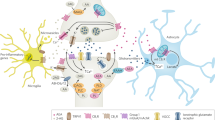
Abstract
Anxiety and stress-related psychiatric disorders are highly prevalent, have uncertain aetiologies and are only partially responsive to available therapies. Elucidating fundamental mechanisms that regulate anxiety, fear and stress responsivity could reveal new insights into disease mechanisms and offer opportunities for therapeutic development. Endocannabinoid (eCB) signalling has been shown to modulate innate avoidance behaviour, conditioned defensive behaviour and responsivity to stress in preclinical and human experimental studies. Furthermore, recent studies utilizing eCB biosensors, intersectional genetics and optogenetic-assisted circuit mapping have identified synaptic, cellular and circuit-level mechanisms by which eCBs affect these biobehavioural processes. These data suggest that eCB-deficient states could represent a stress-susceptibility endophenotype while pharmacological eCB augmentation could represent emerging approaches for the treatment of affective and stress-related neuropsychiatric disorders. In addition, several cortical–cortical and cortical–subcortical circuits have been identified as key substrates by which eCB signalling affects avoidance behaviour and stress responsivity. Taken together, the reviewed data offer new insights into the potential contribution of eCB signalling systems to the pathophysiology of anxiety and stress-related disorders and reveal fundamental roles for eCB signalling in the modulation of anxiety, fear and stress responsivity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Steel, Z. et al. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int. J. Epidemiol. 43, 476–493 (2014).
GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet 390, 1211–1259 (2017).
Gold, P. W. The organization of the stress system and its dysregulation in depressive illness. Mol. Psychiatry 20, 32–47 (2015).
Heim, C. & Nemeroff, C. B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry 49, 1023–1039 (2001).
Nemeroff, C. B. The preeminent role of early untoward experience on vulnerability to major psychiatric disorders: the nature-nurture controversy revisited and soon to be resolved. Mol. Psychiatry 4, 106–108 (1999).
Tafet, G. E. & Nemeroff, C. B. The links between stress and depression: psychoneuroendocrinological, genetic, and environmental interactions. J. Neuropsychiatry Clin. Neurosci. 28, 77–88 (2016).
Yehuda, R., Halligan, S. L. & Grossman, R. Childhood trauma and risk for PTSD: relationship to intergenerational effects of trauma, parental PTSD, and cortisol excretion. Dev. Psychopathol. 13, 733–753 (2001).
Nelson, C. A. et al. Adversity in childhood is linked to mental and physical health throughout life. BMJ 371, m3048 (2020).
Kumsta, R. The role of stress in the biological embedding of experience. Psychoneuroendocrinology 156, 106364 (2023).
Gonzalez, A. The impact of childhood maltreatment on biological systems: implications for clinical interventions. Paediatr. Child Health 18, 415–418 (2013).
Craske, M. G. et al. Anxiety disorders. Nat. Rev. Dis. Prim. 3, 17024 (2017).
Loerinc, A. G. et al. Response rates for CBT for anxiety disorders: need for standardized criteria. Clin. Psychol. Rev. 42, 72–82 (2015).
Scholten, W. D., Batelaan, N. M., van Oppen, P., Smit, J. H. & van Balkom, A. J. Discontinuation of antidepressants in remitted anxiety disorder patients: the need for strategies to prevent relapse. Psychother. Psychosom. 82, 399–400 (2013).
Lorimer, B., Kellett, S., Nye, A. & Delgadillo, J. Predictors of relapse and recurrence following cognitive behavioural therapy for anxiety-related disorders: a systematic review. Cogn. Behav. Ther. 50, 1–18 (2021).
Kano, M., Ohno-Shosaku, T., Hashimotodani, Y., Uchigashima, M. & Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 89, 309–380 (2009).
Crocq, M. A. History of cannabis and the endocannabinoid system. Dialogues Clin. Neurosci. 22, 223–228 (2020).
Ohno-Shosaku, T. & Kano, M. Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr. Opin. Neurobiol. 29, 1–8 (2014).
Castillo, P. E., Younts, T. J., Chavez, A. E. & Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 76, 70–81 (2012).
Araque, A., Castillo, P. E., Manzoni, O. J. & Tonini, R. Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology 124, 13–24 (2017).
Augustin, S. M. & Lovinger, D. M. Functional relevance of endocannabinoid-dependent synaptic plasticity in the central nervous system. ACS Chem. Neurosci. 9, 2146–2161 (2018).
Kwee, C. M. B. et al. Anxiolytic effects of endocannabinoid enhancing compounds: a systematic review and meta-analysis. Eur. Neuropsychopharmacol. 72, 79–94 (2023).
Patel, S., Hill, M. N., Cheer, J. F., Wotjak, C. T. & Holmes, A. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci. Biobehav. Rev. 76, 56–66 (2017).
Hill, M. N., Campolongo, P., Yehuda, R. & Patel, S. Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology 43, 80–102 (2018).
Piomelli, D. et al. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597). CNS Drug Rev. 12, 21–38 (2006).
Mangieri, R. A. & Piomelli, D. Enhancement of endocannabinoid signaling and the pharmacotherapy of depression. Pharmacol. Res. 56, 360–366 (2007).
Korem, N., Zer-Aviv, T. M., Ganon-Elazar, E., Abush, H. & Akirav, I. Targeting the endocannabinoid system to treat anxiety-related disorders. J. Basic Clin. Physiol. Pharmacol. 27, 193–202 (2016).
Lu, H. C. & Mackie, K. Review of the endocannabinoid system. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 6, 607–615 (2021).
Katona, I. & Freund, T. F. Multiple functions of endocannabinoid signaling in the brain. Annu. Rev. Neurosci. 35, 529–558 (2012).
Cadas, H., Gaillet, S., Beltramo, M., Venance, L. & Piomelli, D. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J. Neurosci. 16, 3934–3942 (1996).
Cadas, H., di Tomaso, E. & Piomelli, D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J. Neurosci. 17, 1226–1242 (1997).
Di Marzo, V. et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372, 686–691 (1994).
Hussain, Z., Uyama, T., Tsuboi, K. & Ueda, N. Mammalian enzymes responsible for the biosynthesis of N-acylethanolamines. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1862, 1546–1561 (2017).
Tsuboi, K. et al. Enzymatic formation of N-acylethanolamines from N-acylethanolamine plasmalogen through N-acylphosphatidylethanolamine-hydrolyzing phospholipase D-dependent and -independent pathways. Biochim. Biophys. Acta 1811, 565–577 (2011).
Mock, E. D. et al. Discovery of a NAPE-PLD inhibitor that modulates emotional behavior in mice. Nat. Chem. Biol. 16, 667–675 (2020). Study demonstrating that pharmacological inhibition of AEA production impairs fear extinction and increases stress hormone release.
