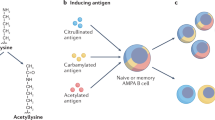
Abstract
Glycosylation has a profound influence on protein activity and cell biology through a variety of mechanisms, such as protein stability, receptor interactions and signal transduction. In many rheumatic diseases, a shift in protein glycosylation occurs, and is associated with inflammatory processes and disease progression. For example, the Fc-glycan composition on (auto)antibodies is associated with disease activity, and the presence of additional glycans in the antigen-binding domains of some autoreactive B cell receptors can affect B cell activation. In addition, changes in synovial fibroblast cell-surface glycosylation can alter the synovial microenvironment and are associated with an altered inflammatory state and disease activity in rheumatoid arthritis. The development of our understanding of the role of glycosylation of plasma proteins (particularly (auto)antibodies), cells and tissues in rheumatic pathological conditions suggests that glycosylation-based interventions could be used in the treatment of these diseases.
Key points
-
Autoantigen-specific IgG in patients with rheumatic diseases has a distinct N-glycosylation signature in the fragment crystallizable (Fc) domain, characterized by fucosylation without sialylation or galactosylation.
-
In rheumatoid arthritis (RA) and anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis, autoantigen-specific IgG, as well as autoreactive B cell receptors, are extensively N-glycosylated in the fragment antigen-binding (Fab) domain.
-
Specific Fc and Fab IgG glycan signatures are associated with RA disease activity and remission.
-
Mechanistic evidence is lacking on how fucosylated, agalactosylated IgG Fc glycans possibly cause a pro-inflammatory phenotype in humans.
-
RA is associated with reduction of cell-surface sialylation of synovial fibroblasts, affecting their interactions with galectin-3 and resulting in a cytokine-induced switch towards a pro-inflammatory phenotype.
-
Glycan-based therapies could intervene in inflammatory processes by alteration of glycosylation, or by specific targeting and depletion of autoreactive B cells and autoantibodies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
23 February 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41584-023-00922-8
References
Jefferis, R. Recombinant proteins and monoclonal antibodies. Adv. Biochem. Eng. Biotechnol. 175, 281–318 (2021).
Haan, N. et al. Developments and perspectives in high-throughput protein glycomics: enabling the analysis of thousands of samples. Glycobiology 32, 651–663 (2022).
Marshall, R. D. Glycoproteins. Ann. Rev. Biochem. 41, 673–702 (1972).
Zielinska, D. F., Gnad, F., Wisniewski, J. R. & Mann, M. Precision mapping of an in vivo N-glycoproteome reveals rigid topological and sequence constraints. Cell 141, 897–907 (2010).
Bause, E. & Legler, G. The role of the hydroxy amino acid in the triplet sequence Asn-Xaa-Thr(Ser) for the N-glycosylation step during glycoprotein biosynthesis. Biochem. J. 195, 639–644 (1981).
Varki, A. Essentials of Glycobiology 4th edn (ed. Inglis, J.) (Cold Spring Harbor Laboratory Press, 2022).
Lis, H. & Sharon, N. Protein glycosylation. Struct. Funct. Asp. Eur. J. Biochem. 218, 1–27 (1993).
Parekh, R. B. et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 316, 452–457 (1985).
Reiding, K. R. et al. Serum protein N-glycosylation changes with rheumatoid arthritis disease activity during and after pregnancy. Front. Med. 4, 241 (2017).
Albrecht, S., Unwin, L., Muniyappa, M. & Rudd, P. M. Glycosylation as a marker for inflammatory arthritis. Cancer Biomark. 14, 17–28 (2014).
de Haan, N., Falck, D. & Wuhrer, M. Monitoring of immunoglobulin N- and O-glycosylation in health and disease. Glycobiology 30, 226–240 (2020).
Bakovic, M. P. et al. High-throughput IgG Fc N-glycosylation profiling by mass spectrometry of glycopeptides. J. Proteome Res. 12, 821–831 (2013).
Bondt, A. et al. Immunoglobulin G (IgG) Fab glycosylation analysis using a new mass spectrometric high-throughput profiling method reveals pregnancy-associated changes. Mol. Cell. Proteom. 13, 3029–3039 (2014).
