constriction, improved CBF and decreased brain hypoxia in a mouse model of AD, suggesting reduction of pericyte Ca2+ is a strategy for enhancing CBF and cognitive function early in AD.
Iturria-Medina, Y., Sotero, R. C., Toussaint, P. J., Mateos-Pérez, J. M. & Evans, A. C. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 7, 11934 (2016). This study shows that CBF reduction is a very early event in AD and therefore possibly a key driver of disease progression.
Nation, D. A. et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 25, 270–276 (2019).
Schager, B. & Brown, C. E. Susceptibility to capillary plugging can predict brain region specific vessel loss with aging. J. Cereb. Blood Flow. Metab. 40, 2475–2490 (2020). This study showed that capillary stalls predict long-term capillary loss.
Graff, B. J., Harrison, S. L., Payne, S. J. & El-Bouri, W. K. Regional cerebral blood flow changes in healthy ageing and Alzheimer’s disease: a narrative review. Cerebrovasc. Dis. 52, 11–20 (2023).
Leidhin, C. N. et al. Age-related normative changes in cerebral perfusion: data from the irish longitudinal study on ageing (TILDA). Neuroimage 229, 117741 (2021).
Leenders, K. L. et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 113, 27–47 (1990).
Chen, J. J., Rosas, H. D. & Salat, D. H. Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage 55, 468–478 (2011).
Deery, H. A. et al. The association of regional cerebral blood flow and glucose metabolism in normative ageing and insulin resistance. Sci. Rep. 14, 14574 (2024).
Dai, W. et al. Abnormal regional cerebral blood flow in cognitively normal elderly subjects with hypertension. Stroke 39, 349–354 (2008).
Mann, D. M., Eaves, N. R., Marcyniuk, B. & Yates, P. O. Quantitative changes in cerebral cortical microvasculature in ageing and dementia. Neurobiol. Aging 7, 321–330 (1986).
Bell, M. A. & Ball, M. J. Morphometric comparison of hippocampal microvasculature in ageing and demented people: diameters and densities. Acta Neuropathol. 53, 299–318 (1981).
Li, Y. et al. Aging-associated changes in cerebral vasculature and blood flow as determined by quantitative optical coherence tomography angiography. Neurobiol. Aging 70, 148–159 (2018).
Bennett, H. C. et al. Aging drives cerebrovascular network remodeling and functional changes in the mouse brain. Nat. Commun. 15, 6398 (2024).
Sun, Z., Li, C., Wisniewski, T. W., Haacke, E. M. & Ge, Y. In vivo detection of age-related tortuous cerebral small vessels using ferumoxytol-enhanced 7T MRI. Aging Dis. 15, 1913–1926 (2024).
Zhu, Y.-S., Tseng, B. Y., Shibata, S., Levine, B. D. & Zhang, R. Increases in cerebrovascular impedance in older adults. J. Appl. Physiol. 111, 376–381 (2011).
Franklin, S. S. et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 96, 308–315 (1997).
Hart, E. C., Joyner, M. J., Wallin, B. G. & Charkoudian, N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J. Physiol. 590, 2069–2079 (2012).
Mitchell, G. F. et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the age, gene/environment susceptibility-Reykjavik study. Brain 134, 3398–3407 (2011).
Scuteri, A. et al. Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J. Hypertens. 25, 1035–1040 (2007).
Thorin-Trescases, N. et al. Impact of pulse pressure on cerebrovascular events leading to age-related cognitive decline. Am. J. Physiol. Circ. Physiol. 314, H1214–H1224 (2018).
Verheggen, I. C. M. et al. Increase in blood–brain barrier leakage in healthy, older adults. GeroScience 42, 1183–1193 (2020).
Montagne, A. et al. Blood–brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302 (2015).
Lim, X. R. et al. Endothelial Piezo1 channel controls mechano-feedback control of brain blood flow. Nat. Commun. 15, 8686 (2024).
Tarumi, T., Shah, F., Tanaka, H. & Haley, A. P. Association between central elastic artery stiffness and cerebral perfusion in deep subcortical gray and white matter. Am. J. Hypertens. 24, 1108–1113 (2011).
Sorond, F. A. et al. Cerebrovascular hemodynamics, gait, and falls in an elderly population: MOBILIZE Boston study. Neurology 74, 1627–1633 (2010).
Zaletel, M., Strucl, M., Pretnar-Oblak, J. & Zvan, B. Age-related changes in the relationship between visual evoked potentials and visually evoked cerebral blood flow velocity response. Funct. Neurol. 20, 115–120 (2005).
Carey, B. J., Eames, P. J., Blake, M. J., Panerai, R. B. & Potter, J. F. Dynamic cerebral autoregulation is unaffected by aging. Stroke 31, 2895–2900 (2000).
Mehagnoul-Schipper, D. J., Vloet, L. C., Colier, W. N., Hoefnagels, W. H. & Jansen, R. W. Cerebral oxygenation declines in healthy elderly subjects in response to assuming the upright position. Stroke 31, 1615–1620 (2000).
Rosenberg, A. J. et al. Aging reduces cerebral blood flow regulation following an acute hypertensive stimulus. J. Appl. Physiol. 128, 1186–1195 (2020).