Leung, D., Saghatelian, A., Simon, G. M. & Cravatt, B. F. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry 45, 4720–4726 (2006).
Liu, J. et al. A biosynthetic pathway for anandamide. Proc. Natl Acad. Sci. USA 103, 13345–13350 (2006).
Bisogno, T. et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 163, 463–468 (2003).
Murataeva, N., Straiker, A. & Mackie, K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br. J. Pharmacol. 171, 1379–1391 (2014).
Jung, K. M. et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat. Commun. 3, 1080 (2012).
Tanimura, A. et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase α mediates retrograde suppression of synaptic transmission. Neuron 65, 320–327 (2010).
Shonesy, B. C. et al. Genetic disruption of 2-arachidonoylglycerol synthesis reveals a key role for endocannabinoid signaling in anxiety modulation. Cell Rep. 9, 1644–1653 (2014). This study provided critical evidence supporting increased anxiety-like behaviour in mutant mice with deficient 2-AG signalling.
Gao, Y. et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J. Neurosci. 30, 2017–2024 (2010).
Yoshida, T. et al. Localization of diacylglycerol lipase-α around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J. Neurosci. 26, 4740–4751 (2006).
Liu, Z. et al. Deficiency in endocannabinoid synthase DAGLB contributes to early onset Parkinsonism and murine nigral dopaminergic neuron dysfunction. Nat. Commun. 13, 3490 (2022).
Viader, A. et al. A chemical proteomic atlas of brain serine hydrolases identifies cell type-specific pathways regulating neuroinflammation. eLife 5, e12345 (2016).
Fowler, C. J. Transport of endocannabinoids across the plasma membrane and within the cell. FEBS J. 280, 1895–1904 (2013).
Nicolussi, S. & Gertsch, J. Endocannabinoid transport revisited. Vitam. Horm. 98, 441–485 (2015).
Kaczocha, M. & Haj-Dahmane, S. Mechanisms of endocannabinoid transport in the brain. Br. J. Pharmacol. 179, 4300–4310 (2022).
Kaczocha, M., Glaser, S. T. & Deutsch, D. G. Identification of intracellular carriers for the endocannabinoid anandamide. Proc. Natl Acad. Sci. USA 106, 6375–6380 (2009).
Haj-Dahmane, S. et al. Fatty-acid-binding protein 5 controls retrograde endocannabinoid signaling at central glutamate synapses. Proc. Natl Acad. Sci. USA 115, 3482–3487 (2018).
Fauzan, M. et al. Fatty acid-binding protein 5 modulates brain endocannabinoid tone and retrograde signaling in the striatum. Front. Cell Neurosci. 16, 936939 (2022).
Oubraim, S. et al. Astrocytic FABP5 mediates retrograde endocannabinoid transport at central synapses. iScience 28, 112342 (2025).
Albarran, E. et al. Postsynaptic synucleins mediate endocannabinoid signaling. Nat. Neurosci. 26, 997–1007 (2023).
Nakamura, Y. et al. Cocaine-induced endocannabinoid signaling mediated by sigma-1 receptors and extracellular vesicle secretion. eLife 8, https://doi.org/10.7554/eLife.47209 (2019).
Straub, V. M. et al. The endocannabinoid 2-arachidonoylglycerol is released and transported on demand via extracellular microvesicles. Proc. Natl Acad. Sci. USA 122, e2421717122 (2025).
Gabrielli, M. et al. Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep. 16, 213–220 (2015).
Desarnaud, F., Cadas, H. & Piomelli, D. Anandamide amidohydrolase activity in rat brain microsomes. Identification and partial characterization. J. Biol. Chem. 270, 6030–6035 (1995).
Cravatt, B. F. et al. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384, 83–87 (1996).
Ueda, N., Kurahashi, Y., Yamamoto, S. & Tokunaga, T. Partial purification and characterization of the porcine brain enzyme hydrolyzing and synthesizing anandamide. J. Biol. Chem. 270, 23823–23827 (1995).
Hillard, C. J., Wilkison, D. M., Edgemond, W. S. & Campbell, W. B. Characterization of the kinetics and distribution of N-arachidonylethanolamine (anandamide) hydrolysis by rat brain. Biochim. Biophys. Acta 1257, 249–256 (1995).
Giang, D. K. & Cravatt, B. F. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc. Natl Acad. Sci. USA 94, 2238–2242 (1997).
Cravatt, B. F. et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl Acad. Sci. USA 98, 9371–9376 (2001).
Kathuria, S. et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 9, 76–81 (2003). This study demonstrated the ability of pharmacological FAAH inhibition to reduce anxiety-like behaviour in preclinical models.
Dincheva, I. et al. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat. Commun. 6, 6395 (2015). A study providing cross-species evidence that a loss of function in genetic variants in FAAH reduces anxiety, enhances fear extinction and increases cortical–amygdala circuit function.
Marusak, H. A. et al. Endocannabinoid dysregulation and PTSD in urban adolescents: associations with anandamide concentrations and FAAH genotype. Psychopharmacology https://doi.org/10.1007/s00213-024-06717-3 (2024).
de Roon-Cassini, T. A. et al. Circulating endocannabinoids and genetic polymorphisms as predictors of posttraumatic stress disorder symptom severity: heterogeneity in a community-based cohort. Transl. Psychiatry 12, 48 (2022).
Dinh, T. P. et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl Acad. Sci. USA 99, 10819–10824 (2002).
Long, J. Z. et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol. 5, 37–44 (2009).
Schlosburg, J. E. et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat. Neurosci. 13, 1113–1119 (2010).
Marrs, W. R. et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat. Neurosci. 13, 951–957 (2010).
Ross, R. A. Anandamide and vanilloid TRPV1 receptors. Br. J. Pharmacol. 140, 790–801 (2003).
Sigel, E. et al. The major central endocannabinoid directly acts at GABA(A) receptors. Proc. Natl Acad. Sci. USA 108, 18150–18155 (2011).
Turu, G. & Hunyady, L. Signal transduction of the CB1 cannabinoid receptor. J. Mol. Endocrinol. 44, 75–85 (2010).
Jin, W. et al. Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J. Neurosci. 19, 3773–3780 (1999).
Daigle, T. L., Kwok, M. L. & Mackie, K. Regulation of CB1 cannabinoid receptor internalization by a promiscuous phosphorylation-dependent mechanism. J. Neurochem. 106, 70–82 (2008).
Morgan, D. J. et al. Mutation of putative GRK phosphorylation sites in the cannabinoid receptor 1 (CB1R) confers resistance to cannabinoid tolerance and hypersensitivity to cannabinoids in mice. J. Neurosci. 34, 5152–5163 (2014).