Bond, A. et al. A detailed lectin analysis of IgG glycosylation, demonstrating disease specific changes in terminal galactose and N-acetylglucosamine. J. Autoimmun. 10, 77–85 (1997).
Kristic, J. et al. Glycans are a novel biomarker of chronological and biological ages. J. Gerontol. A Biol. Sci. Med. Sci. 69, 779–789 (2014).
Keusch, J., Levy, Y., Shoenfeld, Y. & Youinou, P. Analysis of different glycosylation states in IgG subclasses. Clin. Chim. Acta 252, 147–158 (1996).
Chen, G. et al. Human IgG Fc-glycosylation profiling reveals associations with age, sex, female sex hormones and thyroid cancer. J. Proteom. 75, 2824–2834 (2012).
Watson, M., Rudd, P. M., Bland, M., Dwek, R. A. & Axford, J. S. Sugar printing rheumatic diseases: a potential method for disease differentiation using immunoglobulin G oligosaccharides. Arthritis Rheum. 42, 1682–1690 (1999).
Ercan, A. et al. Multiple juvenile idiopathic arthritis subtypes demonstrate proinflammatory IgG glycosylation. Arthritis Rheum. 64, 3025–3033 (2012).
Vuckovic, F. et al. Association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome. Arthritis Rheumatol. 67, 2978–2989 (2015).
Leirisalo-Repo, M., Hernandez-Munoz, H. E. & Rook, G. A. Agalactosyl IgG is elevated in patients with active spondyloarthropathy. Rheumatol. Int. 18, 171–176 (1999).
Holland, M. et al. Hypogalactosylation of serum IgG in patients with ANCA-associated systemic vasculitis. Clin. Exp. Immunol. 129, 183–190 (2002).
Parekh, R. et al. A comparative analysis of disease-associated changes in the galactosylation of serum IgG. J. Autoimmun. 2, 101–114 (1989).
Trbojevic Akmacic, I. et al. Inflammatory bowel disease associates with proinflammatory potential of the immunoglobulin G glycome. Inflamm. Bowel Dis. 21, 1237–1247 (2015).
Tomana, M., Schrohenloher, R. E., Koopman, W. J., Alarcon, G. S. & Paul, W. A. Abnormal glycosylation of serum IgG from patients with chronic inflammatory diseases. Arthritis Rheum. 31, 333–338 (1988).
Wuhrer, M. et al. Pro-inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid. J. Neuroinflammation 12, 235 (2015).
Selman, M. H. et al. IgG fc N-glycosylation changes in Lambert-Eaton myasthenic syndrome and myasthenia gravis. J. Proteome Res. 10, 143–152 (2011).
Ercan, A. et al. Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis Rheum. 62, 2239–2248 (2010).
Rook, G. A. et al. Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy. J. Autoimmun. 4, 779–794 (1991).
van de Geijn, F. E. et al. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res. Ther. 11, R193 (2009).
Rombouts, Y. et al. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann. Rheum. Dis. 74, 234–241 (2015).
Espy, C. et al. Sialylation levels of anti-proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener’s). Arthritis Rheum. 63, 2105–2115 (2011).
Wuhrer, M. et al. Skewed Fc glycosylation profiles of anti-proteinase 3 immunoglobulin G1 autoantibodies from granulomatosis with polyangiitis patients show low levels of bisection, galactosylation, and sialylation. J. Proteome Res. 14, 1657–1665 (2015).
Fickentscher, C. et al. The pathogenicity of anti-beta2GP1-IgG autoantibodies depends on Fc glycosylation. J. Immunol. Res. 2015, 638129 (2015).
Pasek, M. et al. Galactosylation of IgG from rheumatoid arthritis (RA) patients–changes during therapy. Glycoconj. J. 23, 463–471 (2006).
Gindzienska-Sieskiewicz, E. et al. Changes of glycosylation of IgG in rheumatoid arthritis patients treated with methotrexate. Adv. Med. Sci. 61, 193–197 (2016).
Selman, M. H. et al. Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination. Mol. Cell. Proteom. 11, M111 014563 (2012).
Kemna, M. J. et al. Galactosylation and sialylation levels of IgG predict relapse in patients with PR3-ANCA associated vasculitis. EBioMedicine 17, 108–118 (2017).