Xing, C.-Y. et al. Arterial pressure, heart rate, and cerebral hemodynamics across the adult life span. Hypertension 69, 712–720 (2017).
Toth, P. et al. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am. J. Physiol. Circ. Physiol. 305, H1698–H1708 (2013).
Toth, P. et al. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J. Cereb. Blood Flow. Metab. 34, 1887–1897 (2014).
Zhu, Y.-S. et al. Cerebral vasomotor reactivity during hypo- and hypercapnia in sedentary elderly and masters athletes. J. Cereb. Blood Flow. Metab. 3, 1190–1196 (2013).
Gupta, A. et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke 43, 2884–2891 (2012).
Silvestrini, M. et al. Cerebrovascular reactivity and cognitive decline in patients with Alzheimer disease. Stroke 37, 1010–1015 (2006).
Sam, K. et al. Cerebrovascular reactivity and white matter integrity. Neurology 87, 2333–2339 (2016).
Portegies, M. L. P., de Bruijn, R. F. A. G., Hofman, A., Koudstaal, P. J. & Ikram, M. A. Cerebral vasomotor reactivity and risk of mortality: the Rotterdam study. Stroke 45, 42–47 (2014).
Sorond, F. A., Hurwitz, S., Salat, D. H., Greve, D. N. & Fisher, N. D. L. Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology 81, 904–909 (2013).
Tarantini, S. et al. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J. Cereb. Blood Flow. Metab. 35, 1871–1881 (2015).
Tarantini, S. et al. Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. GeroScience 39, 601–614 (2017).
Park, L., Anrather, J., Girouard, H., Zhou, P. & Iadecola, C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J. Cereb. Blood Flow. Metab. 27, 1908–1918 (2007).
Stobart, J. L. L., Lu, L., Anderson, H. D. I., Mori, H. & Anderson, C. M. Astrocyte-induced cortical vasodilation is mediated by D-serine and endothelial nitric oxide synthase. Proc. Natl Acad. Sci. USA 110, 3149–3154 (2013).
Toth, P. et al. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am. J. Physiol. Heart Circ. Physiol. 306, H299–H308 (2014).
Tarantini, S. et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 24, 101192 (2019).
Csiszar, A., Yabluchanskiy, A., Ungvari, A., Ungvari, Z. & Tarantini, S. Overexpression of catalase targeted to mitochondria improves neurovascular coupling responses in aged mice. GeroScience 41, 609–617 (2019).
Toth, L. et al. Age-related decline in circulating IGF-1 associates with impaired neurovascular coupling responses in older adults. GeroScience 44, 2771–2783 (2022).
Toth, P. et al. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell 14, 1034–1044 (2015).
Tarantini, S. et al. Endothelial deficiency of insulin-like growth factor-1 receptor (IGF1R) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. GeroScience 43, 2387–2394 (2021).
Mathiesen, C., Brazhe, A., Thomsen, K. & Lauritzen, M. Spontaneous calcium waves in Bergman glia increase with age and hypoxia and may reduce tissue oxygen. J. Cereb. Blood Flow. Metab. 33, 161–169 (2013).
Zarate, S. M., Huntington, T. E., Bagher, P. & Srinivasan, R. Aging reduces calreticulin expression and alters spontaneous calcium signals in astrocytic endfeet of the mouse dorsolateral striatum. NPJ Aging 9, 5 (2023).
Ding, F. et al. Astrocytes exhibit diverse Ca2+ changes at subcellular domains during brain aging. Front. Aging Neurosci. 14, 1029533 (2022).
Senatorov, V. V. J. et al. Blood–brain barrier dysfunction in aging induces hyperactivation of TGFβ signaling and chronic yet reversible neural dysfunction. Sci. Transl. Med. 11, eaaw8283 (2019).
Bisht, K. et al. Capillary-associated microglia regulate vascular structure and function through PANX1-P2RY12 coupling in mice. Nat. Commun. 12, 5289 (2021).
Császár, E. et al. Microglia modulate blood flow, neurovascular coupling, and hypoperfusion via purinergic actions. J. Exp. Med. 219, e20211071 (2022).
Heneka, M. T. et al. Neuroinflammation in Alzheimer disease. Nat. Rev. Immunol. 25, 321–352 (2025).
Li, X. et al. Transcriptional and epigenetic decoding of the microglial aging process. Nat. Aging 3, 1288–1311 (2023).
Mattsson, N. et al. Association of brain amyloid-β with cerebral perfusion and structure in Alzheimer’s disease and mild cognitive impairment. Brain 137, 1550–1561 (2024). This study reported that Aβ pathology is associated with reduced CBF early in disease and with brain atrophy in later stages, suggesting reduced CBF as an early driver of pathology and a target for preventing disease progression.
Wierenga, C. E., Hays, C. C. & Zlatar, Z. Z. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. J. Alz. Dis. 42, S411–S419 (2014).
Marshall, R. S. et al. Recovery of brain function during induced cerebral hypoperfusion. Brain 124, 1208–1217 (2001).
Wang, X. et al. Cerebrovascular hypoperfusion induces spatial memory impairment, synaptic changes, and amyloid-β oligomerization in rats. J. Alz. Dis. 21, 813–822 (2010).