Nyilas, R. et al. Enzymatic machinery for endocannabinoid biosynthesis associated with calcium stores in glutamatergic axon terminals. J. Neurosci. 28, 1058–1063 (2008).
Rivera, P. et al. Localization of peroxisome proliferator-activated receptor alpha (PPARα) and N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) in cells expressing the Ca2+-binding proteins calbindin, calretinin, and parvalbumin in the adult rat hippocampus. Front. Neuroanat. 8, 12 (2014).
Egertova, M., Simon, G. M., Cravatt, B. F. & Elphick, M. R. Localization of N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) expression in mouse brain: a new perspective on N-acylethanolamines as neural signaling molecules. J. Comp. Neurol. 506, 604–615 (2008).
Reguero, L. et al. Subcellular localization of NAPE-PLD and DAGL-α in the ventromedial nucleus of the hypothalamus by a preembedding immunogold method. Histochem. Cell Biol. 141, 543–550 (2014).
Tsou, K. et al. Fatty acid amide hydrolase is located preferentially in large neurons in the rat central nervous system as revealed by immunohistochemistry. Neurosci. Lett. 254, 137–140 (1998).
Omiya, Y. et al. VGluT3-expressing CCK-positive basket cells construct invaginating synapses enriched with endocannabinoid signaling proteins in particular cortical and cortex-like amygdaloid regions of mouse brains. J. Neurosci. 35, 4215–4228 (2015).
Uchigashima, M. et al. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J. Neurosci. 27, 3663–3676 (2007).
Yoshida, T. et al. Unique inhibitory synapse with particularly rich endocannabinoid signaling machinery on pyramidal neurons in basal amygdaloid nucleus. Proc. Natl Acad. Sci. USA 108, 3059–3064 (2011).
Tanimura, A. et al. Synapse type-independent degradation of the endocannabinoid 2-arachidonoylglycerol after retrograde synaptic suppression. Proc. Natl Acad. Sci. USA 109, 12195–12200 (2012).
Docs, K. et al. Selective axonal and glial distribution of monoacylglycerol lipase immunoreactivity in the superficial spinal dorsal horn of rodents. Brain Struct. Funct. 220, 2625–2637 (2015).
Ludanyi, A. et al. Complementary synaptic distribution of enzymes responsible for synthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in the human hippocampus. Neuroscience 174, 50–63 (2011).
Uchigashima, M. et al. Molecular and morphological configuration for 2-arachidonoylglycerol-mediated retrograde signaling at mossy cell-granule cell synapses in the dentate gyrus. J. Neurosci. 31, 7700–7714 (2011).
Grabner, G. F. et al. Deletion of monoglyceride lipase in astrocytes attenuates lipopolysaccharide-induced neuroinflammation. J. Biol. Chem. 291, 913–923 (2016).
Egertova, M. & Elphick, M. R. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J. Comp. Neurol. 422, 159–171 (2000).
Marsicano, G. & Lutz, B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur. J. Neurosci. 11, 4213–4225 (1999).
Eggan, S. M., Melchitzky, D. S., Sesack, S. R., Fish, K. N. & Lewis, D. A. Relationship of cannabinoid CB1 receptor and cholecystokinin immunoreactivity in monkey dorsolateral prefrontal cortex. Neuroscience 169, 1651–1661 (2010).
Katona, I. et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J. Neurosci. 19, 4544–4558 (1999).
Katona, I. et al. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J. Neurosci. 21, 9506–9518 (2001).
Winters, B. D. et al. Cannabinoid receptor 1-expressing neurons in the nucleus accumbens. Proc. Natl Acad. Sci. USA 109, E2717–E2725 (2012).
Dudok, B. et al. Cell-specific STORM super-resolution imaging reveals nanoscale organization of cannabinoid signaling. Nat. Neurosci. 18, 75–86 (2015).
Maroso, M. et al. Cannabinoid control of learning and memory through HCN channels. Neuron 89, 1059–1073 (2016).
Metna-Laurent, M. & Marsicano, G. Rising stars: modulation of brain functions by astroglial type-1 cannabinoid receptors. Glia 63, 353–364 (2015).
Gutierrez-Rodriguez, A. et al. Localization of the cannabinoid type-1 receptor in subcellular astrocyte compartments of mutant mouse hippocampus. Glia 66, 1417–1431 (2018).
Bosier, B. et al. Astroglial CB1 cannabinoid receptors regulate leptin signaling in mouse brain astrocytes. Mol. Metab. 2, 393–404 (2013).
Jimenez-Blasco, D. et al. Glucose metabolism links astroglial mitochondria to cannabinoid effects. Nature 583, 603–608 (2020).
Soria-Gomez, E. et al. Subcellular specificity of cannabinoid effects in striatonigral circuits. Neuron 109, 1513–1526.e11 (2021).
Benard, G. et al. Mitochondrial CB(1) receptors regulate neuronal energy metabolism. Nat. Neurosci. 15, 558–564 (2012).
Benito, C. et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J. Neurosci. 23, 11136–11141 (2003).
Benito, C. et al. Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. J. Neurosci. 27, 2396–2402 (2007).
Nunez, E. et al. Glial expression of cannabinoid CB2 receptors and fatty acid amide hydrolase are beta amyloid-linked events in Down’s syndrome. Neuroscience 151, 104–110 (2008).
Lopez, A. et al. Cannabinoid CB2 receptors in the mouse brain: relevance for Alzheimer’s disease. J. Neuroinflamm. 15, 158 (2018).
Jung, K. M. et al. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol. Pharmacol. 68, 1196–1202 (2005).
Jung, K. M. et al. A key role for diacylglycerol lipase-α in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Mol. Pharmacol. 72, 612–621 (2007).
Ohno-Shosaku, T., Shosaku, J., Tsubokawa, H. & Kano, M. Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur. J. Neurosci. 15, 953–961 (2002).
Maejima, T. et al. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase C β4 signaling cascade in the cerebellum. J. Neurosci. 25, 6826–6835 (2005).
Hashimotodani, Y. et al. Phospholipase Cβ serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron 45, 257–268 (2005).
Ohno-Shosaku, T., Maejima, T. & Kano, M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron 29, 729–738 (2001).
Wilson, R. I. & Nicoll, R. A. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410, 588–592 (2001).
Kreitzer, A. C. & Regehr, W. G. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 29, 717–727 (2001).
Heifets, B. D. & Castillo, P. E. Endocannabinoid signaling and long-term synaptic plasticity. Annu. Rev. Physiol. 71, 283–306 (2009).
Heifets, B. D., Chevaleyre, V. & Castillo, P. E. Interneuron activity controls endocannabinoid-mediated presynaptic plasticity through calcineurin. Proc. Natl Acad. Sci. USA 105, 10250–10255 (2008).