Bondt, A. et al. IgA N- and O-glycosylation profiling reveals no association with the pregnancy-related improvement in rheumatoid arthritis. Arthritis Res. Ther. 19, 160 (2017).
Bondt, A. et al. Longitudinal monitoring of immunoglobulin A glycosylation during pregnancy by simultaneous MALDI-FTICR-MS analysis of N- and O-glycopeptides. Sci. Rep. 6, 27955 (2016).
Steffen, U. et al. IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat. Commun. 11, 120 (2020).
Scherer, H. U. et al. Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 62, 1620–1629 (2010).
Lardinois, O. M. et al. Immunoglobulins G from patients with ANCA-associated vasculitis are atypically glycosylated in both the Fc and Fab regions and the relation to disease activity. PLoS ONE 14, e0213215 (2019).
Kiyoshi, M., Tsumoto, K., Ishii-Watabe, A. & Caaveiro, J. M. M. Glycosylation of IgG-Fc: a molecular perspective. Int. Immunol. 29, 311–317 (2017).
Shinkawa, T. et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 278, 3466–3473 (2003).
Ferrara, C., Stuart, F., Sondermann, P., Brunker, P. & Umana, P. The carbohydrate at FcγRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J. Biol. Chem. 281, 5032–5036 (2006).
Shields, R. L. et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277, 26733–26740 (2002).
Larsen, M. D. et al. Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science 371, eabc8378 (2021).
Pongracz, T., Vidarsson, G. & Wuhrer, M. Antibody glycosylation in COVID-19. Glycoconj. J. 39, 335–344 (2022).
Karsten, C. M. et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat. Med. 18, 1401–1406 (2012).
Heyl, K. A., Karsten, C. M. & Slevogt, H. Galectin-3 binds highly galactosylated IgG1 and is crucial for the IgG1 complex mediated inhibition of C5aReceptor induced immune responses. Biochem. Biophys. Res. Commun. 479, 86–90 (2016).
Ohmi, Y. et al. Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat. Commun. 7, 11205 (2016).
Anthony, R. M., Kobayashi, T., Wermeling, F. & Ravetch, J. V. Intravenous gammaglobulin suppresses inflammation through a novel TH2 pathway. Nature 475, 110–113 (2011).
Sondermann, P., Pincetic, A., Maamary, J., Lammens, K. & Ravetch, J. V. General mechanism for modulating immunoglobulin effector function. Proc. Natl Acad. Sci. USA 110, 9868–9872 (2013).
Rademacher, T. W., Williams, P. & Dwek, R. A. Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc. Natl Acad. Sci. USA 91, 6123–6127 (1994).
Dekkers, G. et al. Decoding the human immunoglobulin G-glycan repertoire reveals a spectrum of Fc-receptor- and complement-mediated-effector activities. Front. Immunol. 8, 877 (2017).
Peschke, B., Keller, C. W., Weber, P., Quast, I. & Lunemann, J. D. Fc-galactosylation of human immunoglobulin gamma isotypes improves C1q binding and enhances complement-dependent cytotoxicity. Front. Immunol. 8, 646 (2017).
van Osch, T. L. J. et al. Fc galactosylation promotes hexamerization of human IgG1, leading to enhanced classical complement activation. J. Immunol. 207, 1545–1554 (2021).
Yu, X., Vasiljevic, S., Mitchell, D. A., Crispin, M. & Scanlan, C. N. Dissecting the molecular mechanism of IVIg therapy: the interaction between serum IgG and DC-SIGN is independent of antibody glycoform or Fc domain. J. Mol. Biol. 425, 1253–1258 (2013).
Crispin, M., Yu, X. & Bowden, T. A. Crystal structure of sialylated IgG Fc: implications for the mechanism of intravenous immunoglobulin therapy. Proc. Natl Acad. Sci. USA 110, E3544–E3546 (2013).
Ahmed, A. A. et al. Structural characterization of anti-inflammatory immunoglobulin G Fc proteins. J. Mol. Biol. 426, 3166–3179 (2014).
Campbell, I. K. et al. Therapeutic effect of IVIG on inflammatory arthritis in mice is dependent on the Fc portion and independent of sialylation or basophils. J. Immunol. 192, 5031–5038 (2014).
