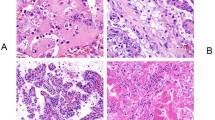
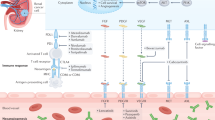
Abstract
Sarcomatoid dedifferentiation is an uncommon feature that can occur in most histological subtypes of renal cell carcinomas (RCCs) and carries a decidedly poor prognosis. Historically, conventional treatments for sarcomatoid RCCs (sRCCs) have shown little efficacy, and median survival is commonly 6–13 months. Despite being first described in 1968, the mechanisms driving sarcomatoid dedifferentiation remain poorly understood, and information and treatment options available to physicians and patients are limited. When diagnosed at an early stage, surgical intervention remains the treatment of choice. However, preoperative identification through routine imaging or biopsy is unreliable and most patients present with advanced disease and systemic symptoms. For these patients, the role of cytoreductive nephrectomy is disputed. The expansion of immunotherapies approved for RCCs has generated a search for biomarkers that might be indicative of treatment response in sRCCs, although a proven effective systemic agent remains elusive. PDL1 expression is increased in sarcomatoid dedifferentiated renal tumours, which suggests that patients with sRCCs could benefit from PD1 and/or PDL1 immune checkpoint blockade therapy. Treatment outcomes for sarcomatoid tumours have remained relatively consistent compared with other RCCs, but further investigation of the tumour–immune cell microenvironment might yield insights into further therapeutic possibilities.
Key Points
-
Sarcomatoid dedifferentiation is not considered to be a unique histological subtype of renal cell carcinomas (RCCs); rather, it can be present within any subtype of RCCs.
-
Sarcomatoid dedifferentiation appears in ~4% of all RCCs, but is present in ~20% of all metastatic RCCs. According to WHO guidelines, any RCC with sarcomatoid dedifferentiation is a WHO–International Society of Urological Pathology grade 4 lesion.
-
Sarcomatoid dedifferentiation is often heterogeneously present within RCCs, making routine imaging and biopsy unreliable for preoperative detection. Surgical resection for localized disease is the standard of care, with subsequent close monitoring of patients following surgery.
-
In patients with metastatic disease, conventional therapies such as surgery and systemic agents have been ineffective and overall 5-year survival remains at 23.5–33%.
-
Previous genomic analyses have failed to identify definitive mutational drivers of disease. However, sarcomatoid RCCs (sRCCs) have been shown to have higher PD1 and PDL1 expression than other subtypes of RCCs. Newer combinations of immune checkpoint inhibitor immunotherapies could yield improved responses and outcomes.
-
Studies investigating sRCCs are limited by patient numbers owing to the low incidence of sRCCs and their advanced stage at presentation. Multi-institutional efforts to establish a consensus on treatment recommendations based on highly powered data are essential.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34 (2019).
Capitanio, U. et al. Epidemiology of renal cell carcinoma. Eur. Urol. 75, 74–84 (2019).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Motzer, R. J. et al. Kidney cancer, version 2.2017, NCCN clinical practice guidelines in oncology. J. Natl Compr. Canc. Netw. 15, 804–834 (2017).
Delahunt, B. Sarcomatoid renal carcinoma: the final common dedifferentiation pathway of renal epithelial malignancies. Pathology 31, 185–190 (1999).
de Peralta-Venturina, M. et al. Sarcomatoid differentiation in renal cell carcinoma: a study of 101 cases. Am. J. Surg. Pathol. 25, 275–284 (2001).
Mian, B. M. et al. Prognostic factors and survival of patients with sarcomatoid renal cell carcinoma. J. Urol. 167, 65–70 (2002).
Shuch, B. et al. Quality of pathological reporting for renal cell cancer: implications for systemic therapy, prognostication and surveillance. BJU Int. 108, 343–348 (2011).
Shuch, B. et al. Histologic evaluation of metastases in renal cell carcinoma with sarcomatoid transformation and its implications for systemic therapy. Cancer 116, 616–624 (2010).
Cheville, J. C. et al. Sarcomatoid renal cell carcinoma: an examination of underlying histologic subtype and an analysis of associations with patient outcome. Am. J. Surg. Pathol. 28, 435–441 (2004).
Kim, T. et al. Using percentage of sarcomatoid differentiation as a prognostic factor in renal cell carcinoma. Clin. Genitourin. Cancer 13, 225–230 (2015).
Brookman-May, S. et al. Prognostic effect of sarcomatoid dedifferentiation in patients with surgically treated renal cell carcinoma: a matched-pair analysis. Clin. Genitourin. Cancer 11, 465–470 (2013).
Gu, L. et al. Presence of sarcomatoid differentiation as a prognostic indicator for survival in surgically treated metastatic renal cell carcinoma. J. Cancer Res. Clin. Oncol. 143, 499–508 (2017).
