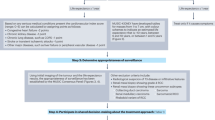
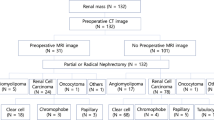
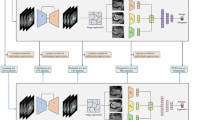
Abstract
Advancements in imaging modalities have increased the frequency of renal mass discovery. Imaging has typically been considered sufficient to guide management for a large proportion of these tumours, but renal mass biopsies (RMBs) have an increasing role in determining malignancy and can be a valuable tool for preventing unnecessary surgery in patients with benign tumours. A structured approach should be used to help to navigate the expanding repertoire of renal tumours, many of which are molecularly defined. In terms of tumour subtyping, the pathologist’s strategy should focus on stratifying patients into clinically different prognostic groups according to our current knowledge of tumour behaviour, including benign, low-grade or indolent, intermediate malignant or highly aggressive. Crucial pathological features and morphological mimicry of tumours can alter the tumour’s prognostic group. Thus, pathologists and urologists can use RMB to select patients with tumours at a reduced risk of progression, which can be safely managed with active surveillance within a tailored imaging schedule, versus tumours for which ablation or surgical intervention is indicated. RMB is also crucial in the oncological setting to distinguish between different high-grade tumours and guide tailored management strategies.
Key points
-
Renal mass biopsy (RMB) is a safe procedure that has a valuable role in the management of patients with small renal masses (cT1), as well as those with hereditary, advanced or metastatic disease.
-
RMB yields accurate pathological information that can reduce unnecessary surgical interventions for benign or indolent renal tumours, favour nephron-sparing surgery for tumours with malignant behaviour and guide patients with highly aggressive renal neoplasms to radical surgery or neoadjuvant therapy.
-
The increasing complexity of renal tumour classification is challenging to apply in clinical practice when tumours are classified according to their morphological and molecular features alone, without incorporation of prognostic outcomes.
-
A definitive diagnosis might not be absolutely crucial in RMB, but a diagnostic approach to stratifying renal neoplasms into the correct prognostic group is pivotal in ensuring correct management.
-
Pathologists need to be aware of overlapping features between well-recognized types of renal tumours and some of the rare and newly recognized renal cell carcinoma entities to avoid misdiagnosis and mismanagement.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Cancer Research UK. Kidney cancer incidence statistics. Cancer Research UK https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/kidney-cancer/incidence (2015).
Smittenaar, C. R., Petersen, K. A., Stewart, K. & Moitt, N. Cancer incidence and mortality projections in the UK until 2035. Br. J. Cancer 115, 1147–1155 (2016).
Leppert, J. T. et al. Utilization of renal mass biopsy in patients with renal cell carcinoma. Urology 83, 774–780 (2014).
Chan, V. W.-S. et al. The changing trends of image-guided biopsy of small renal masses before intervention — an analysis of European multinational prospective EuRECA registry. Eur. Radiol. 32, 4667–4678 (2022).
Kutikov, A. et al. Renal mass biopsy: always, sometimes, or never? Eur. Urol. 70, 403–406 (2016).
Kim, J. H. et al. Association of prevalence of benign pathologic findings after partial nephrectomy with preoperative imaging patterns in the United States from 2007 to 2014. JAMA Surg. 154, 225–231 (2019).
Neves, J. B. et al. Contemporary surgical management of renal oncocytoma: a nation’s outcome. BJU Int. 121, 893–899 (2018).
Campbell, S. C. et al. Renal mass and localized renal cancer: evaluation, management, and follow-up: AUA guideline: part I. J. Urol. 206, 199–208 (2021).
Campbell, S. C. et al. Renal mass and localized renal cancer: evaluation, management, and follow-up: AUA guideline: part II. J. Urol. 206, 209–218 (2021).
Ljungberg, B. et al. European Association of Urology guidelines on renal cell carcinoma: the 2022 update. Eur. Urol. 82, 399–410 (2022).
Finelli, A. et al. Management of small renal masses: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 35, 668–680 (2017).
Iguchi, T. et al. Image-guided core biopsy of 2-cm or smaller renal tumors. Diagn. Interv. Imaging 101, 715–720 (2020).