Aamand, R. et al. Cerebral microvascular changes in healthy carriers of the APOE-ɛ4 Alzheimer’s disease risk gene. Proc. Natl Acad. Sci. USA Nexus 3, pgae369 (2016).
Suri, S. et al. Reduced cerebrovascular reactivity in young adults carrying the APOE ε4 allele. Alzheimers Dement. 11, 648–657 (2015).
Michels, L. et al. Arterial spin labeling imaging reveals widespread and Aβ-independent reductions in cerebral blood flow in elderly apolipoprotein epsilon-4 carriers. J. Cereb. Blood Flow. Metab. 36, 581–595 (2016).
Madsen, L. S. et al. Capillary dysfunction in healthy elderly APOE ε4 carriers with raised brain Aβ deposition. Alzheimers Dement. 20, 459–471 (2024).
Montagne, A. et al. APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature 581, 71–76 (2020). Human ApoE4 carriers show BBB breakdown in hippocampus, and CSF levels of the pericyte protein PDGFRβ predict future cognitive decline.
Sivanandam, T. M. & Thakur, M. K. Traumatic brain injury: a risk factor for Alzheimer’s disease. Neurosci. Biobehav. Rev. 36, 1376–1381 (2012).
Pacholko, A. & Iadecola, C. Hypertension, neurodegeneration, and cognitive decline. Hypertension 81, 991–1007 (2024).
Bachmann, D. et al. Hypertension and cerebral blood flow in the development of Alzheimer’s disease. Alzheimer’s Dement. 20, 7729–7744 (2024).
Cifuentes, D. et al. Hypertension accelerates the progression of Alzheimer-like pathology in a mouse model of the disease. Hypertension 65, 218–224 (2015).
Thalman, S. et al. A preliminary study of cerebral blood flow, aging and dementia in people with Down syndrome. J. Intellect. Disabil. Res. 64, 934–945 (2020).
Madsen, L. S. et al. Capillary function progressively deteriorates in prodromal Alzheimer’s disease: a longitudinal MRI perfusion study. Aging Brain 2, 100035 (2022).
Madsen, L. S. et al. Capillary dysfunction correlates with cortical amyloid load in early Alzheimer’s disease. Neurobiol. Aging 123, 1–9 (2023).
Zhang, X. et al. Hypoxia-inducible factor 1α (HIF-1α)-mediated hypoxia increases BACE1 expression and β-amyloid generation. J. Biol. Chem. 282, 10873–10880 (2007).
Eskildsen, S. F. et al. Increased cortical capillary transit time heterogeneity in Alzheimer’s disease: a DSC-MRI perfusion study. Neurobiol. Aging 50, 107–118 (2017). In addition to reduced CBF in people with AD, this study identified areas of increased capillary transit time heterogeneity, conceivably evoked by variable pericyte-mediated constriction, that correlated with white matter hyperintensities and severity of cognitive impairment.
Gould, I. G., Tsai, P., Kleinfeld, D. & linninger, A. The capillary bed offers the largest hemodynamic resistance to the cortical blood supply. J. Cereb. Blood Flow. Metab. 37, 52–68 (2017). Using microscopy data and computational modelling, capillaries were shown to confer the bulk of the resistance in the vascular bed, suggesting that pericyte-mediated constriction of capillaries may have a large effect on CBF.
Ruiz-Uribe, N. E. et al. Vascular oxidative stress causes neutrophil arrest in brain capillaries, leading to decreased cerebral blood flow and contributing to memory impairment in a mouse model of Alzheimer’s disease. Preprint at bioRxiv https://doi.org/10.1101/2023.02.15.528710 (2023).
Park, L. et al. Brain perivascular macrophages initiate the neurovascular dysfunction of Alzheimer Aβ peptides. Circ. Res. 121, 258–269 (2017).
Uekawa, K. et al. Border-associated macrophages promote cerebral amyloid angiopathy and cognitive impairment through vascular oxidative stress. Mol. Neurodegener. 18, 73 (2024).
Anfray, A. et al. A cell-autonomous role for border-associated macrophages in ApoE4 neurovascular dysfunction and susceptibility to white matter injury. Nat. Neurosci. 27, 2138–2151 (2024).
Park, L. et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc. Natl Acad. Sci. USA 105, 1347–1352 (2008).
Palmer, J. C., Baig, S., Kehoe, P. G. & Love, S. Endothelin-converting enzyme-2 is increased in Alzheimer’s disease and up-regulated by Aβ. Am. J. Pathol. 175, 262–270 (2009).
Korte, N. et al. The Ca2+-gated channel TMEM16A amplifies capillary pericyte contraction and reduces cerebral blood flow after ischemia. J. Clin. Invest. 132, e154118 (2022).
Mughal, A. et al. Pathogenic soluble tau peptide disrupts endothelial calcium signaling and vasodilation in the brain microvasculature. Proc. Natl Acad. Sci. USA 44, 680–688 (2024).
Visser, D. et al. Tau pathology as a determinant of changes in atrophy and cerebral blood flow: a multi-modal longitudinal imaging study. Eur. J. Nucl. Med. Mol. Imaging 50, 2409–2419 (2023).