Alevizakos, M., Gaitanidis, A., Nasioudis, D., Msaouel, P. & Appleman, L. J. Sarcomatoid renal cell carcinoma: population-based study of 879 patients. Clin. Genitourin. Cancer 17, e447–e453 (2019).
Shuch, B. et al. Cytoreductive nephrectomy for kidney cancer with sarcomatoid histology–is up-front resection indicated and, if not, is it avoidable? J. Urol. 182, 2164–2171 (2009).
Korenbaum, C. et al. Treatments, outcomes, and validity of prognostic scores in patients with sarcomatoid renal cell carcinoma: a 20-year single-institution experience. Clin. Genitourin. Cancer 16, e577–e586 (2018).
Cangiano, T. et al. Sarcomatoid renal cell carcinoma: biologic behavior, prognosis, and response to combined surgical resection and immunotherapy. J. Clin. Oncol. 17, 523–528 (1999).
Ro, J. Y., Ayala, A. G., Sella, A., Samuels, M. L. & Swanson, D. A. Sarcomatoid renal cell carcinoma: clinicopathologic. A study of 42 cases. Cancer 59, 516–526 (1987).
Keskin, S. K. et al. Outcomes of patients with renal cell carcinoma and sarcomatoid dedifferentiation treated with nephrectomy and systemic therapies: comparison between the cytokine and targeted therapy eras. J. Urol. 198, 530–537 (2017).
Lucca, I., Klatte, T., Fajkovic, H., de Martino, M. & Shariat, S. F. Gender differences in incidence and outcomes of urothelial and kidney cancer. Nat. Rev. Urol. 12, 585–592 (2015).
Zhang, B. Y. et al. A novel prognostic model for patients with sarcomatoid renal cell carcinoma. BJU Int. 115, 405–411 (2015).
Adibi, M. et al. Percentage of sarcomatoid component as a prognostic indicator for survival in renal cell carcinoma with sarcomatoid dedifferentiation. Urol. Oncol. 33, 427.e417–423 (2015).
Trudeau, V. et al. Comparison of oncologic outcomes between sarcomatoid and clear cell renal cell carcinoma. World J. Urol. 34, 1429–1436 (2016).
Merrill, M. M. et al. Clinically nonmetastatic renal cell carcinoma with sarcomatoid dedifferentiation: natural history and outcomes after surgical resection with curative intent. Urol. Oncol. 33, 166.e121–169 (2015).
Russo, P. et al. Survival rates after resection for localized kidney cancer: 1989 to 2004. Cancer 113, 84–96 (2008).
Shuch, B. et al. Impact of pathological tumour characteristics in patients with sarcomatoid renal cell carcinoma. BJU Int. 109, 1600–1606 (2012).
Weisel, W., Dockerty, M. B. & Priestley, J. T. Sarcoma of the kidney. J. Urol. 50, 564–573 (1943).
Lee-Tsün, H. & Willis, R. A. Renal carcino-sarcoma, true and false. J. Pathol. Bacteriol. 85, 139–144 (1963).
Farrow, G. M., Harrison, E. G. Jr & Utz, D. C. Sarcomas and sarcomatoid and mixed malignant tumors of the kidney in adults. 3. Cancer 22, 556–563 (1968).
Thoenes, W., Storkel, S. & Rumpelt, H. J. Histopathology and classification of renal cell tumors (adenomas, oncocytomas and carcinomas). The basic cytological and histopathological elements and their use for diagnostics. Pathol. Res. Pract. 181, 125–143 (1986).
Storkel, S. et al. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer 80, 987–989 (1997).
Kovacs, G. et al. The Heidelberg classification of renal cell tumours. J. Pathol. 183, 131–133 (1997).
Delahunt, B. et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am. J. Surg. Pathol. 37, 1490–1504 (2013).
Moch, H., Cubilla, A. L., Humphrey, P. A., Reuter, V. E. & Ulbright, T. M. The 2016 WHO classification of tumours of the urinary system and male genital organs — part A: renal, penile, and testicular tumours. Eur. Urol. 70, 93–105 (2016).
Shuch, B., Bratslavsky, G., Linehan, W. M. & Srinivasan, R. Sarcomatoid renal cell carcinoma: a comprehensive review of the biology and current treatment strategies. Oncologist 17, 46–54 (2012).
Sella, A., Logothetis, C. J., Ro, J. Y., Swanson, D. A. & Samuels, M. L. Sarcomatoid renal cell carcinoma. A treatable entity. Cancer 60, 1313–1318 (1987).
Abel, E. J. et al. Limitations of preoperative biopsy in patients with metastatic renal cell carcinoma: comparison to surgical pathology in 405 cases. BJU Int. 110, 1742–1746 (2012).