Leontyev, D. et al. Sialylation-independent mechanism involved in the amelioration of murine immune thrombocytopenia using intravenous gammaglobulin. Transfusion 52, 1799–1805 (2012).
Tjon, A. S. et al. Intravenous immunoglobulin treatment in humans suppresses dendritic cell function via stimulation of IL-4 and IL-13 production. J. Immunol. 192, 5625–5634 (2014).
Bayry, J., Bansal, K., Kazatchkine, M. D. & Kaveri, S. V. DC-SIGN and alpha2,6-sialylated IgG Fc interaction is dispensable for the anti-inflammatory activity of IVIg on human dendritic cells. Proc. Natl Acad. Sci. USA 106, E24 (2009).
Guhr, T. et al. Enrichment of sialylated IgG by lectin fractionation does not enhance the efficacy of immunoglobulin G in a murine model of immune thrombocytopenia. PLoS ONE 6, e21246 (2011).
Malhotra, R. et al. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat. Med. 1, 237–243 (1995).
Nimmerjahn, F., Anthony, R. M. & Ravetch, J. V. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc. Natl Acad. Sci. USA 104, 8433–8437 (2007).
van de Geijn, F. E. et al. Mannose-binding lectin does not explain the course and outcome of pregnancy in rheumatoid arthritis. Arthritis Res. Ther. 13, R10 (2011).
van de Geijn, F. E. et al. Mannose-binding lectin polymorphisms are not associated with rheumatoid arthritis–confirmation in two large cohorts. Rheumatology 47, 1168–1171 (2008).
Lippold, S., Nicolardi, S., Wuhrer, M. & Falck, D. Proteoform-resolved FcRIIIa binding assay for Fab glycosylated monoclonal antibodies achieved by affinity chromatography mass spectrometry of Fc moieties. Front. Chem. 7, 698 (2019).
Quast, I. et al. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J. Clin. Invest. 125, 4160–4170 (2015).
Wei, B. et al. Fc galactosylation follows consecutive reaction kinetics and enhances immunoglobulin G hexamerization for complement activation. mAbs 13, 1893427 (2021).
Bartsch, Y. C. et al. IgG Fc sialylation is regulated during the germinal center reaction following immunization with different adjuvants. J. Allergy Clin. Immunol. 146, 652–666.e11 (2020).
Pfeifle, R. et al. Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat. Immunol. 18, 104–113 (2017).
Jones, M. B. et al. B-cell-independent sialylation of IgG. Proc. Natl Acad. Sci. USA 113, 7207–7212 (2016).
Glendenning, L. M., Zhou, J. Y., Reynero, K. M. & Cobb, B. A. Divergent Golgi trafficking limits B cell-mediated IgG sialylation. J. Leukoc. Biol. https://doi.org/10.1002/JLB.3MA0522-731R (2022).
Axford, J. S. et al. Reduced B-cell galactosyltransferase activity in rheumatoid arthritis. Lancet 2, 1486–1488 (1987).
Wang, J. et al. Fc-glycosylation of IgG1 is modulated by B-cell stimuli. Mol. Cell. Proteom. 10, M110 004655 (2011).
Engdahl, C. et al. Estrogen induces St6gal1 expression and increases IgG sialylation in mice and patients with rheumatoid arthritis: a potential explanation for the increased risk of rheumatoid arthritis in postmenopausal women. Arthritis Res. Ther. 20, 84 (2018).
Mijakovac, A. et al. Effects of estradiol on immunoglobulin G glycosylation: mapping of the downstream signaling mechanism. Front. Immunol. 12, 680227 (2021).
Ercan, A. et al. Estrogens regulate glycosylation of IgG in women and men. JCI Insight 2, e89703 (2017).
Lagattuta, K. A. & Nigrovic, P. A. Estrogen-driven changes in immunoglobulin G Fc glycosylation. Exp. Suppl. 112, 341–361 (2021).
van de Bovenkamp, F. S., Hafkenscheid, L., Rispens, T. & Rombouts, Y. The emerging importance of IgG Fab glycosylation in immunity. J. Immunol. 196, 1435–1441 (2016).
Anumula, K. R. Quantitative glycan profiling of normal human plasma derived immunoglobulin and its fragments Fab and Fc. J. Immunol. Methods 382, 167–176 (2012).