Rubinski, A. et al. Lower cerebral perfusion is associated with tau-PET in the entorhinal cortex across the Alzheimer’s continuum. Neurobiol. Aging 102, 111–118 (2021).
Davis, H. & Attwell, D. A tight squeeze: how do we make sense of small changes in microvascular diameter? J. Phys. 601, 2263–2272 (2023).
Gutiérrez-Jiménez, E. et al. Effect of electrical forepaw stimulation on capillary transit-time heterogeneity (CTH). J. Cereb. Blood Flow. Metab. 36, 2072–2086 (2016).
Secomb, T. W. & Pries, A. R. Blood viscosity in microvessels: experiment and theory. C. R. Phys. 14, 470–478 (2013).
Reeson, P., Schager, B., Van Sprengel, M. & Brown, C. E. Behavioral and neural activity-dependent recanalization of plugged capillaries in the brain of adult and aged mice. Front. Cell. Neurosci. 16, 876746 (2022).
Cruz Hernández, J. C. et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat. Neurosci. 22, 413–420 (2019). This study discovered that part of the CBF reduction in AD mice is caused by neutrophils adhering to capillary walls and blocking blood flow, suggesting that preventing neutrophil adhesion may improve CBF in AD.
Yoon, J. H. et al. Increased capillary stalling is associated with endothelial glycocalyx loss in subcortical vascular dementia. J. Cereb. Blood Flow. Metab. 42, 1383–1397 (2022).
Frohman, E. M., Frohman, T. C., Gupta, S., de Fougerolles, A. & van den Noort, S. Expression of intercellular adhesion molecule 1 (ICAM-1) in Alzheimer’s disease. J. Neurol. Sci. 106, 105–111 (1991).
Mestas, J. & Hughes, C. C. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
Procter, T. V., Williams, A. & Montagne, A. Interplay between brain pericytes and endothelial cells in dementia. Am. J. Pathol. 191, 1917–1931 (2021).
Zenaro, E. et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 21, 880–886 (2015).
Bracko, O. et al. Increasing cerebral blood flow improves cognition into late stages in Alzheimer’s disease mice. J. Cereb. Blood Flow. Metab. 40, 1441–1452 (2020).
Buée, L. et al. Pathological alterations of the cerebral microvasculature in Alzheimer’s disease and related dementing disorders. Acta Neuropathol. 87, 469–480 (1994).
Stefanova, N. A., Maksimova, K. Y., Rudnitskaya, E. A., Muraleva, N. A. & Kolosova, N. G. Association of cerebrovascular dysfunction with the development of Alzheimer’s disease-like pathology in OXYS rats. BMC Genom. 19, 75 (2018).
Berthiaume, A. A. et al. Pericyte remodeling is deficient in the aged brain and contributes to impaired capillary flow and structure. Nat. Commun. 13, 5912 (2022). This study shows that pericyte and capillary remodelling after injury are slower in the ageing brain, suggesting a mechanism contributing to CBF decrease with age.
Hampel, H. et al. The β-secretase BACE1 in Alzheimer’s disease. Biol. Psychiatry 89, 745–756 (2021).
Gupta, A. & Iadecola, C. Impaired Aβ clearance: a potential link between atherosclerosis and Alzheimer’s disease. Front. Aging Neurosci. 7, 115 (2015).
Shibata, M. et al. Clearance of Alzheimer’s amyloid-β1-40 peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J. Clin. Invest. 106, 1489–1499 (2000).
Aldea, R., Weller, R. O., Wilcock, D. M., Carare, R. O. & Richardson, G. Cerebrovascular smooth muscle cells as the drivers of intramural periarterial drainage of the brain. Front. Aging Neurosci. 11, 1 (2019).
van Veluw, S. J. et al. Vasomotion as a driving force for paravascular clearance in the awake mouse brain. Neuron 105, 549–561 (2020). This study shows that clearance of extracellular protein is driven by spontaneous vasomotion, suggesting that promoting vasomotion may aid Aβ clearance.
Viswanathan, A. & Greenberg, S. M. Cerebral amyloid angiopathy in the elderly. Ann. Neurol. 70, 871–880 (2011).
McGowan, E. et al. Aβ42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron 47, 191–199 (2005).
Kakuda, N. et al. Distinct deposition of amyloid® species in brains with Alzheimer’s disease pathology visualized with MALDI imaging mass spectrometry. Acta Neuropathol. Commun. 5, 73 (2017).
Gkanatsiou, E. et al. A distinct brain beta amyloid signature in cerebral amyloid angiopathy compared to Alzheimer’s disease. Neurosci. Lett. 701, 125–131 (2019).
Long, J. M. & Holtzman, D. M. Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179, 312–339 (2019).
Crary, J. F. et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 128, 755–766 (2014).
Park, J. H. et al. The effect of chronic cerebral hypoperfusion on the pathology of Alzheimer’s disease: a positron emission tomography study in rats. Sci. Rep. 9, 14102 (2019).
Halliday, M. R. et al. Accelerated pericyte degeneration and blood–brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow. Metab. 36, 216–227 (2016).
van de Haar, H. J. et al. Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging. Neurobiol. Aging 45, 190–196 (2016).