Schieda, N. et al. Diagnosis of sarcomatoid renal cell carcinoma with CT: evaluation by qualitative imaging features and texture analysis. AJR Am. J. Roentgenol 204, 1013–1023 (2015).
Rosenkrantz, A. B., Chandarana, H. & Melamed, J. MRI findings of sarcomatoid renal cell carcinoma in nine cases. Clin. Imaging 35, 459–464 (2011).
Takeuchi, M. et al. Characteristic MRI findings of sarcomatoid renal cell carcinoma dedifferentiated from clear cell renal carcinoma: radiological-pathological correlation. Clin. Imaging 37, 908–912 (2013).
Takeuchi, M. et al. MRI for differentiation of renal cell carcinoma with sarcomatoid component from other renal tumor types. Abdom. Imaging 40, 112–119 (2015).
Jeong, D. et al. Quantification of sarcomatoid differentiation in renal cell carcinoma on magnetic resonance imaging. Quant. Imaging Med. Surg. 8, 373–382 (2018).
Fuser, D., Hedberg, M. L., Dehner, L. P., Dehdashti, F. & Siegel, B. A. Extensive metastatic Sarcomatoid renal cell carcinoma evaluated by (18)F-FDG PET/CT: a case report and review of literature. J. Kidney Cancer VHL 5, 1–6 (2018).
Thambugala, G. M., Mohamed, A., O’Neill, G. F. & Fulham, M. J. Sarcomatoid renal cell carcinoma: rapid dissemination detected on FDG PET-CT. Australas. Radiol. 50, 604–606 (2006).
Hyodo, T. et al. Widespread metastases from sarcomatoid renal cell carcinoma detected by 18F-FDG positron emission tomography/computed tomography. Japn. J. Radiol. 27, 111–114 (2009).
Nadebaum, D. P., Hofman, M. S., Mitchell, C. A., Siva, S. & Hicks, R. J. Oligometastatic renal cell carcinoma with sarcomatoid differentiation demonstrating variable imaging phenotypes on 68Ga-PSMA and 18F-FDG PET/CT: a case report and review of the literature. Clin. Genitourin. Cancer 16, 1–5 (2018).
Vogel, C. et al. Imaging in suspected renal-cell carcinoma: systematic review. Clin. Genitourin. Cancer 17, e345–e355 (2019).
Donat, S. M. et al. Follow-up for clinically localized renal neoplasms: AUA guideline. J. Urol. 190, 407–416 (2013).
Escudier, B. et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 25, iii49–iii56 (2014).
Ljungberg, B. et al. in European Association of Urology Guidelines. 2018 Edition. Vol. presented at the EAU Annual Congress Copenhagen 2018 (European Association of Urology Guidelines Office, 2018).
Kuroda, N., Toi, M., Hiroi, M. & Enzan, H. Review of sarcomatoid renal cell carcinoma with focus on clinical and pathobiological aspects. Histol. Histopathol. 18, 551–555 (2003).
Reuter, V. E. Sarcomatoid lesions of the urogenital tract. Semin. Diagn. Pathol. 10, 188–201 (1993).
Kwak, C. et al. Sarcomatoid differentiation as a prognostic factor for immunotherapy in metastatic renal cell carcinoma. J. Surg. Oncol. 95, 317–323 (2007).
Leibovich, B. C. et al. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J. Urol. 183, 1309–1315 (2010).
Casuscelli, J. et al. Chromophobe renal cell carcinoma: results from a large single-institution series. Clin. Genitourin. Cancer 17, 373–379.e374 (2019).
Akhtar, M., Tulbah, A., Kardar, A. H. & Ali, M. A. Sarcomatoid renal cell carcinoma: the chromophobe connection. Am. J. Surg. Pathol. 21, 1188–1195 (1997).
Kuroda, N. et al. Acquired cystic disease-associated renal cell carcinoma with sarcomatoid change and rhabdoid features. Ann. Diagn. Pathol. 15, 462–466 (2011).
Chen, Y. B. et al. Hereditary leiomyomatosis and renal cell carcinoma syndrome-associated renal cancer: recognition of the syndrome by pathologic features and the utility of detecting aberrant succination by immunohistochemistry. Am. J. Surg. Pathol. 38, 627–637 (2014).
Udager, A. M. et al. Hereditary leiomyomatosis and renal cell carcinoma (HLRCC): a rapid autopsy report of metastatic renal cell carcinoma. Am. J. Surg. Pathol. 38, 567–577 (2014).
He, H. & Magi-Galluzzi, C. Epithelial-to-mesenchymal transition in renal neoplasms. Adv. Anat. Pathol. 21, 174–180 (2014).
Tong, G. X. et al. Expression of PAX8 in normal and neoplastic renal tissues: an immunohistochemical study. Mod. Pathol. 22, 1218–1227 (2009).