Endo, T., Wright, A., Morrison, S. L. & Kobata, A. Glycosylation of the variable region of immunoglobulin G — site specific maturation of the sugar chains. Mol. Immunol. 32, 931–940 (1995).
Dunn-Walters, D., Boursier, L. & Spencer, J. Effect of somatic hypermutation on potential N-glycosylation sites in human immunoglobulin heavy chain variable regions. Mol. Immunol. 37, 107–113 (2000).
Koers, J. et al. Biased N-glycosylation site distribution and acquisition across the antibody V region during B cell maturation. J. Immunol. 202, 2220–2228 (2019).
van de Bovenkamp, F. S. et al. Adaptive antibody diversification through N-linked glycosylation of the immunoglobulin variable region. Proc. Natl Acad. Sci. USA 115, 1901–1906 (2018).
van de Bovenkamp, F. S. et al. Variable domain N-linked glycans acquired during antigen-specific immune responses can contribute to Immunoglobulin G antibody stability. Front. Immunol. 9, 740 (2018).
Courtois, F., Agrawal, N. J., Lauer, T. M. & Trout, B. L. Rational design of therapeutic mAbs against aggregation through protein engineering and incorporation of glycosylation motifs applied to bevacizumab. mAbs 8, 99–112 (2016).
Leibiger, H., Wustner, D., Stigler, R. D. & Marx, U. Variable domain-linked oligosaccharides of a human monoclonal IgG: structure and influence on antigen binding. Biochem. J. 338, 529–538 (1999).
Tachibana, H., Kim, J. Y. & Shirahata, S. Building high affinity human antibodies by altering the glycosylation on the light chain variable region in N-acetylglucosamine-supplemented hybridoma cultures. Cytotechnology 23, 151–159 (1997).
Coloma, M. J., Trinh, R. K., Martinez, A. R. & Morrison, S. L. Position effects of variable region carbohydrate on the affinity and in vivo behavior of an anti-(1→6) dextran antibody. J. Immunol. 162, 2162–2170 (1999).
Sabouri, Z. et al. Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proc. Natl Acad. Sci. USA 111, E2567–E2575 (2014).
Hafkenscheid, L. et al. Structural analysis of variable domain glycosylation of anti-citrullinated protein antibodies in rheumatoid arthritis reveals the presence of highly sialylated glycans. Mol. Cell. Proteom. 16, 278–287 (2017).
Rombouts, Y. et al. Extensive glycosylation of ACPA-IgG variable domains modulates binding to citrullinated antigens in rheumatoid arthritis. Ann. Rheum. Dis. 75, 578–585 (2016).
Vergroesen, R. D. et al. B-cell receptor sequencing of anti-citrullinated protein antibody (ACPA) IgG-expressing B cells indicates a selective advantage for the introduction of N-glycosylation sites during somatic hypermutation. Ann. Rheum. Dis. 77, 956–958 (2018).
Vergroesen, R. D. et al. N-Glycosylation site analysis of citrullinated antigen-specific B-cell receptors indicates alternative selection pathways during autoreactive B-cell development. Front. Immunol. 10, 2092 (2019).
Kissel, T. et al. IgG anti-citrullinated protein antibody variable domain glycosylation increases before the onset of rheumatoid arthritis and stabilizes thereafter: a cross-sectional study encompassing ~1,500 samples. Arthritis Rheumatol. 74, 1147–1158 (2022).
Hafkenscheid, L. et al. N-Linked glycans in the variable domain of IgG anti-citrullinated protein antibodies predict the development of rheumatoid arthritis. Arthritis Rheumatol. 71, 1626–1633 (2019).
Kissel, T. et al. On the presence of HLA-SE alleles and ACPA-IgG variable domain glycosylation in the phase preceding the development of rheumatoid arthritis. Ann. Rheum. Dis. 78, 1616–1620 (2019).
Kissel, T. et al. Genetic predisposition (HLA-SE) is associated with ACPA-IgG variable domain glycosylation in the predisease phase of RA. Ann. Rheum. Dis. 81, 141–143 (2022).
Suwannalai, P. et al. Anti-citrullinated protein antibodies have a low avidity compared with antibodies against recall antigens. Ann. Rheum. Dis. 70, 373–379 (2011).