Fernández-Klett, F. et al. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J. Cereb. Blood Flow. Metab. 33, 428–439 (2013).
Hall, C. N. et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55–60 (2014). This study demonstrates that capillary pericytes actively regulate cerebral blood flow, fundamentally shifting our understanding of brain microcirculation, and shows that brief ischaemia can trigger pericyte-mediated capillary constriction that leads to a prolonged decrease of CBF.
Zille, M. et al. The impact of endothelial cell death in the brain and its role after stroke: a systematic review. Cell Stress. 3, 330–347 (2019).
Wilhelmus, M. M. et al. Lipoprotein receptor-related protein-1 mediates amyloid-beta-mediated cell death of cerebrovascular cells. Am. J. Pathol. 171, 1989–1999 (2007).
Rushworth, G. F. & Megson, I. L. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 141, 150–159 (2014).
Lovell, M. A. et al. Calcium channel blockers, progression to dementia, and effects on amyloid beta peptide production. Oxid. Med. Cell. Longev. 2015, 787805 (2015).
Wu, C. L. & Wen, S. H. A 10-year follow-up study of the association between calcium channel blocker use and the risk of dementia in elderly hypertensive patients. Medicine 95, e4593 (2016).
Law, C. S. & Yeong, K. Y. Repurposing antihypertensive drugs for the management of Alzheimer’s disease. Curr. Med. Chem. 28, 1716–1730 (2021).
Paris, D. et al. Nilvadipine antagonizes both Aβ vasoactivity in isolated arteries, and the reduced cerebral blood flow in APPsw transgenic mice. Brain Res. 999, 53–61 (2004).
de Jong, D. L. et al. Effects of nilvadipine on cerebral blood flow in patients with Alzheimer disease: a randomized trial. Hypertension 74, 413–420 (2019).
Abdullah, L. et al. The influence of baseline Alzheimer’s disease severity on cognitive decline and CSF biomarkers in the NILVAD trial. Front. Neurol. 11, 149 (2020). This study suggests that calcium channel blockers may be most effective at slowing cognitive decline early in Alzheimer disease.
Špiranec, K. et al. Endothelial C-type natriuretic peptide acts on pericytes to regulate microcirculatory flow and blood pressure. Circulation 138, 494–508 (2018).
Ammendola, A., Geiselhoringer, A., Hofmann, F. & Schlossmann, J. Molecular determinants of the interaction between the inositol 1,4,5-trisphosphate receptor-associated cGMP kinase substrate (IRAG) and cGMP kinase Ibeta. J. Biol. Chem. 276, 24153–24159 (2001).
Sheng, M. et al. Sildenafil improves vascular and metabolic function in patients with Alzheimer’s disease. J. Alz. Dis. 60, 1351–1364 (2017).
Gohel, D. et al. Sildenafil as a candidate drug for Alzheimer’s disease: real-world patient data observation and mechanistic observations from patient-induced pluripotent stem cell-derived neurons. J. Alz. Dis. 98, 643–657 (2024).
Adesuyan, M. et al. Phosphodiesterase type 5 inhibitors in men with erectile dysfunction and the risk of Alzheimer’s disease. Neurology 102, e209131 (2024).
Cortes-Canteli, M. et al. Long-term dabigatran treatment delays Alzheimer’s disease pathogenesis in the TgCRND8 mouse model. J. Am. Coll. Cardiol. 74, 1910–1923 (2019).
Friberg, L. & Rosenqvist, M. Less dementia with oral anticoagulation in atrial fibrillation. Eur. Heart J. 39, 453–460 (2018).
Iadecola, C. et al. Vascular cognitive impairment and dementia: JACC scientific expert panel. J. Am. Coll. Cardiol. 73, 3326–3344 (2019).
Dichgans, M. & Leys, D. Vascular cognitive impairment. Circ. Res. 120, 573–591 (2017).
ter Telgte, A. et al. Cerebral small vessel disease — from a focal to a global perspective. Nat. Rev. Neurol. 14, 387–398 (2018).
Wardlaw, J. M., Smith, C. & Dichgans, M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 18, 684–696 (2019).
Duncombe, J. et al. Chronic cerebral hypoperfusion: a key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin. Sci. 131, 2451–2468 (2017).
Koizumi, K. et al. Apoε4 disrupts neurovascular regulation and undermines white matter integrity and cognitive function. Nat. Commun. 9, 3816 (2018). Introducing human Apoε4 into mice lowers CBF and leads to increased white matter damage when blood flow is reduced further experimentally.
Stewart, C. R., Stringer, M. S., Shi, Y., Thrippleton, M. J. & Wardlaw, J. M. Associations between white matter hyperintensity burden, cerebral blood flow and transit time in small vessel disease: an updated meta-analysis. Front. Neurol. 12, 647848 (2021). This study demonstrates that greater white matter hyperintensity burden in SVD is linked to lower cerebral blood flow, highlighting impaired perfusion as a key disease feature and potential target for intervention.
Shi, Y. et al. Small vessel disease is associated with altered cerebrovascular pulsatility but not resting cerebral blood flow. J. Cereb. Blood Flow. Metab. 40, 85–99 (2020).