Younts, T. J. et al. Presynaptic protein synthesis is required for long-term plasticity of GABA release. Neuron 92, 479–492 (2016).
Sjostrom, P. J., Turrigiano, G. G. & Nelson, S. B. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron 39, 641–654 (2003).
Pan, B. et al. Blockade of 2-arachidonoylglycerol hydrolysis by selective monoacylglycerol lipase inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) enhances retrograde endocannabinoid signaling. J. Pharmacol. Exp. Ther. 331, 591–597 (2009).
Martin, H. G. et al. Endocannabinoids mediate muscarinic acetylcholine receptor-dependent long-term depression in the adult medial prefrontal cortex. Front. Cell Neurosci. 9, 457 (2015).
Wang, Y. et al. Monoacylglycerol lipase inhibitors produce pro- or antidepressant responses via hippocampal CA1 GABAergic synapses. Mol. Psychiatry 22, 215–226 (2017).
Zhong, P. et al. Genetic deletion of monoacylglycerol lipase alters endocannabinoid-mediated retrograde synaptic depression in the cerebellum. J. Physiol. 589, 4847–4855 (2011).
Viader, A. et al. Metabolic interplay between astrocytes and neurons regulates endocannabinoid action. Cell Rep. 12, 798–808 (2015).
Marcus, D. J. et al. Endocannabinoid signaling collapse mediates stress-induced amygdalo-cortical strengthening. Neuron 105, 1062–1076.e6 (2020).
Bluett, R. J. et al. Endocannabinoid signalling modulates susceptibility to traumatic stress exposure. Nat. Commun. 8, 14782 (2017).
Bedse, G. et al. Functional redundancy between canonical endocannabinoid signaling systems in the modulation of anxiety. Biol. Psychiatry 82, 488–499 (2017).
Liu, X. et al. Coordinated regulation of endocannabinoid-mediated retrograde synaptic suppression in the cerebellum by neuronal and astrocytic monoacylglycerol lipase. Sci. Rep. 6, 35829 (2016).
Lee, S. H. et al. Multiple forms of endocannabinoid and endovanilloid signaling regulate the tonic control of GABA release. J. Neurosci. 35, 10039–10057 (2015).
Chevaleyre, V., Heifets, B. D., Kaeser, P. S., Sudhof, T. C. & Castillo, P. E. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1α. Neuron 54, 801–812 (2007).
Marinelli, S. et al. The endocannabinoid 2-arachidonoylglycerol is responsible for the slow self-inhibition in neocortical interneurons. J. Neurosci. 28, 13532–13541 (2008).
Bacci, A., Huguenard, J. R. & Prince, D. A. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature 431, 312–316 (2004).
Marinelli, S., Pacioni, S., Cannich, A., Marsicano, G. & Bacci, A. Self-modulation of neocortical pyramidal neurons by endocannabinoids. Nat. Neurosci. 12, 1488–1490 (2009).
Jensen, K. R., Berthoux, C., Nasrallah, K. & Castillo, P. E. Multiple cannabinoid signaling cascades powerfully suppress recurrent excitation in the hippocampus. Proc. Natl Acad. Sci. USA 118, e2017590118 (2021).
Martin-Fernandez, M. et al. Synapse-specific astrocyte gating of amygdala-related behavior. Nat. Neurosci. 20, 1540–1548 (2017).
Navarrete, M. & Araque, A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68, 113–126 (2010).
Gomez-Gonzalo, M. et al. Endocannabinoids induce lateral long-term potentiation of transmitter release by stimulation of gliotransmission. Cereb. Cortex 25, 3699–3712 (2015).
Chavez, A. E., Chiu, C. Q. & Castillo, P. E. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat. Neurosci. 13, 1511–1518 (2010).
Grueter, B. A., Brasnjo, G. & Malenka, R. C. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat. Neurosci. 13, 1519–1525 (2010).
Beckers, T. & Craske, M. G. Avoidance and decision making in anxiety: an introduction to the special issue. Behav. Res. Ther. 96, 1–2 (2017).
Trew, J. L. Exploring the roles of approach and avoidance in depression: an integrative model. Clin. Psychol. Rev. 31, 1156–1168 (2011).
Gencturk, S. & Unal, G. Rodent tests of depression and anxiety: construct validity and translational relevance. Cogn. Affect. Behav. Neurosci. 24, 191–224 (2024).
Rosso, M. et al. Reliability of common mouse behavioural tests of anxiety: a systematic review and meta-analysis on the effects of anxiolytics. Neurosci. Biobehav. Rev. 143, 104928 (2022).
Martin, M., Ledent, C., Parmentier, M., Maldonado, R. & Valverde, O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology 159, 379–387 (2002). This study demonstrated that deletion of CB1 receptors worsens the behavioural consequences of stress.
Haller, J., Bakos, N., Szirmay, M., Ledent, C. & Freund, T. F. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur. J. Neurosci. 16, 1395–1398 (2002).
Haller, J., Varga, B., Ledent, C., Barna, I. & Freund, T. F. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur. J. Neurosci. 19, 1906–1912 (2004).
Bura, S. A., Burokas, A., Martin-Garcia, E. & Maldonado, R. Effects of chronic nicotine on food intake and anxiety-like behaviour in CB(1) knockout mice. Eur. Neuropsychopharmacol. 20, 369–378 (2010).
Gamble-George, J. C. et al. Dissociable effects of CB1 receptor blockade on anxiety-like and consummatory behaviors in the novelty-induced hypophagia test in mice. Psychopharmacology 228, 401–409 (2013).
Navarro, M. et al. Acute administration of the CB1 cannabinoid receptor antagonist SR 141716 A induces anxiety-like responses in the rat. Neuroreport 8, 491–496 (1997).
Patel, S. & Hillard, C. J. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J. Pharmacol. Exp. Ther. 318, 304–311 (2006).
Rodgers, R. J., Evans, P. M. & Murphy, A. Anxiogenic profile of AM-251, a selective cannabinoid CB1 receptor antagonist, in plus-maze-naive and plus-maze-experienced mice. Behav. Pharmacol. 16, 405–413 (2005).
Bowers, M. E. & Ressler, K. J. Sex-dependence of anxiety-like behavior in cannabinoid receptor 1 (Cnr1) knockout mice. Behav. Brain Res. 300, 65–69 (2016).
Griebel, G., Stemmelin, J. & Scatton, B. Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biol. Psychiatry 57, 261–267 (2005).
Woodward, T. J. et al. Genetic deletion of NAPE-PLD induces context-dependent dysregulation of anxiety-like behaviors, stress responsiveness, and HPA-axis functionality in mice. Preprint at bioRxiv https://doi.org/10.1101/2024.09.10.612324 (2024).