Ozcan, A., de la Roza, G., Ro, J. Y., Shen, S. S. & Truong, L. D. PAX2 and PAX8 expression in primary and metastatic renal tumors: a comprehensive comparison. Arch. Pathol. Lab. Med. 136, 1541–1551 (2012).
Tickoo, S. K. et al. Immunohistochemical expression of hypoxia inducible factor-1alpha and its downstream molecules in sarcomatoid renal cell carcinoma. J. Urol. 177, 1258–1263 (2007).
Kutikov, A. et al. Renal mass biopsy: always, sometimes, or never? Eur. Urol. 70, 403–406 (2016).
Auger, M. et al. Fine-needle aspiration cytology of sarcomatoid renal cell carcinoma: a morphologic and immunocytochemical study of 15 cases. Diagn. Cytopathol. 9, 46–51 (1993).
Abel, E. J. et al. Multi-quadrant biopsy technique improves diagnostic ability in large heterogeneous renal masses. J. Urol. 194, 886–891 (2015).
Manley, B. J. & Hsieh, J. J. Sarcomatoid renal cell carcinoma: genomic insights from sequencing of matched sarcomatous and carcinomatous components. Transl. Cancer Res. 5, S160–s165 (2016).
Conant, J. L., Peng, Z., Evans, M. F., Naud, S. & Cooper, K. Sarcomatoid renal cell carcinoma is an example of epithelial–mesenchymal transition. J. Clin. Pathol. 64, 1088–1092 (2011).
Jones, T. D. et al. Clonal divergence and genetic heterogeneity in clear cell renal cell carcinomas with sarcomatoid transformation. Cancer 104, 1195–1203 (2005).
Wick, M. R. & Swanson, P. E. Carcinosarcomas: current perspectives and an historical review of nosological concepts. Semin. Diagn. Pathol. 10, 118–127 (1993).
Kalluri, R. EMT: when epithelial cells decide to become mesenchymal-like cells. J. Clin. Invest. 119, 1417–1419 (2009).
Zeisberg, M. & Neilson, E. G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Invest. 119, 1429–1437 (2009).
Iwatsuki, M. et al. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 101, 293–299 (2010).
De Craene, B. & Berx, G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 13, 97–110 (2013).
Huber, M. A., Kraut, N. & Beug, H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 17, 548–558 (2005).
Cano, A. et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76–83 (2000).
Guarino, M., Rubino, B. & Ballabio, G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology 39, 305–318 (2007).
Lamouille, S., Xu, J. & Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196 (2014).
Piloto, S. & Schilling, T. F. Ovo1 links Wnt signaling with N-cadherin localization during neural crest migration. Development 137, 1981–1990 (2010).
Gumbiner, B. M. Regulation of cadherin adhesive activity. J. Cell Biol. 148, 399–404 (2000).
Sircar, K. et al. Biphasic components of sarcomatoid clear cell renal cell carcinomas are molecularly similar to each other, but distinct from, non-sarcomatoid renal carcinomas. J. Pathol. 1, 212–224 (2015).
Hsieh, C. H. et al. Co-existence of epithelioid and fibroblastoid subsets in a sarcomatoid renal carcinoma cell line revealed by clonal studies. Anticancer. Res. 33, 4875–4889 (2013).
Lopez-Lago, M. A. et al. Genomic deregulation during metastasis of renal cell carcinoma implements a myofibroblast-like program of gene expression. Cancer Res. 70, 9682–9692 (2010).
Braga, E. A., Fridman, M. V., Loginov, V. I., Dmitriev, A. A. & Morozov, S. G. Molecular mechanisms in clear cell renal cell carcinoma: role of miRNAs and hypermethylated miRNA genes in crucial oncogenic pathways and processes. Front. Genet. 10, 320 (2019).
Mann, J. et al. Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: implications for wound healing and fibrogenesis. Cell Death Differ. 14, 275–285 (2007).
Dumont, N. et al. Sustained induction of epithelial to mesenchymal transition activates DNA methylation of genes silenced in basal-like breast cancers. Proc. Natl Acad. Sci. USA 105, 14867–14872 (2008).
Xu, M. et al. miR-203 inhibition of renal cancer cell proliferation, migration and invasion by targeting of FGF2. Diagn. Pathol. 10, 24 (2015).
Schneider, M., Hansen, J. L. & Sheikh, S. P. S100A4: a common mediator of epithelial-mesenchymal transition, fibrosis and regeneration in diseases? J. Mol. Med. 86, 507–522 (2008).
Helfman, D. M., Kim, E. J., Lukanidin, E. & Grigorian, M. The metastasis associated protein S100A4: role in tumour progression and metastasis. Br. J. Cancer 92, 1955–1958 (2005).
Oda, H., Nakatsuru, Y. & Ishikawa, T. Mutations of the p53 gene and p53 protein overexpression are associated with sarcomatoid transformation in renal cell carcinomas. Cancer Res. 55, 658–662 (1995).