Gottesmann, R. F., Egle, M., Groechel, R. C. & Mughal, A. Blood pressure and the brain: the conundrum of hypertension and dementia. Cardiovasc. Res. 120, 2360–2372 (2025).
Muller, M. et al. Hypertension and longitudinal changes in cerebral blood flow: the SMART-MR study. Ann. Neurol. 71, 825–833 (2012).
Gottesman, R. F. et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 71, 1218–1227 (2014).
Leopold, I. H., Kety, S. S., Jeffers, W. A., Hafkenshiel, J. H. & Shenkin, H. A. Correlation of the cerebrovascular resistance and the grade of hypertensive retinal findings. Am. J. Ophthalmol. 32, 365–368 (1949).
Greenberg, S. M. Cerebral amyloid angiopathy and vessel dysfunction. Cerebrovasc. Dis. 13, 42–47 (2002).
Chabriat, H. et al. Cerebral hemodynamics in CADASIL before and after acetazolamide challenge assessed with MRI bolus tracking. Stroke 31, 1904–1912 (2000).
Tuominen, S. et al. Positron emission tomography examination of cerebral blood flow and glucose metabolism in young CADASIL patients. Stroke 35, 1063–1067 (2004).
Wang, R., Zhang, J., Shang, J., Wang, F. & Yan, X. Effects of different regional cerebral blood flow on white matter hyperintensity in CADASIL patients. J. Biomed. Res. 36, 368–374 (2022).
Chabriat, H., Joutel, A., Dichgans, M., Tournier-Lasserve, E. & Bousser, M. G. Cadasil. Lancet Neurol. 8, 643–653 (2009).
Fouillade, C., Monet-Leprêtre, M., Baron-Menguy, C. & Joutel, A. Notch signalling in smooth muscle cells during development and disease. Cardiovasc. Res. 95, 138–146 (2012).
Joutel, A. et al. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J. Clin. Invest. 105, 597–605 (2000).
Dupré, N. et al. Protein aggregates containing wild-type and mutant NOTCH3 are major drivers of arterial pathology in CADASIL. J. Clin. Invest. 134, e175789 (2024). This study identifies deposition of the extracellular domain of NOTCH3 as a mechanism to target therapeutically in CADASIL.
Ruchoux, M. M., Kalaria, R. N. & Román, G. C. The pericyte: a critical cell in the pathogenesis of CADASIL. Cereb. Circ. Cogn. Behav. 2, 100031 (2021).
Yamamoto, Y. et al. Brain microvascular accumulation and distribution of the NOTCH3 ectodomain and granular osmiophilic material in CADASIL. J. Neuropathol. Exp. Neurol. 72, 416–431 (2013).
Joutel, A. et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J. Clin. Invest. 120, 433–445 (2010).
Masuda, T. et al. Specification of CNS macrophage subsets occurs postnatally in defined niches. Nature 604, 740–748 (2022).
Van Hove, H. et al. Interleukin-34-dependent perivascular macrophages promote vascular function in the brain. Immunity 58, 1289–1305.e8 (2025).
Shrouder, J. J. et al. Continued dysfunction of capillary pericytes promotes no-reflow after experimental stroke in vivo. Brain 147, 1057–1074 (2024).
Smith, E. E., Schneider, J. A., Wardlaw, J. M. & Greenberg, S. M. Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 11, 272–282 (2012).
Soontornniyomkij, V. et al. Cerebral microinfarcts associated with severe cerebral β‐amyloid angiopathy. Brain Pathol. 20, 459–467 (2010).
Shih, A. Y. et al. The smallest stroke: occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nat. Neurosci. 16, 55–63 (2013). This study demonstrates in mice that microinfarcts can contribute to cognitive decline, by occluding blood flow through a single arteriole.
Schmid, F., Conti, G., Jenny, P. & Weber, B. The severity of microstrokes depends on local vascular topology and baseline perfusion. eLife 10, e60208 (2021).
Akoudad, S. et al. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 73, 934–943 (2016).
Greenberg, S. M. et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 8, 165–174 (2009).
Holland, P. R. et al. Gliovascular disruption and cognitive deficits in a mouse model with features of small vessel disease. J. Cereb. Blood Flow. Metab. 35, 1005–1014 (2015).
Sokoloff, L. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure,and normal values in the conscious and anesthetized albino rat. J. Neurochem. 28, 897–916 (1977).
Harris, J. J. & Attwell, D. The energetics of CNS white matter. J. Neurosci. 32, 356–371 (2012).
Cavaglia, M. et al. Regional variation in brain capillary density and vascular response to ischemia. Brain Res. 910, 81–93 (2001).
Vavilala, M. S., Lee, L. A. & Lam, A. M. Cerebral blood flow and vascular physiology. Anesthesiol. Clin. North. Am. 20, 247–264 (2002).
Stamenkovic, S. et al. Impaired capillary-venous drainage contributes to gliosis and demyelination in white matter during aging. Preprint at bioRxiv https://doi.org/10.1101/2024.02.11.579849 (2025).
Li, B. et al. Two-photon microscopic imaging of capillary red blood cell flux in mouse brain reveals vulnerability of cerebral white matter to hypoperfusion. J. Cereb. Blood Flow. Metab. 40, 501–512 (2020).