Widdershoven, C. V. et al. Renal biopsies performed before versus during ablation of T1 renal tumors: implications for prevention of overtreatment and follow-up. Abdom. Radiol. 46, 373–379 (2021).
Wells, S. A. et al. Renal mass biopsy and thermal ablation: should biopsy be performed before or during the ablation procedure? Abdom. Radiol. 42, 1773–1780 (2017).
Lay, A. H. et al. Oncologic efficacy of radio frequency ablation for small renal masses: clear cell vs papillary subtype. J. Urol. 194, 653–657 (2015).
Danzig, M. R. et al. Active surveillance for small renal masses: a review of the aims and preliminary results of the DISSRM registry. Curr. Urol. Rep. 17, 4 (2016).
Rybicki, F. J. et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am. J. Roentgenol. 180, 1281–1287 (2003).
Davidson, J. C. et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions-part I: review of anticoagulation agents and clinical considerations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J. Vasc. Interv. Radiol. 30, 1155–1167 (2019).
Maturen, K. E. et al. Renal mass core biopsy: accuracy and impact on clinical management. AJR Am. J. Roentgenol. 188, 563–570 (2007).
Sinks, A. et al. Renal mass biopsy mandate is associated with change in treatment decisions. J. Urol. 210, 72–78 (2023).
Marconi, L. et al. Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur. Urol. 69, 660–673 (2016).
Richard, P. O. et al. Renal tumor biopsy for small renal masses: a single-center 13-year experience. Eur. Urol. 68, 1007–1013 (2015).
Turajlic, S. et al. Deterministic evolutionary trajectories influence primary tumor growth: TRACERx renal. Cell 173, 595–610.e11 (2018).
Samplaski, M. K., Zhou, M., Lane, B. R., Herts, B. & Campbell, S. C. Renal mass sampling: an enlightened perspective. Int. J. Urol. 18, 5–19 (2011).
Leveridge, M. J. et al. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur. Urol. 60, 578–584 (2011).
Patel, H. D. et al. Diagnostic accuracy and risks of biopsy in the diagnosis of a renal mass suspicious for localized renal cell carcinoma: systematic review of the literature. J. Urol. 195, 1340–1347 (2016).
Renshaw, A. A., Powell, A., Caso, J. & Gould, E. W. Needle track seeding in renal mass biopsies. Cancer Cytopathol. 127, 358–361 (2019).
Smith, E. H. Complications of percutaneous abdominal fine-needle biopsy. Rev. Radiol. 178, 253–258 (1991).
Herts, B. R. & Baker, M. E. The current role of percutaneous biopsy in the evaluation of renal masses. Semin. Urol. Oncol. 13, 254–261 (1995).
Macklin, P. S. et al. Tumour seeding in the tract of percutaneous renal tumour biopsy: a report on seven cases from a UK tertiary referral centre. Eur. Urol. 75, 861–867 (2019).
Tyagi, R. & Dey, P. Needle tract seeding: an avoidable complication. Diagn. Cytopathol. 42, 636–640 (2014).
Valencia-Guerrero, A. et al. To stage or not to stage: determining the true clinical significance of the biopsy tract through perinephric fat in assessing renal cell carcinoma. Histopathology 78, 951–962 (2021).
Khan, A. A. et al. Percutaneous needle biopsy for indeterminate renal masses: a national survey of UK consultant urologists. BMC Urol. 7, 10 (2007).
Trpkov, K. & Hes, O. New and emerging renal entities: a perspective post-WHO 2016 classification. Histopathology 74, 31–59 (2019).
Delahunt, B. et al. Dataset for the reporting of renal biopsy for tumour: recommendations from the International Collaboration on Cancer Reporting (ICCR). J. Clin. Pathol. 72, 573–578 (2019).
Reuter, V. E., Argani, P., Zhou, M., Delahunt, B. & Members of the ISUP Immunohistochemistry in Diagnostic Urologic Pathology Group. Best practices recommendations in the application of immunohistochemistry in the kidney tumors: report from the International Society of Urologic Pathology consensus conference. Am. J. Surg. Pathol. 38, e35–e49 (2014).
International Agency for Research on Cancer. in WHO Classification of Tumours: Urinary and Male Genital Tumours 5th edn Vol. 8 (eds WHO Classification of Tumours Editorial Board) 31–110 (IARC, 2022).