Kissel, T. et al. Surface Ig variable domain glycosylation affects autoantigen binding and acts as threshold for human autoreactive B cell activation. Sci. Adv. 8, eabm1759 (2022).
Rizzuto, G. et al. Establishment of fetomaternal tolerance through glycan-mediated B cell suppression. Nature 603, 497–502 (2022).
Cao, A. et al. Galectin-9 binds IgM-BCR to regulate B cell signaling. Nat. Commun. 9, 3288 (2018).
Meyer, S. J., Linder, A. T., Brandl, C. & Nitschke, L. B cell Siglecs — news on signaling and its interplay with ligand binding. Front. Immunol. 9, 2820 (2018).
Demetriou, M., Granovsky, M., Quaggin, S. & Dennis, J. W. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 409, 733–739 (2001).
Marth, J. D. & Grewal, P. K. Mammalian glycosylation in immunity. Nat. Rev. Immunol. 8, 874–887 (2008).
Dias, A. M. et al. Metabolic control of T cell immune response through glycans in inflammatory bowel disease. Proc. Natl Acad. Sci. USA 115, E4651–E4660 (2018).
Zhu, D. et al. Acquisition of potential N-glycosylation sites in the immunoglobulin variable region by somatic mutation is a distinctive feature of follicular lymphoma. Blood 99, 2562–2568 (2002).
Linley, A. et al. Lectin binding to surface Ig variable regions provides a universal persistent activating signal for follicular lymphoma cells. Blood 126, 1902–1910 (2015).
Radcliffe, C. M. et al. Human follicular lymphoma cells contain oligomannose glycans in the antigen-binding site of the B-cell receptor. J. Biol. Chem. 282, 7405–7415 (2007).
Coelho, V. et al. Glycosylation of surface Ig creates a functional bridge between human follicular lymphoma and microenvironmental lectins. Proc. Natl Acad. Sci. USA 107, 18587–18592 (2010).
Xu, P. C. et al. Influence of variable domain glycosylation on anti-neutrophil cytoplasmic autoantibodies and anti-glomerular basement membrane autoantibodies. BMC Immunol. 13, 10 (2012).
Hamza, N. et al. Ig gene analysis reveals altered selective pressures on Ig-producing cells in parotid glands of primary Sjogren’s syndrome patients. J. Immunol. 194, 514–521 (2015).
Visser, A., Hamza, N., Kroese, F. G. M. & Bos, N. A. Acquiring new N-glycosylation sites in variable regions of immunoglobulin genes by somatic hypermutation is a common feature of autoimmune diseases. Ann. Rheum. Dis. 77, e69 (2018).
Huijbers, M. G. et al. MuSK myasthenia gravis monoclonal antibodies: valency dictates pathogenicity. Neurol. Neuroimmunol. Neuroinflamm. 6, e547 (2019).
Mandel-Brehm, C. et al. Elevated N-linked glycosylation of IgG V regions in myasthenia gravis disease subtypes. J. Immunol. 207, 2005–2014 (2021).
Pereira, M. S. et al. Glycans as key checkpoints of T cell activity and function. Front. Immunol. 9, 2754 (2018).
Kanyo, N. et al. Glycocalyx regulates the strength and kinetics of cancer cell adhesion revealed by biophysical models based on high resolution label-free optical data. Sci. Rep. 10, 22422 (2020).
Shurer, C. R. et al. Physical principles of membrane shape regulation by the glycocalyx. Cell 177, 1757–1770.e21 (2019).
Paszek, M. J. et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature 511, 319–325 (2014).
Alves, I., Fernandes, A., Santos-Pereira, B., Azevedo, C. M. & Pinho, S. S. Glycans as a key factor in self and nonself discrimination: impact on the breach of immune tolerance. FEBS Lett. 596, 1485–1502 (2022).
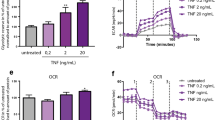
Wang, Y. et al. Loss of α2-6 sialylation promotes the transformation of synovial fibroblasts into a pro-inflammatory phenotype in arthritis. Nat. Commun. 12, 2343 (2021).