Jenniches, I. et al. Anxiety, stress, and fear response in mice with reduced endocannabinoid levels. Biol. Psychiatry 79, 858–868 (2016).
Bortolato, M. et al. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol. Psychiatry 62, 1103–1110 (2007).
Moreira, F. A., Kaiser, N., Monory, K. & Lutz, B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology 54, 141–150 (2008).
Busquets-Garcia, A. et al. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol. Psychiatry 70, 479–486 (2011). This was an early study demonstrating the anxiolytic-like effects of 2-AG augmentation via MGL inhibition.
Scherma, M. et al. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology 54, 129–140 (2008).
Haller, J. et al. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology 204, 607–616 (2009).
Naidu, P. S. et al. Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology 192, 61–70 (2007).
Sciolino, N. R., Zhou, W. & Hohmann, A. G. Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol. Res. 64, 226–234 (2011). This was an early study demonstrating the anxiolytic-like effects of 2-AG augmentation via MGL inhibition.
Bedse, G. et al. Therapeutic endocannabinoid augmentation for mood and anxiety disorders: comparative profiling of FAAH, MAGL and dual inhibitors. Transl. Psychiatry 8, 92 (2018).
Imperatore, R. et al. Genetic deletion of monoacylglycerol lipase leads to impaired cannabinoid receptor CB1R signaling and anxiety-like behavior. J. Neurochem. 135, 799–813 (2015).
McEwen, B. S. Mood disorders and allostatic load. Biol. Psychiatry 54, 200–207 (2003).
McEwen, B. S. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. N. Y. Acad. Sci. 1032, 1–7 (2004).
McEwen, B. S. & Gianaros, P. J. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 1186, 190–222 (2010).
Bedse, G., Hill, M. N. & Patel, S. 2-Arachidonoylglycerol modulation of anxiety and stress adaptation: from grass roots to novel therapeutics. Biol. Psychiatry 88, 520–530 (2020).
Petrie, G. N., Nastase, A. S., Aukema, R. J. & Hill, M. N. Endocannabinoids, cannabinoids and the regulation of anxiety. Neuropharmacology 195, 108626 (2021).
Degroot, A. & Nomikos, G. G. Genetic deletion and pharmacological blockade of CB1 receptors modulates anxiety in the shock-probe burying test. Eur. J. Neurosci. 20, 1059–1064 (2004).
Steiner, M. A. et al. Impaired cannabinoid receptor type 1 signaling interferes with stress-coping behavior in mice. Pharmacogenom. J. 8, 196–208 (2008).
Aso, E. et al. BDNF impairment in the hippocampus is related to enhanced despair behavior in CB1 knockout mice. J. Neurochem. 105, 565–572 (2008).
Aso, E., Ozaita, A., Serra, M. A. & Maldonado, R. Genes differentially expressed in CB1 knockout mice: involvement in the depressive-like phenotype. Eur. Neuropsychopharmacol. 21, 11–22 (2011).
Adamczyk, P., Golda, A., McCreary, A. C., Filip, M. & Przegalinski, E. Activation of endocannabinoid transmission induces antidepressant-like effects in rats. J. Physiol. Pharmacol. 59, 217–228 (2008). A study demonstrating antidepressant-like effects of FAAH inhibition using preclinical models.
Gobbi, G. et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc. Natl Acad. Sci. USA 102, 18620–18625 (2005).
Vinod, K. Y. et al. Dysfunction in fatty acid amide hydrolase is associated with depressive-like behavior in Wistar Kyoto rats. PLoS One 7, e36743 (2012).
Haller, J., Goldberg, S. R., Pelczer, K. G., Aliczki, M. & Panlilio, L. V. The effects of anandamide signaling enhanced by the FAAH inhibitor URB597 on coping styles in rats. Psychopharmacology 230, 353–362 (2013).
Griebel, G. et al. The selective reversible FAAH inhibitor, SSR411298, restores the development of maladaptive behaviors to acute and chronic stress in rodents. Sci. Rep. 8, 2416 (2018).
Heinz, D. E., Genewsky, A. & Wotjak, C. T. Enhanced anandamide signaling reduces flight behavior elicited by an approaching robo-beetle. Neuropharmacology 126, 233–241 (2017).
Pavon, F. J. et al. Selective inhibition of monoacylglycerol lipase is associated with passive coping behavior and attenuation of stress-induced dopamine release in the medial prefrontal cortex. Neurobiol. Stress. 14, 100293 (2021).
Kondev, V. et al. The endocannabinoid 2-arachidonoylglycerol bidirectionally modulates acute and protracted effects of predator odor exposure. Biol. Psychiatry 92, 739–749 (2022).
Lomazzo, E. et al. Therapeutic potential of inhibitors of endocannabinoid degradation for the treatment of stress-related hyperalgesia in an animal model of chronic pain. Neuropsychopharmacology 40, 488–501 (2015).
Duan, T. et al. Fatty acid amide hydrolase inhibitors produce rapid anti-anxiety responses through amygdala long-term depression in male rodents. J. Psychiatry Neurosci. 42, 230–241 (2017).
Bluett, R. J. et al. Central anandamide deficiency predicts stress-induced anxiety: behavioral reversal through endocannabinoid augmentation. Transl. Psychiatry 4, e408 (2014).
Rossi, S. et al. Preservation of striatal cannabinoid CB1 receptor function correlates with the antianxiety effects of fatty acid amide hydrolase inhibition. Mol. Pharmacol. 78, 260–268 (2010).
Danandeh, A. et al. Effects of fatty acid amide hydrolase inhibitor URB597 in a rat model of trauma-induced long-term anxiety. Psychopharmacology 235, 3211–3221 (2018).
Fotio, Y., Mabou Tagne, A., Jung, K. M. & Piomelli, D. Fatty acid amide hydrolase inhibition alleviates anxiety-like symptoms in a rat model used to study post-traumatic stress disorder. Psychopharmacology 242, 1541–1551 (2025).
Hill, M. N. et al. Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol. Psychiatry 18, 1125–1135 (2013). This study demonstrated that chronic FAAH inhibition prevents behavioural and physiological responses to repeated homotypic stress.
Zhong, P. et al. Monoacylglycerol lipase inhibition blocks chronic stress-induced depressive-like behaviors via activation of mTOR signaling. Neuropsychopharmacology 39, 1763–1776 (2014).
Sumislawski, J. J., Ramikie, T. S. & Patel, S. Reversible gating of endocannabinoid plasticity in the amygdala by chronic stress: a potential role for monoacylglycerol lipase inhibition in the prevention of stress-induced behavioral adaptation. Neuropsychopharmacology 36, 2750–2761 (2011).