Linehan, W. M., Lerman, M. I. & Zbar, B. Identification of the von Hippel-Lindau (VHL) gene. Its role in renal cancer. JAMA 273, 564–570 (1995).
Clark, P. E. The role of VHL in clear-cell renal cell carcinoma and its relation to targeted therapy. Kidney Int. 76, 939–945 (2009).
Bi, M. et al. Genomic characterization of sarcomatoid transformation in clear cell renal cell carcinoma. Proc. Natl Acad. Sci. USA 113, 2170–2175 (2016).
Malouf, G. G. et al. Genomic characterization of renal cell carcinoma with sarcomatoid dedifferentiation pinpoints recurrent genomic alterations. Eur. Urol. 70, 348–357 (2016).
Wei, S. & Al-Saleem, T. The pathology and molecular genetics of sarcomatoid renal cell carcinoma: a mini-review. J. Kidney Cancer VHL 4, 19–23 (2017).
Wang, Z. et al. Sarcomatoid renal cell carcinoma has a distinct molecular pathogenesis, driver mutation profile, and transcriptional landscape. Clin. Cancer Res. 23, 6686–6696 (2017).
Bergerot, P., Agarwal, N., Pal, S. K. & Jones, J. Sarcomatoid renal cell carcinoma: the apple doesn’t fall far from the tree. Clin. Cancer Res. 23, 6381–6383 (2017).
Creighton, C. J. et al. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499, 43–49 (2013).
Gupta, S. et al. JAK2/PD-L1/PD-L2 (9p24.1) amplifications in renal cell carcinomas with sarcomatoid transformation: implications for clinical management. Mod. Pathol. https://doi.org/10.1038/s41379-019-0269-x (2019).
Kawakami, F. et al. Programmed cell death ligand 1 and tumor-infiltrating lymphocyte status in patients with renal cell carcinoma and sarcomatoid dedifferentiation. Cancer 123, 4823–4831 (2017).
Wasser, C. R. & Herz, J. Reelin: neurodevelopmental architect and homeostatic regulator of excitatory synapses. J. Biol. Chem. 292, 1330–1338 (2017).
Yuan, Y., Chen, H., Ma, G., Cao, X. & Liu, Z. Reelin is involved in transforming growth factor-beta1-induced cell migration in esophageal carcinoma cells. PLoS One 7, e31802 (2012).
Kulkarni, A., Chang, M. T., Vissers, J. H. A., Dey, A. & Harvey, K. F. The Hippo pathway as a driver of select human cancers. Trends Cancer 6, 781–796 (2020).
Meng, Z., Moroishi, T. & Guan, K. L. Mechanisms of Hippo pathway regulation. Genes Dev. 30, 1–17 (2016).
Chen, Y. B. et al. Molecular analysis of aggressive renal cell carcinoma with unclassified histology reveals distinct subsets. Nat. Commun. 7, 13131 (2016).
Zhao, B., Tumaneng, K. & Guan, K. L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 13, 877–883 (2011).
Shao, Diane D. et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell 158, 171–184 (2014).
Lamar, J. M. et al. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc. Natl Acad. Sci. USA 109, E2441–E2450 (2012).
Liu, Y. et al. YAP modulates TGF-β1-induced simultaneous apoptosis and EMT through upregulation of the EGF receptor. Sci. Rep. 7, 45523 (2017).
Li, Z. et al. The Hippo transducer TAZ promotes epithelial to mesenchymal transition and cancer stem cell maintenance in oral cancer. Mol. Oncol. 9, 1091–1105 (2015).
Wang, Y., Liu, J., Ying, X., Lin, P. C. & Zhou, B. P. Twist-mediated epithelial-mesenchymal transition promotes breast tumor cell invasion via inhibition of hippo pathway. Sci. Rep. 6, 24606 (2016).
Malouf, G. G. et al. Molecular characterization of sarcomatoid clear cell renal cell carcinoma unveils new candidate oncogenic drivers. Sci. Rep. 10, 701 (2020).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Shin, S. J. et al. Clinicopathologic analysis of PD-L1 and PD-L2 expression in renal cell carcinoma: association with oncogenic proteins status. Ann. Surg. Oncol. 23, 694–702 (2016).
Chen, L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 4, 336–347 (2004).
Weinstock, M. & McDermott, D. Targeting PD-1/PD-L1 in the treatment of metastatic renal cell carcinoma. Ther. Adv. Urol. 7, 365–377 (2015).
Thompson, R. H. et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 66, 3381–3385 (2006).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012).
Thompson, R. H. et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin. Cancer Res. 13, 1757–1761 (2007).
Choueiri, T. K. et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann. Oncol. 25, 2178–2184 (2014).