Yang, X., Lehotay, M., Anastassiades, T., Harrison, M. & Brockhausen, I. The effect of TNF-α on glycosylation pathways in bovine synoviocytes. Biochem. Cell Biol. 82, 559–568 (2004).
Filer, A. et al. Galectin 3 induces a distinctive pattern of cytokine and chemokine production in rheumatoid synovial fibroblasts via selective signaling pathways. Arthritis Rheum. 60, 1604–1614 (2009).
Pearson, M. J. et al. Endogenous galectin-9 suppresses apoptosis in human rheumatoid arthritis synovial fibroblasts. Sci. Rep. 8, 12887 (2018).
Forsman, H. et al. Galectin 3 aggravates joint inflammation and destruction in antigen-induced arthritis. Arthritis Rheum. 63, 445–454 (2011).
Ohshima, S. et al. Galectin 3 and its binding protein in rheumatoid arthritis. Arthritis Rheum. 48, 2788–2795 (2003).
Pichler, K. M. et al. Galectin network in osteoarthritis: galectin-4 programs a pathogenic signature of gene and effector expression in human chondrocytes in vitro. Histochem. Cell Biol. 157, 139–151 (2022).
Isozaki, T. et al. Fucosyltransferase 1 mediates angiogenesis, cell adhesion and rheumatoid arthritis synovial tissue fibroblast proliferation. Arthritis Res. Ther. 16, R28 (2014).
Xu, D. et al. Tumor necrosis factor-alpha up-regulates the expression of beta1,4-galactosyltransferase-I in human fibroblast-like synoviocytes. Inflammation 34, 531–538 (2011).
Przybysz, M., Maszczak, D., Borysewicz, K., Szechinski, J. & Katnik-Prastowska, I. Relative sialylation and fucosylation of synovial and plasma fibronectins in relation to the progression and activity of rheumatoid arthritis. Glycoconj. J. 24, 543–550 (2007).
Flowers, S. A. et al. Lubricin binds cartilage proteins, cartilage oligomeric matrix protein, fibronectin and collagen II at the cartilage surface. Sci. Rep. 7, 13149 (2017).
Estrella, R. P., Whitelock, J. M., Packer, N. H. & Karlsson, N. G. The glycosylation of human synovial lubricin: implications for its role in inflammation. Biochem. J. 429, 359–367 (2010).
Alves, I. et al. Protein mannosylation as a diagnostic and prognostic biomarker of lupus nephritis: an unusual glycan neoepitope in systemic lupus erythematosus. Arthritis Rheumatol. 73, 2069–2077 (2021).
Urushihara, M. et al. Sisters with α-mannosidosis and systemic lupus erythematosus. Eur. J. Pediatr. 163, 192–195 (2004).
Chui, D. et al. Genetic remodeling of protein glycosylation in vivo induces autoimmune disease. Proc. Natl Acad. Sci. USA 98, 1142–1147 (2001).
van Kooyk, Y. & Rabinovich, G. A. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 9, 593–601 (2008).
Cai, M. et al. DC-SIGN expression on podocytes and its role in inflammatory immune response of lupus nephritis. Clin. Exp. Immunol. 183, 317–325 (2016).
Noguchi, S. et al. Reduction of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase activity and sialylation in distal myopathy with rimmed vacuoles. J. Biol. Chem. 279, 11402–11407 (2004).
Malicdan, M. C., Noguchi, S., Hayashi, Y. K., Nonaka, I. & Nishino, I. Prophylactic treatment with sialic acid metabolites precludes the development of the myopathic phenotype in the DMRV-hIBM mouse model. Nat. Med. 15, 690–695 (2009).
Carrillo, N. et al. Safety and efficacy of N-acetylmannosamine (ManNAc) in patients with GNE myopathy: an open-label phase 2 study. Genet. Med. 23, 2067–2075 (2021).
McGovern, D. P. et al. Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn’s disease. Hum. Mol. Genet. 19, 3468–3476 (2010).
Goto, Y. et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 345, 1254009 (2014).
Morosi, L. G. et al. Control of intestinal inflammation by glycosylation-dependent lectin-driven immunoregulatory circuits. Sci. Adv. 7, eabf8630 (2021).
Costa, A. F., Campos, D., Reis, C. A. & Gomes, C. Targeting glycosylation: a new road for cancer drug discovery. Trends Cancer 6, 757–766 (2020).