Micu, I. et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature 439, 988–992 (2005).
Nave, K. Myelination and the trophic support of long axons. Nat. Rev. Neurosci. 11, 275–283 (2010).
Huang, S. et al. New insights into the roles of oligodendrocytes regulation in ischemic stroke recovery. Neurobiol. Dis. 184, 106200 (2023).
Hamilton, N. B., Kolodziejczyk, K., Kougioumtzidou, E. & Attwell, D. Proton-gated Ca2+-permeable TRP channels damage myelin in conditions mimicking ischaemia. Nature 529, 523–527 (2016).
Choi, B. R. et al. Characterization of white matter injury in a rat model of chronic cerebral hypoperfusion. Stroke 47, 542–547 (2016).
Holland, P. R. et al. MRI is a sensitive marker of subtle white matter pathology in hypoperfused mice. Neurobiol. Aging 32, 2325.e1–2325.e6 (2011).
Bouhrara, M. et al. Association of cerebral blood flow with longitudinal changes in cerebral microstructural integrity in the coronary artery risk development in young adults (CARDIA) study. JAMA Netw. Open. 5, e2231189–e2231189 (2022). This study demonstrates that reduced CBF in healthy young adults is associated with faster decline in white and grey matter microstructural integrity, highlighting the impact of chronic reduction of CBF on brain health.
Bouhrara, M. et al. Association of cerebral blood flow with myelin content in cognitively unimpaired adults. BMJ Neurol. Open. 2, e000053 (2020).
Chen, J. J., Rosas, H. D. & Salat, D. H. The relationship between cortical blood flow and sub-cortical white-matter health across the adult age span. PLoS ONE 8, e56733 (2013).
Han, X. et al. Predicting white matter hyperintensity progression and cognitive decline in patients with cerebral smal vessel disease: a magnetic resonance-based habitat analysis. Quant. Imaging Med. Surg. 14, 6621–6634 (2024).
Van Dalen, J. W. et al. White matter hyperintensity volume and cerebral perfusion in older individuals with hypertension using arterial spin-labeling. AJNR Am. J. Neuroradiol. 37, 1824–1830 (2016).
Prins, N. D. & Scheltens, P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat. Rev. Neurol. 11, 157–165 (2015).
Lin, M. et al. Longitudinal changes in white matter free water in cerebral small vessel disease: relationship to cerebral blood flow and white matter fiber alterations. J. Cereb. Blood Flow. Metab. 45, 932–944 (2025).
Park, M., Moon, Y., Han, S., Kim, H. K. & Moon, W. Myelin loss in white matter hyperintensities and normal-appearing white matter of cognitively impaired patients: a quantitative synthetic magnetic resonance imaging study. Eur. Radiol. 29, 4914–4921 (2019).
Holland, C. M. et al. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke 39, 1127–1133 (2008).
Hu, H. Y. et al. White matter hyperintensities and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 36 prospective studies. Neurosci. Biobehav. Rev. 120, 16–27 (2021).
Biesbroek, J. M., Weaver, N. A. & Biessels, G. J. Lesion location and cognitive impact of cerebral small vessel disease. Clin. Sci. 131, 715–728 (2017).
Van der Veen, P. H. et al. Longitudinal relationship between cerebral small-vessel disease and cerebral blood flow: the second manifestations of arterial disease-magnetic resonance study. Stroke 465, 1233–1238 (2015).
Kremer, R., Williams, A. & Wardlaw, J. Endothelial cells as key players in cerebral small vessel disease. Nat. Rev. Neurosci. 26, 179–188 (2025).
Attwell, D. et al. Glial and neuronal control of brain blood flow. Nature 468, 232–243 (2010).
Hashimoto, A., Miyakoda, G., Hirose, Y. & Mori, T. Activation of endothelial nitric oxide synthase by cilostazol via a cAMP/protein kinase A- and phosphatidylinositol 3-kinase/Akt-dependent mechanism. Atherosclerosis 189, 350–357 (2006).
Matsumoto, S., Ohama, R., Hoei, T., Tojo, R. & Nakamura, T. Two cases showing that cilostazol administration leads to an increase in cerebral blood flow and has a positive effect on rehabilitation. Cureus 16, e56376 (2024).
Webb, A. J. S. et al. Cerebrovascular effects of sildenafil in small vessel disease: the OxHARP trial. J. Circ. Res. 135, 320–331 (2024).
Maki, T. et al. Angiogenic and vasoprotective effects of adrenomedullin on prevention of cognitive decline after chronic cerebral hypoperfusion in mice. Stroke 42, 1122–1128 (2011).
Washida, K. et al. A multicenter, single-arm, phase II clinical trial of adrenomedullin in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Cereb. Circ. Cogn. Behav. 6, 100211 (2024).
Tryambake, D. et al. Intensive blood pressure lowering increases cerebral blood flow in older subjects with hypertension. Hypertension 61, 1309–1315 (2013).
Kopczak, A. et al. (2023). Effect of blood pressure-lowering agents on microvascular function in people with small vessel diseases (TREAT-SVDs): a multicentre, open-label, randomised, crossover trial. Lancet Neurol. 22, 991–1004 (2023).