Lim, J. et al. Endocannabinoid modulation of predator stress-induced long-term anxiety in rats. Neuropsychopharmacology 41, 1329–1339 (2016).
Ivy, D. et al. Cannabinoid CB2 receptors mediate the anxiolytic-like effects of monoacylglycerol lipase inhibition in a rat model of predator-induced fear. Neuropsychopharmacology 45, 1330–1338 (2020).
Kamprath, K. et al. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J. Neurosci. 26, 6677–6686 (2006).
Marsicano, G. et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature 418, 530–534 (2002). This study describes a key role for CB1 receptors in the modulation of conditioned fear extinction.
Reich, C. G., Mohammadi, M. H. & Alger, B. E. Endocannabinoid modulation of fear responses: learning and state-dependent performance effects. J. Psychopharmacol. 22, 769–777 (2008).
Pamplona, F. A., Bitencourt, R. M. & Takahashi, R. N. Short- and long-term effects of cannabinoids on the extinction of contextual fear memory in rats. Neurobiol. Learn. Mem. 90, 290–293 (2008).
Suzuki, A. et al. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 24, 4787–4795 (2004).
Pickens, C. L. & Theberge, F. R. Blockade of CB1 receptors prevents retention of extinction but does not increase low preincubated conditioned fear in the fear incubation procedure. Behav. Pharmacol. 25, 23–31 (2014).
Mizuno, I., Matsuda, S., Tohyama, S. & Mizutani, A. The role of the cannabinoid system in fear memory and extinction in male and female mice. Psychoneuroendocrinology 138, 105688 (2022).
Cavener, V. S. et al. Inhibition of diacylglycerol lipase impairs fear extinction in mice. Front. Neurosci. 12, 479 (2018).
Ramos-Medina, L., Rosas-Vidal, L. E. & Patel, S. Pharmacological diacylglycerol lipase inhibition impairs contextual fear extinction in mice. Psychopharmacology 241, 569–584 (2024).
Bellocchio, L. et al. Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB1 receptor blockade. Proc. Natl Acad. Sci. USA 110, 4786–4791 (2013).
Gunduz-Cinar, O. et al. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol. Psychiatry 18, 813–823 (2013). A study demonstrating that pharmacological FAAH inhibition facilitates fear extinction.
Morena, M. et al. Enhancing endocannabinoid neurotransmission augments the efficacy of extinction training and ameliorates traumatic stress-induced behavioral alterations in rats. Neuropsychopharmacology 43, 1284–1296 (2018).
Fidelman, S., Mizrachi Zer-Aviv, T., Lange, R., Hillard, C. J. & Akirav, I. Chronic treatment with URB597 ameliorates post-stress symptoms in a rat model of PTSD. Eur. Neuropsychopharmacol. 28, 630–642 (2018).
Hartley, N. D. et al. 2-Arachidonoylglycerol signaling impairs short-term fear extinction. Transl. Psychiatry 6, e749 (2016).
Llorente-Berzal, A. et al. 2-AG promotes the expression of conditioned fear via cannabinoid receptor type 1 on GABAergic neurons. Psychopharmacology 232, 2811–2825 (2015).
Hill, M. N. et al. Prolonged glucocorticoid treatment decreases cannabinoid CB1 receptor density in the hippocampus. Hippocampus 18, 221–226 (2008).
Patel, S., Kingsley, P. J., Mackie, K., Marnett, L. J. & Winder, D. G. Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology 34, 2699–2709 (2009).
Wamsteeker, J. I., Kuzmiski, J. B. & Bains, J. S. Repeated stress impairs endocannabinoid signaling in the paraventricular nucleus of the hypothalamus. J. Neurosci. 30, 11188–11196 (2010).
Wamsteeker Cusulin, J. I., Senst, L., Teskey, G. C. & Bains, J. S. Experience salience gates endocannabinoid signaling at hypothalamic synapses. J. Neurosci. 34, 6177–6181 (2014).
Hohmann, A. G. et al. An endocannabinoid mechanism for stress-induced analgesia. Nature 435, 1108–1112 (2005).
Patel, S., Roelke, C. T., Rademacher, D. J. & Hillard, C. J. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur. J. Neurosci. 21, 1057–1069 (2005).
Tomas-Roig, J. et al. Social defeat leads to changes in the endocannabinoid system: an overexpression of calreticulin and motor impairment in mice. Behav. Brain Res. 303, 34–43 (2016).
Dubreucq, S. et al. Genetic dissection of the role of cannabinoid type-1 receptors in the emotional consequences of repeated social stress in mice. Neuropsychopharmacology 37, 1885–1900 (2012).
Bouter, Y. et al. Chronic psychosocial stress causes increased anxiety-like behavior and alters endocannabinoid levels in the brain of C57Bl/6 J mice. Cannabis Cannabinoid Res. 5, 51–61 (2020).
Hill, M. N., Ho, W. S., Meier, S. E., Gorzalka, B. B. & Hillard, C. J. Chronic corticosterone treatment increases the endocannabinoid 2-arachidonylglycerol in the rat amygdala. Eur. J. Pharmacol. 528, 99–102 (2005).
Gray, J. M. et al. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J. Neurosci. 35, 3879–3892 (2015). This study demonstrated that CRF-induced AEA deficiency-derived anxiety-like behaviour can be reversed by pharmacological FAAH inhibition.
Dow-Edwards, D. Sex differences in the interactive effects of early life stress and the endocannabinoid system. Neurotoxicol. Teratol. 80, 106893 (2020).
Craft, R. M., Marusich, J. A. & Wiley, J. L. Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sci. 92, 476–481 (2013).
Schlienz, N. J., Budney, A. J., Lee, D. C. & Vandrey, R. Cannabis withdrawal: a review of neurobiological mechanisms and sex differences. Curr. Addict. Rep. 4, 75–81 (2017).
Viveros, M. P. et al. The endocannabinoid system in critical neurodevelopmental periods: sex differences and neuropsychiatric implications. J. Psychopharmacol. 26, 164–176 (2012).
Blanton, H. L. et al. Sex differences and the endocannabinoid system in pain. Pharmacol. Biochem. Behav. 202, 173107 (2021).
Vecchiarelli, H. A. et al. Sex and stressor modality influence acute stress-induced dynamic changes in corticolimbic endocannabinoid levels in adult Sprague Dawley rats. Neurobiol. Stress 20, 100470 (2022).
Monory, K. et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 51, 455–466 (2006).
Rey, A. A., Purrio, M., Viveros, M. P. & Lutz, B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA(B) receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology 37, 2624–2634 (2012).