Caliò, A., Segala, D., Munari, E., Brunelli, M. & Martignoni, G. MiT family translocation renal cell carcinoma: from the early descriptions to the current knowledge. Cancers 11, 1110 (2019).
Wang, A.-X. et al. TFEB rearranged renal cell carcinoma: pathological and molecular characterization of 10 cases, with novel clinical implications: a single center 10-year experience. Biomedicines 11, 245 (2023).
Johnson, D. C. et al. Preoperatively misclassified, surgically removed benign renal masses: a systematic review of surgical series and United States population level burden estimate. J. Urol. 193, 30–35 (2015).
Henske, E. P., Jóźwiak, S., Kingswood, J. C., Sampson, J. R. & Thiele, E. A. Tuberous sclerosis complex. Nat. Rev. Dis. Primers 2, 16035 (2016).
Flum, A. S. et al. Update on the diagnosis and management of renal angiomyolipoma. J. Urol. 195, 834–846 (2016).
Milner, J. et al. Fat poor renal angiomyolipoma: patient, computerized tomography and histological findings. J. Urol. 176, 905–909 (2006).
Aquilina, J. et al. Epithelioid angiomyolipomas of the kidney: rare renal tumours associated with poor prognoses. Urology 176, 102–105 (2023).
Kuroda, N. et al. Renal leiomyoma: an immunohistochemical, ultrastructural and comparative genomic hybridization study. Histol. Histopathol. 22, 883–888 (2007).
Kinney, S. N. et al. Metanephric adenoma: the utility of immunohistochemical and cytogenetic analyses in differential diagnosis, including solid variant papillary renal cell carcinoma and epithelial-predominant nephroblastoma. Mod. Pathol. 28, 1236–1248 (2015).
Yin, X. et al. Atypical metanephric adenoma: shares similar histopathological features and molecular changes of metanephric adenoma and epithelial-predominant Wilms’ tumor. Front. Oncol. 12, 1020456 (2022).
Cancer Genome Atlas Research Network. et al. Comprehensive molecular characterization of papillary renal-cell carcinoma. N. Engl. J. Med. 374, 135–145 (2016).
Kuroda, N. et al. ALK rearranged renal cell carcinoma (ALK-RCC): a multi-institutional study of twelve cases with identification of novel partner genes CLIP1, KIF5B and KIAA1217. Mod. Pathol. 33, 2564–2579 (2020).
World Health Organization. International Classification of Diseases for Oncology (ICD-O) (WHO, 2013).
Warren, H., Neves, J. B. & Tran, M. G. B. Renal oncocytoma: landscape of diagnosis and management. BJU Int. 128, 685–687 (2021).
Moch, H. et al. The 2022 World Health Organization classification of tumours of the urinary system and male genital organs — part a: renal, penile, and testicular tumours. Eur. Urol. 82, 458–468 (2022).
Neves, J. B. et al. Growth and renal function dynamics of renal oncocytomas in patients on active surveillance. BJU Int. 128, 722–727 (2021).
Abdessater, M. et al. Renal oncocytoma: an algorithm for diagnosis and management. Urology 143, 173–180 (2020).
Deledalle, F.-X. et al. Active surveillance for biopsy proven renal oncocytomas: outcomes and feasibility. Urology 156, 185–190 (2021).
Williamson, S. R. et al. Diagnostic criteria for oncocytic renal neoplasms: a survey of urologic pathologists. Hum. Pathol. 63, 149–156 (2017).
Branger, N. et al. Oncocytoma on renal mass biopsy: is it still the same histology when surgery is performed? Results from UroCCR-104 study. World J. Urol. 41, 483–489 (2023).
Wobker, S. E. & Williamson, S. R. Modern pathologic diagnosis of renal oncocytoma. J. Kidney Cancer VHL 4, 1–12 (2017).
Trpkov, K. et al. New developments in existing WHO entities and evolving molecular concepts: the Genitourinary Pathology Society (GUPS) update on renal neoplasia. Mod. Pathol. 34, 1392–1424 (2021).
Gupta, S., Rowsey, R. A., Cheville, J. C. & Jimenez, R. E. Morphologic overlap between low-grade oncocytic tumor and eosinophilic variant of chromophobe renal cell carcinoma. Hum. Pathol. 119, 114–116 (2022).