Joseph, R. W. et al. PD-1 and PD-L1 expression in renal cell carcinoma with sarcomatoid differentiation. Cancer Immunol. Res. 3, 1303–1307 (2015).
Mickisch, G. H., Garin, A., van Poppel, H., de Prijck, L. & Sylvester, R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet 358, 966–970 (2001).
Flanigan, R. C. et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N. Engl. J. Med. 345, 1655–1659 (2001).
Aslam, M. Z. & Matthews, P. N. Cytoreductive nephrectomy for metastatic renal cell carcinoma: a review of the historical literature and its role in the era of targeted molecular therapy. ISRN Urol. 2014, 717295 (2014).
Hanna, N. et al. Survival analyses of patients with metastatic renal cancer treated with targeted therapy with or without cytoreductive nephrectomy: a national cancer data base study. J. Clin. Oncol. 34, 3267–3275 (2016).
Petrelli, F. et al. Cytoreductive nephrectomy in metastatic renal cell carcinoma treated with targeted therapies: a systematic review with a meta-analysis. Clin. Genitourin. Cancer 14, 465–472 (2016).
Heng, D. Y. et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur. Urol. 66, 704–710 (2014).
Kutikov, A. et al. Use of systemic therapy and factors affecting survival for patients undergoing cytoreductive nephrectomy. BJU Int. 106, 218–223 (2010).
Thomas, A. Z. et al. The role of metastasectomy in patients with renal cell carcinoma with sarcomatoid dedifferentiation: a matched controlled analysis. J. Urol. 196, 678–684 (2016).
Motzer, R. J. & Russo, P. Cytoreductive nephrectomy — patient selection is key. N. Engl. J. Med. 379, 481–482 (2018).
Ljungberg, B. et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur. Urol. 67, 913–924 (2015).
Deschavanne, P. J. & Fertil, B. A review of human cell radiosensitivity in vitro. Int. J. Radiat. Oncol. Biol. Phys. 34, 251–266 (1996).
Tunio, M. A., Hashmi, A. & Rafi, M. Need for a new trial to evaluate postoperative radiotherapy in renal cell carcinoma: a meta-analysis of randomized controlled trials. Ann. Oncol. 21, 1839–1845 (2010).
van der Werf-Messing, B. Proceedings: carcinoma of the kidney. Cancer 32, 1056–1061 (1973).
Siva, S. et al. Radiotherapy for renal cell carcinoma: renaissance of an overlooked approach. Nat. Rev. Urol. 14, 549 (2017).
Eminaga, O., Akbarov, I., Wille, S. & Engelmann, U. Does postoperative radiation therapy impact survival in non-metastatic sarcomatoid renal cell carcinoma? A SEER-based study. Int. Urol. Nephrol. 47, 1653–1663 (2015).
Alt, A. L. et al. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer 117, 2873–2882 (2011).
Dabestani, S. et al. Local treatments for metastases of renal cell carcinoma: a systematic review. Lancet Oncol. 15, e549–e561 (2014).
Correa, R. J. M. et al. The emerging role of stereotactic ablative radiotherapy for primary renal cell carcinoma: a systematic review and meta-analysis. Eur. Urol. Focus. (2019).
Staehler, M. et al. Single fraction radiosurgery for the treatment of renal tumors. J. Urol. 193, 771–775 (2015).
Frick, M. A., Chhabra, A. M., Lin, L. & Simone, C. B. II, First ever use of proton stereotactic body radiation therapy delivered with curative intent to bilateral synchronous primary renal cell carcinomas. Cureus 9, e1799 (2017).
Golshayan, A. R. et al. Metastatic sarcomatoid renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. J. Clin. Oncol. 27, 235–241 (2009).
Voss, M. H. et al. Treatment outcome with mTOR inhibitors for metastatic renal cell carcinoma with nonclear and sarcomatoid histologies. Ann. Oncol. 25, 663–668 (2014).
Pinedo, H. M. & Verweij, J. The treatment of soft tissue sarcomas with focus on chemotherapy: a review. Radiother. Oncol. 5, 193–205 (1986).
Culine, S., Bekradda, M., Terrier-Lacombe, M. J. & Droz, J. P. Treatment of sarcomatoid renal cell carcinoma: is there a role for chemotherapy? Eur. Urol. 27, 138–141 (1995).
Escudier, B. et al. Doxorubicin and ifosfamide in patients with metastatic sarcomatoid renal cell carcinoma: a phase II study of the Genitourinary Group of the French Federation of Cancer Centers. J. Urol. 168, 959–961 (2002).
Nanus, D. M., Garino, A., Milowsky, M. I., Larkin, M. & Dutcher, J. P. Active chemotherapy for sarcomatoid and rapidly progressing renal cell carcinoma. Cancer 101, 1545–1551 (2004).