Dube, D. H. & Bertozzi, C. R. Glycans in cancer and inflammation — potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 4, 477–488 (2005).
Nandakumar, K. S. et al. Endoglycosidase treatment abrogates IgG arthritogenicity: importance of IgG glycosylation in arthritis. Eur. J. Immunol. 37, 2973–2982 (2007).
Albert, H., Collin, M., Dudziak, D., Ravetch, J. V. & Nimmerjahn, F. In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner. Proc. Natl Acad. Sci. USA 105, 15005–15009 (2008).
Collin, M., Shannon, O. & Bjorck, L. IgG glycan hydrolysis by a bacterial enzyme as a therapy against autoimmune conditions. Proc. Natl Acad. Sci. USA 105, 4265–4270 (2008).
Nandakumar, K. S. et al. Dominant suppression of inflammation by glycan-hydrolyzed IgG. Proc. Natl Acad. Sci. USA 111, 15851 (2014).
Trastoy, B. et al. Structural basis for the recognition of complex-type N-glycans by Endoglycosidase S. Nat. Commun. 9, 1874 (2018).
Bartsch, Y. C. et al. Sialylated autoantigen-reactive IgG antibodies attenuate disease development in autoimmune mouse models of lupus nephritis and rheumatoid arthritis. Front. Immunol. 9, 1183 (2018).
Verhelst, X. et al. Protein glycosylation as a diagnostic and prognostic marker of chronic inflammatory gastrointestinal and liver diseases. Gastroenterology 158, 95–110 (2020).
Araujo, L., Khim, P., Mkhikian, H., Mortales, C. L. & Demetriou, M. Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation. eLife 6, e21330 (2017).
Salvatore, S. et al. A pilot study of N-acetyl glucosamine, a nutritional substrate for glycosaminoglycan synthesis, in paediatric chronic inflammatory bowel disease. Aliment. Pharmacol. Ther. 14, 1567–1579 (2000).
Mkhikian, H. et al. Genetics and the environment converge to dysregulate N-glycosylation in multiple sclerosis. Nat. Commun. 2, 334 (2011).
Brandt, A. U. et al. Association of a marker of N-acetylglucosamine with progressive multiple sclerosis and neurodegeneration. JAMA Neurol. 78, 842–852 (2021).
Pereira, N. A., Chan, K. F., Lin, P. C. & Song, Z. The “less-is-more” in therapeutic antibodies: afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. mAbs 10, 693–711 (2018).
Macauley, M. S., Crocker, P. R. & Paulson, J. C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 14, 653–666 (2014).
Kristyanto, H. et al. Multifunctional, multivalent PIC polymer scaffolds for targeting antigen-specific, autoreactive B cells. ACS Biomater. Sci. Eng. 8, 1486–1493 (2022).
Caianiello, D. F. et al. Bifunctional small molecules that mediate the degradation of extracellular proteins. Nat. Chem. Biol. 17, 947–953 (2021).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
R.E.M.T. and T.W.J.H. are mentioned inventors on a patent application on ACPA-IgG V-domain glycosylation. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Salomé Pinho, Mattias Collin and Peter Nigrovic for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kissel, T., Toes, R.E.M., Huizinga, T.W.J. et al. Glycobiology of rheumatic diseases. Nat Rev Rheumatol 19, 28–43 (2023). https://doi.org/10.1038/s41584-022-00867-4
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41584-022-00867-4
This article is cited by
-
Targeting heterogeneous depression with trazodone prolonged release: from neuropharmacology to clinical application
Annals of General Psychiatry (2025)
-
Antibody glycosylation in neuroimmune diseases
Journal of Translational Medicine (2025)
-
Development and multi-center validation of machine learning models based on targeted metabolomics for rheumatoid arthritis
Journal of Translational Medicine (2025)
-
Proteomic analysis of hydrogen peroxide-treated human chondrocytes shows endoplasmic reticulum stress, cytoskeleton remodeling, and altered secretome composition
Cell Communication and Signaling (2025)
-
Immunoglobulin G N-glycosylation predicts long-term risk of ischemic stroke: a nested case-control study
Journal of Translational Medicine (2025)