Haring, M., Kaiser, N., Monory, K. & Lutz, B. Circuit specific functions of cannabinoid CB1 receptor in the balance of investigatory drive and exploration. PLoS One 6, e26617 (2011).
De Giacomo, V., Ruehle, S., Lutz, B., Haring, M. & Remmers, F. Cell type-specific genetic reconstitution of CB1 receptor subsets to assess their role in exploratory behaviour, sociability, and memory. Eur. J. Neurosci. 55, 939–951 (2022).
Steiner, M. A., Marsicano, G., Wotjak, C. T. & Lutz, B. Conditional cannabinoid receptor type 1 mutants reveal neuron subpopulation-specific effects on behavioral and neuroendocrine stress responses. Psychoneuroendocrinology 33, 1165–1170 (2008).
Metna-Laurent, M. et al. Bimodal control of fear-coping strategies by CB1 cannabinoid receptors. J. Neurosci. 32, 7109–7118 (2012).
Lesuis, S. L. et al. Stress disrupts engram ensembles in lateral amygdala to generalize threat memory in mice. Cell 188, 121–140.e20 (2025).
Haring, M. et al. Cannabinoid type-1 receptor signaling in central serotonergic neurons regulates anxiety-like behavior and sociability. Front. Behav. Neurosci. 9, 235 (2015).
Busquets-Garcia, A. et al. Peripheral and central CB1 cannabinoid receptors control stress-induced impairment of memory consolidation. Proc. Natl Acad. Sci. USA 113, 9904–9909 (2016).
Baddenhausen, S., Lutz, B. & Hofmann, C. Cannabinoid type-1 receptor signaling in dopaminergic engrailed-1 expressing neurons modulates motivation and depressive-like behavior. Front. Mol. Neurosci. 17, 1379889 (2024).
Mineka, S. & Kihlstrom, J. F. Unpredictable and uncontrollable events: a new perspective on experimental neurosis. J. Abnorm. Psychol. 87, 256–271 (1978).
Grupe, D. W. & Nitschke, J. B. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat. Rev. Neurosci. 14, 488–501 (2013).
Blanchard, D. C. & Canteras, N. S. Uncertainty and anxiety: evolution and neurobiology. Neurosci. Biobehav. Rev. 162, 105732 (2024).
Avery, S. N., Clauss, J. A. & Blackford, J. U. The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology 41, 126–141 (2016).
Davis, M., Walker, D. L. & Lee, Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 352, 1675–1687 (1997).
Knight, L. K. & Depue, B. E. New frontiers in anxiety research: the translational potential of the bed nucleus of the stria terminalis. Front. Psychiatry 10, 510 (2019).
Puente, N. et al. Localization and function of the cannabinoid CB1 receptor in the anterolateral bed nucleus of the stria terminalis. PLoS One 5, e8869 (2010).
Lange, M. D. et al. Cannabinoid CB1 receptors in distinct circuits of the extended amygdala determine fear responsiveness to unpredictable threat. Mol. Psychiatry 22, 1422–1430 (2017).
Kim, S. Y. et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496, 219–223 (2013).
Lowery-Gionta, E. G. et al. Chronic stress dysregulates amygdalar output to the prefrontal cortex. Neuropharmacology 139, 68–75 (2018).
Felix-Ortiz, A. C., Burgos-Robles, A., Bhagat, N. D., Leppla, C. A. & Tye, K. M. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 321, 197–209 (2016).
Burgos-Robles, A. et al. Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat. Neurosci. 20, 824–835 (2017).
Yuen, E. Y. et al. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc. Natl Acad. Sci. USA 106, 14075–14079 (2009).
Moghaddam, B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol. Psychiatry 51, 775–787 (2002).
Bukalo, O. et al. Prefrontal inputs to the amygdala instruct fear extinction memory formation. Sci. Adv. 1, e1500251 (2015).
Gunduz-Cinar, O. et al. A cortico-amygdala neural substrate for endocannabinoid modulation of fear extinction. Neuron 111, 3053–3067.e3010 (2023).
Jackson, A. D. et al. Amygdala-hippocampus somatostatin interneuron beta-synchrony underlies a cross-species biomarker of emotional state. Neuron 112, 1182–1195.e5 (2024).
Deji, C. et al. The basolateral amygdala to ventral hippocampus circuit controls anxiety-like behaviors induced by morphine withdrawal. Front. Cell Neurosci. 16, 894886 (2022).
Zhang, Q. et al. The slack channel regulates anxiety-like behaviors via basolateral amygdala glutamatergic projections to ventral hippocampus. J. Neurosci. 42, 3049–3064 (2022).
Pi, G. et al. Posterior basolateral amygdala to ventral hippocampal CA1 drives approach behaviour to exert an anxiolytic effect. Nat. Commun. 11, 183 (2020).
Kondev, V. et al. Endocannabinoid release at ventral hippocampal-amygdala synapses regulates stress-induced behavioral adaptation. Cell Rep. 42, 113027 (2023).
Al Jowf, G. I., Snijders, C., Rutten, B. P. F., de Nijs, L. & Eijssen, L. M. T. The molecular biology of susceptibility to post-traumatic stress disorder: highlights of epigenetics and epigenomics. Int. J. Mol. Sci. 22, 10743 (2021).
Yehuda, R. Biological factors associated with susceptibility to posttraumatic stress disorder. Can. J. Psychiatry 44, 34–39 (1999).
Yehuda, R. & LeDoux, J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron 56, 19–32 (2007).
Kim, J., Pignatelli, M., Xu, S., Itohara, S. & Tonegawa, S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat. Neurosci. 19, 1636–1646 (2016).
Beyeler, A. et al. Organization of valence-encoding and projection-defined neurons in the basolateral amygdala. Cell Rep. 22, 905–918 (2018).
Piantadosi, S. C. et al. Holographic stimulation of opposing amygdala ensembles bidirectionally modulates valence-specific behavior via mutual inhibition. Neuron 112, 593–610.e5 (2024).
Hill, M. N. et al. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology 38, 2952–2961 (2013).
Gowatch, L. C. et al. Endocannabinoids and stress-related neurospsychiatric disorders: a systematic review and meta-analysis of basal concentrations and response to acute psychosocial stress. Cannabis Cannabinoid Res. 9, 1217–1234 (2024).
Hillard, C. J. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 43, 155–172 (2018).
Russo, E. B. Clinical endocannabinoid deficiency reconsidered: current research supports the theory in migraine, fibromyalgia, irritable bowel, and other treatment-resistant syndromes. Cannabis Cannabinoid Res. 1, 154–165 (2016).
Mayo, L. M. et al. Elevated anandamide, enhanced recall of fear extinction, and attenuated stress responses following inhibition of fatty acid amide hydrolase: a randomized, controlled experimental medicine trial. Biol. Psychiatry 87, 538–547 (2020). This study in humans showed that pharmacological FAAH inhibition enhances fear extinction and attenuates stress responses.