Haas, N. B. et al. A phase II trial of doxorubicin and gemcitabine in renal cell carcinoma with sarcomatoid features: ECOG 8802. Med. Oncol. 29, 761–767 (2012).
Dutcher, J. P. & Nanus, D. Long-term survival of patients with sarcomatoid renal cell cancer treated with chemotherapy. Med. Oncol. 28, 1530–1533 (2011).
Staehler, M. et al. Sorafenib after combination therapy with gemcitabine plus doxorubicine in patients with sarcomatoid renal cell carcinoma: a prospective evaluation. Eur. J. Med. Res. 15, 287–291 (2010).
Kyriakopoulos, C. E. et al. Outcome of patients with metastatic sarcomatoid renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Clin. Genitourin. Cancer 13, e79–e85 (2015).
Molina, A. M. et al. Sarcomatoid-variant renal cell carcinoma: treatment outcome and survival in advanced disease. Am. J. Clin. Oncol. 34, 454–459 (2011).
Michaelson, M. D. et al. Phase 2 trial of sunitinib and gemcitabine in patients with sarcomatoid and/or poor-risk metastatic renal cell carcinoma. Cancer 121, 3435–3443 (2015).
Maiti, A. et al. Phase 2 trial of capecitabine, gemcitabine, and bevacizumab in sarcomatoid renal-cell carcinoma. Clin. Genitourin. Cancer (2017).
Jonasch, E. et al. Treatment of metastatic renal carcinoma patients with the combination of gemcitabine, capecitabine and bevacizumab at a tertiary cancer centre. BJU Int. 107, 741–747 (2011).
Haas, N. B. et al. ECOG 1808: Randomized phase II trial of sunitinib with or without gemcitabine in advanced kidney cancer with sarcomatoid features. J. Clin. Oncol. 34, 4511–4511 (2016).
Achkar, T. et al. High-dose interleukin 2 in patients with metastatic renal cell carcinoma with sarcomatoid features. PLoS One 12, e0190084 (2017).
Figlin, R. et al. Interleukin-2-based immunotherapy for the treatment of metastatic renal cell carcinoma: an analysis of 203 consecutively treated patients. Cancer J. Sci. Am. 3, S92–S97 (1997).
Rosenberg, S. A., Yang, J. C., White, D. E. & Steinberg, S. M. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann. Surg. 228, 307–319 (1998).
Fyfe, G. et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. 13, 688–696 (1995).
Coppin, C. et al. Immunotherapy for advanced renal cell cancer. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD001425.pub2 (2004).
Derosa, L. et al. Safety of available treatment options for renal cell carcinoma. Expert Opin. Drug Saf. 15, 1097–1106 (2016).
Negrier, S. et al. Recombinant human interleukin-2, recombinant human interferon Alfa-2a, or both in metastatic renal-cell carcinoma. N. Engl. J. Med. 338, 1272–1278 (1998).
Amin, A. & White, R. L. Interleukin-2 in renal cell carcinoma: a has-been or a still-viable option? J. Kidney Cancer VHL 1, 74–83 (2014).
Taube, J. M. et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 20, 5064–5074 (2014).
McGregor, B. A. et al. Results of a multicenter phase ii study of atezolizumab and bevacizumab for patients with metastatic renal cell carcinoma with variant histology and/or sarcomatoid features. J. Clin. Oncol. 38, 63–70 (2020).
Rouvinov, K. et al. Rapid response to nivolumab in a patient with sarcomatoid transformation of chromophobe renal cell carcinoma. Clin. Genitourin. Cancer 15, e1127–e1130 (2017).
Geynisman, D. M. Anti-programmed cell death protein 1 (PD-1) antibody nivolumab leads to a dramatic and rapid response in papillary rnal cell carcinoma with sarcomatoid and rhabdoid features. Eur. Urol. 68, 912–914 (2015).
El Mouallem, N., Smith, S. C. & Paul, A. K. Complete response of a patient with metastatic sarcomatoid renal cell carcinoma to a programmed death-1 checkpoint inhibitor. J. Oncol. Pract. 14, 511–513 (2018).
McDermott, D. F. et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J. Clin. Oncol. 34, 833–842 (2016).
Motzer, R. J. et al. Nivolumab plus Ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378, 1277–1290 (2018).
Tannir, N. M. et al. Efficacy and safety of nivolumab plus ipilimumab versus sunitinib in first-line treatment of patients with advanced sarcomatoid renal cell carcinoma. Clin.Cancer Res. https://doi.org/10.1158/1078-0432.Ccr-20-2063 (2020).
McDermott DF, M. R. et al. CheckMate 214 retrospective analyses of nivolumab plus ipilimumab or sunitinib in IMDC intermediate/poor-risk patients with previously untreated advanced renal cell carcinoma with sarcomatoid features. Abstract presented at: The Seventeenth International Kidney Cancer Symposium;2-3, 2018 (American Society of Clinical Oncology, Miami, Florida, 2018).