Binder, N. F. et al. Leptomeningeal collaterals regulate reperfusion in ischemic stroke and rescue the brain from futile recanalization. Neuron 112, 1456–1472 (2024).
Schaeffer, S. & Iadecola, C. Revisiting the vascular unit. Nat. Neurosci. 24, 1198–1209 (2021).
Rajani, R. M. et al. Selective suppression of oligodendrocyte-derived amyloid beta rescues neuronal dysfunction in Alzheimer’s disease. PLoS Biol. 22, e3002727 (2024). This study demonstrates that oligodendrocytes contribute to Aβ production and neuronal dysfunction in AD.
Sasmita, A. O. et al. Oligodendrocytes produce amyloid-β and contribute to plaque formation alongside neurons in Alzheimer’s disease model mice. Nat. Neurosci. 27, 1668–1674 (2024). This study demonstrates that oligodendrocytes contribute to Aβ production in AD.
Hartmann, D. A., Coelho-Santos, V. & Shih, A. Pericyte control of blood flow across microvascular zones in the central nervous system. Ann. Rev. Physiol. 84, 331–354 (2022).
Sweeney, M. D., Ayyadurai, S. & Zlokovic, B. V. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci. 19, 771–783 (2016).
Durham, J. T., Surks, H. K., Dulmovits, B. M. & Herman, I. M. Pericyte contractility controls endothelial cell cycle progression and sprouting: insights into angiogenic switch mechanics. Am. J. Physiol. Cell Physiol. 307, C878–C892 (2014).
Zhu, H. Y., Hong, F. F. & Yang, S. L. The roles of nitric oxide synthase/nitric oxide pathway in the pathology of vascular dementia and related therapeutic approaches. Int. J. Mol. Sc. 22, 4540 (2021).
Debette, S. & Marcus, H. S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 341, c3666 (2010).
Knowles, J. K. et al. Adaptive and maladaptive myelination in health and disease. Nat. Rev. Neurol. 18, 735–746 (2022).
Attwell, D., Mishra, A., Hall, C. N., O’Farrell, F. M. & Dalkara, T. What is a pericyte? J. Cereb. Blood Flow. Metab. 36, 451–455 (2016).
Filosa, J. A. et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat. Neurosci. 9, 1397–1403 (2006).
Pereira da Silva, E. A., Baudel, M. M., Navedo, M. F. & Nieves-Cintron, M. Ion channel molecular complexes in vascular smooth muscle. Front. Physiol. 13, 999369 (2022).
Ferris, H. R., Jeffrey, D. A., Birnbaumer, L., Zheng, F. & Dabertrand, F. Increased luminal pressure in brain capillaries drives TRPC3-dependent depolarization and constriction of transitional pericytes. Sci. Signaling 18, ads1903 (2025). This study demonstrates CBF autoregulation at the capillary level, mediated by TRP channels.
Chen, X. et al. Ischemia-reperfusion impairs blood–brain barrier function and alters tight junction protein expression in the ovine fetus. Neuroscience 226, 89–100 (2012).
Zhang, X. et al. Glial growth factor 2 treatment alleviates ischemia and reperfusion-damaged integrity of the blood-brain barrier through decreasing Mfsd2a/caveolin-1-mediated transcellular and Pdlim5/YAP/TAZ-mediated paracellular permeability. Acta Pharm. Sin. 45, 2241–2252 (2024).
Nadal, A., Fuentes, E., Pastor, J. & McNaughton, P. A. Plasma albumin induces calcium waves in rat cortical astrocytes. Glia 19, 343–351 (1997).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks Craig Brown, Anna Csiszar and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Actomyosin
-
The myosin and actin filaments that generate contraction within a muscle cell.
- Arterioles
-
Larger blood vessels ringed by smooth muscle cells that feed blood to capillaries.
- Capillaries
-
The smallest blood vessels, where nutrient and gas exchange occurs with the tissue.
- Cell adhesion molecules
-
Molecules that join cells together, including endothelial cells.
- Cerebrovascular resistance
-
The ratio of pressure to blood flow across a brain vascular bed.
- Monocytes
-
An immune cell type in the blood, the largest type of white blood cell.
- Neutrophils
-
A type of immune cell in the blood that engulfs and destroys bacteria.
- Pressure autoregulation
-
Reflex contraction (or relaxation) of contractile cells evoked when the blood pressure rises (or falls) that tends to keep the downstream pressure constant.
- Reactive oxygen species
-
(ROS). Reactive molecules formed by immune cells to kill bacteria or by mitochondria when energy supply is limited that can damage lipids and proteins.
- Transit time
-
The time taken for blood to pass, for example, from the start to the end of the capillary bed.
- White matter hyperintensities
-
White areas on magnetic resonance images of the brain that may indicate BBB leakage or fluid accumulation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anderle, S., Dixon, M., Quintela-Lopez, T. et al. The vascular contribution to cognitive decline in ageing and dementia. Nat. Rev. Neurosci. 26, 591–606 (2025). https://doi.org/10.1038/s41583-025-00950-1
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41583-025-00950-1
This article is cited by
-
Dysfunctional respiration as a risk factor for Alzheimer disease: a hypothesis
Metabolic Brain Disease (2026)