Crombie, K. M. et al. The influence of FAAH genetic variation on physiological, cognitive, and neural signatures of fear acquisition and extinction learning in women with PTSD. Neuroimage Clin. 33, 102922 (2022).
Schmidt, M. E. et al. The effects of inhibition of fatty acid amide hydrolase (FAAH) by JNJ-42165279 in social anxiety disorder: a double-blind, randomized, placebo-controlled proof-of-concept study. Neuropsychopharmacology 46, 1004–1010 (2021).
Jazz Pharmaceuticals Provides Update on Phase 2 Trial of Investigational JZP150 in Adult Patients with Post-Traumatic Stress Disorder https://investor.jazzpharma.com/news-releases/news-release-details/jazz-pharmaceuticals-provides-update-phase-2-trial (Jazz Pharmaceuticals, 2023).
Mayo, L. M. et al. The efficacy of elevating anandamide via inhibition of fatty acid amide hydrolase (FAAH) combined with internet-delivered cognitive behavioral therapy in the treatment of post-traumatic stress disorder: a randomized, placebo-controlled clinical trial. Neuropsychopharmacology 80, 1564–1572 (2025).
Muller-Vahl, K. R. et al. Endocannabinoid modulation using monoacylglycerol lipase inhibition in tourette syndrome: a phase 1 randomized, placebo-controlled study. Pharmacopsychiatry 55, 148–156 (2022).
Jiang, M. et al. A monoacylglycerol lipase inhibitor showing therapeutic efficacy in mice without central side effects or dependence. Nat. Commun. 14, 8039 (2023).
Carnevali, L. et al. Enhancement of peripheral fatty acyl ethanolamide signaling prevents stress-induced social avoidance and anxiety-like behaviors in male rats. Psychopharmacology 242, 997–1009 (2025).
Mielnik, C. A., Lam, V. M. & Ross, R. A. CB1 allosteric modulators and their therapeutic potential in CNS disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 106, 110163 (2021).
Khurana, L., Mackie, K., Piomelli, D. & Kendall, D. A. Modulation of CB1 cannabinoid receptor by allosteric ligands: pharmacology and therapeutic opportunities. Neuropharmacology 124, 3–12 (2017).
Christensen, R., Kristensen, P. K., Bartels, E. M., Bliddal, H. & Astrup, A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 370, 1706–1713 (2007).
Morena, M. et al. Upregulation of anandamide hydrolysis in the basolateral complex of amygdala reduces fear memory expression and indices of stress and anxiety. J. Neurosci. 39, 1275–1292 (2019).
Shields, B. C. et al. Deconstructing behavioral neuropharmacology with cellular specificity. Science 356, eaaj2161 (2017).
Mondoloni, S., Durand-de Cuttoli, R. & Mourot, A. Cell-specific neuropharmacology. Trends Pharmacol. Sci. 40, 696–710 (2019).
Yasmin, F., Naskar, S., Rosas-Vidal, L. E. & Patel, S. Cannabinoid modulation of central amygdala population dynamics during threat investigation. Preprint at bioRxiv https://doi.org/10.1101/2025.01.21.634174 (2025).
Rosas-Vidal, L. E. et al. Prefrontal correlates of fear generalization during endocannabinoid depletion. J. Clin. Invest. 135, e179881 (2025).
Komorowska-Muller, J. A. & Schmole, A. C. CB2 receptor in microglia: the guardian of self-control. Int. J. Mol. Sci. 22, 19 (2020).
Dong, A. et al. A fluorescent sensor for spatiotemporally resolved imaging of endocannabinoid dynamics in vivo. Nat. Biotechnol. 40, 787–798 (2022).
Marsicano, G. et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302, 84–88 (2003).
Schuele, L. L. et al. Regulation of adult neurogenesis by the endocannabinoid-producing enzyme diacylglycerol lipase alpha (DAGLa). Sci. Rep. 12, 633 (2022).
Shen, C. J. et al. Cannabinoid CB1 receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat. Med. 25, 337–349 (2019).
Guggenhuber, S. et al. Impaired 2-AG signaling in hippocampal glutamatergic neurons: aggravation of anxiety-like behavior and unaltered seizure susceptibility. Int. J. Neuropsychopharmacol. 19, pyv091 (2015).
Qian, T., Wang, H., Xia, X. & Li, Y. Current and emerging methods for probing neuropeptide transmission. Curr. Opin. Neurobiol. 81, 102751 (2023).
Buczynski, M. W. & Parsons, L. H. Quantification of brain endocannabinoid levels: methods, interpretations and pitfalls. Br. J. Pharmacol. 160, 423–442 (2010).
Acknowledgements
The authors would like to thank their long-time colleagues, M. Hill, C. Hillard, L. Mayo, A. Holmes, O. Gunduz-Cinar, B. Shonesy and others, for their thoughtful and spirited discussions on the topics reviewed here over the years. This work is supported by the National Institutes of Health grant MH107435 (S.P.).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no financial conflicts of interest.
Peer review
Peer review information
Nature Reviews Neuroscience thanks Xiao-Ming Li and the other, anonymous, reviewers for their contribution to the peer review of this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Allostasis
-
The active processes that the body and brain undergo to re-establish homeostasis after stress exposure.
- Anhedonia
-
The inability to experience pleasure or enjoyment from activities that would normally be pleasurable.
- Elevated-plus maze
-
(EPM). A rodent spatial avoidance task during which rodents can explore closed (safe) and threatening (open) arms, with increases in open-arm exploration suggestive of reduced anxiety-related responses.
- Innate avoidance
-
An organism’s avoidance of locations or stimuli without previous exposure or knowledge of the exposure outcome through previous experience.
- Learned defensive response generation
-
Defensive behaviours, including avoidance, freezing and flight, expressed upon presentation of environmental stimuli that predicts threat as a function of previously formed associations between the stimuli and a negative outcome.
- Stress adaptation
-
Dynamic physiological and behavioural responses to stress exposure across time, some of which contribute to allostasis, while others contribute to stress-induced pathological states.
- Stress coping
-
An animal’s short-term (usually several minutes) response to stress that is generally assessed by forced swimming or tail suspension, with coping responses noted to be active or passive.
- Stress reactivity
-
An animal’s immediate defensive reactions (seconds to minutes) to stress exposure, including avoidance, freezing or flight.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Loomba, N., Patel, S. Circuit mechanisms governing endocannabinoid modulation of affective behaviour and stress adaptation. Nat. Rev. Neurosci. 26, 677–697 (2025). https://doi.org/10.1038/s41583-025-00961-y
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41583-025-00961-y