Rini, B. I. et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of advanced renal cell carcinoma (RCC). J. ImmunoTherapy Cancer 7, 354 (2019).
Rini, B. I. et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1116–1127 (2019).
Rini, B. I. et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for metastatic renal cell carcinoma (mRCC): outcomes in the combined IMDC intermediate/poor risk and sarcomatoid subgroups of the phase 3 KEYNOTE-426 study. J. Clin. Oncol. 37, 4500–4500 (2019).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Motzer, R. J. et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1103–1115 (2019).
Choueiri, T. K. et al. 910PD — Efficacy and biomarker analysis of patients (pts) with advanced renal cell carcinoma (aRCC) with sarcomatoid histology (sRCC): subgroup analysis from the phase III JAVELIN renal 101 trial of first-line avelumab plus axitinib (A+Ax) vs sunitinib (S). Ann. Oncol. 30, v361 (2019).
Rini, B. I. et al. Atezolizumab plus bevacizumab versus sunitinib for patients with untreated metastatic renal cell carcinoma and sarcomatoid features: a prespecified subgroup analysis of the IMmotion151 clinical trial. Eur. Urol. https://doi.org/10.1016/j.eururo.2020.06.021 (2020).
Rini, B. I. et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 393, 2404–2415 (2019).
Iacovelli, R. et al. Patients with sarcomatoid renal cell carcinoma — re-defining the first-line of treatment: A meta-analysis of randomised clinical trials with immune checkpoint inhibitors. Eur. J. Cancer 136, 195–203 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03483883 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03793166 (2019).
Wing, K. et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322, 271–275 (2008).
Griffiths, R. W. et al. Frequency of regulatory T cells in renal cell carcinoma patients and investigation of correlation with survival. Cancer Immunol. Immunother. 56, 1743–1753 (2007).
Kang, M. J. et al. Tumor-infiltrating PD1-positive lymphocytes and FoxP3-positive regulatory T cells predict distant metastatic relapse and survival of clear cell renal cell carcinoma. Transl. Oncol. 6, 282–289 (2013).
Liotta, F. et al. Frequency of regulatory T cells in peripheral blood and in tumour-infiltrating lymphocytes correlates with poor prognosis in renal cell carcinoma. BJU Int. 107, 1500–1506 (2011).
Schvartsman, G., Carneiro, A., Filippi, R. Z., Rao, P. & Msaouel, P. Rapid deep responses with nivolumab plus ipilimumab in papillary renal cell carcinoma with sarcomatoid dedifferentiation. Clin. Genitourin. Cancer 17, 315–318 (2019).
Takahashi, T. et al. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 192, 303–310 (2000).
Al-Ahmadie, H. A. et al. Carbonic anhydrase IX expression in clear cell renal cell carcinoma: an immunohistochemical study comparing 2 antibodies. Am. J. Surg. Pathol. 32, 377–382 (2008).
Author information
Authors and Affiliations
Contributions
K.A.B. researched data for the article. K.A.B., S.G., J.A.K. and A.A.H. wrote the article. All authors made substantial contributions to discussion of the content and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.A.K. is a member of consulting or advisory boards for, and has received honoraria from, Merck, EMD Serono, Genentech, Novartis and Pfizer. J.A.K. has received institutional research funding from Merck, Genentech and Mirati. R.J.M. has received grants and paid consultancy from Eisai, Exelixis, Genentech, Roche, Novartis, Merck and Pfizer, paid consultancy from Astra Zeneca and grants from Bristol Myers Squibb. All other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Urology thanks G. Malouf, M. Staehler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Blum, K.A., Gupta, S., Tickoo, S.K. et al. Sarcomatoid renal cell carcinoma: biology, natural history and management. Nat Rev Urol 17, 659–678 (2020). https://doi.org/10.1038/s41585-020-00382-9
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41585-020-00382-9
This article is cited by
-
Successful treatment of advanced Birt-Hogg-Dubé syndrome-associated renal cell carcinoma with sarcomatoid dedifferentiation using anlotinib combined with PD-1 inhibitor after first-line therapy failure: a case report
World Journal of Surgical Oncology (2025)
-
Clinicopathological-based nomogram prediction and molecular characterization of postoperative recurrence in clinical T1 clear cell renal cell carcinoma
BMC Surgery (2025)
-
Molecular and therapeutic landscape of non-clear cell renal carcinoma
Nature Reviews Urology (2025)
-
Biomarker-informed care for patients with renal cell carcinoma
Nature Cancer (2025)
-
The percentage abundance of sarcomatoid component has a prognostic role in grade 4 non-metastatic clear cell-renal carcinoma
World Journal of Urology (2025)