Trpkov, K. et al. Eosinophilic solid and cystic renal cell carcinoma (ESC RCC): further morphologic and molecular characterization of ESC RCC as a distinct entity. Am. J. Surg. Pathol. 41, 1299–1308 (2017).
Kravtsov, O. et al. Low-grade oncocytic tumor of kidney (CK7-Positive, CD117-Negative): incidence in a single institutional experience with clinicopathological and molecular characteristics. Hum. Pathol. 114, 9–18 (2021).
Chen, Y.-B. et al. Somatic mutations of TSC2 or MTOR characterize a morphologically distinct subset of sporadic renal cell carcinoma with eosinophilic and vacuolated cytoplasm. Am. J. Surg. Pathol. 43, 121–131 (2019).
Truong, L. D. & Shen, S. S. Immunohistochemical diagnosis of renal neoplasms. Arch. Pathol. Lab. Med. 135, 92–109 (2011).
Richard, P. O. et al. Active surveillance for renal neoplasms with oncocytic features is safe. J. Urol. 195, 581–586 (2016).
Miller, B. L. et al. Comparative analysis of surgery, thermal ablation, and active surveillance for renal oncocytic neoplasms. Urology 112, 92–97 (2018).
Samaratunga, H. et al. LOT and HOT … or not. The proliferation of clinically insignificant and poorly characterised types of renal neoplasia. Pathology 54, 842–847 (2022).
Amin, M. B. et al. Low grade oncocytic tumors of the kidney: a clinically relevant approach for the workup and accurate diagnosis. Mod. Pathol. 35, 1306–1316 (2022).
Lobo, J. et al. Eosinophilic solid and cystic renal cell carcinoma and renal cell carcinomas with TFEB alterations: a comparative study. Histopathology 81, 32–43 (2022).
Caliò, A. et al. Cathepsin K: a novel diagnostic and predictive biomarker for renal tumors. Cancers 13, 2441 (2021).
Smith, S. C. et al. A distinctive, low-grade oncocytic fumarate hydratase-deficient renal cell carcinoma, morphologically reminiscent of succinate dehydrogenase-deficient renal cell carcinoma. Histopathology 71, 42–52 (2017).
Hamza, A., Sirohi, D., Smith, S. C. & Amin, M. B. Low-grade oncocytic fumarate hydratase-deficient renal cell carcinoma: an update on biologic potential, morphologic spectrum, and differential diagnosis with other low-grade oncocytic tumors. Adv. Anat. Pathol. 28, 396–407 (2021).
Moch, H., Cubilla, A. L., Humphrey, P. A., Reuter, V. E. & Ulbright, T. M. The 2016 WHO classification of tumours of the urinary system and male genital organs — part A: renal, penile, and testicular tumours. Eur. Urol. 70, 93–105 (2016).
Massari, F. et al. The tumor entity denominated ‘clear cell-papillary renal cell carcinoma’ according to the WHO 2016 new classification, have the clinical characters of a renal cell adenoma as does harbor a benign outcome. Pathol. Oncol. Res. 24, 447–456 (2018).
Williamson, S. R. & Cheng, L. Clear cell renal cell tumors: not all that is ‘clear’ is cancer. Urol. Oncol. 34, 292.e17–22 (2016).
Hakimi, A. A. et al. TCEB1-mutated renal cell carcinoma: a distinct genomic and morphologic subtype. Mod. Pathol. 28, 845 (2015).
Wang, Y. et al. Analysis of clinicopathological and molecular features of ELOC(TCEB1)-mutant renal cell carcinoma. Pathol. Res. Pract. 235, 153960 (2022).
Shah, R. B. et al. ‘Renal cell carcinoma with leiomyomatous stroma’ harbor somatic mutations of TSC1, TSC2, MTOR, and/or ELOC (TCEB1): clinicopathologic and molecular characterization of 18 sporadic tumors supports a distinct entity. Am. J. Surg. Pathol. 44, 571–581 (2020).
Parilla, M. et al. Genetic underpinnings of renal cell carcinoma with leiomyomatous stroma. Am. J. Surg. Pathol. 43, 1135 (2019).
Nathany, S. & Monappa, V. Mucinous tubular and spindle cell carcinoma: a review of histopathology and clinical and prognostic implications. Arch. Pathol. Lab. Med. 144, 115–118 (2020).
Zhao, M., He, X. & Teng, X. Mucinous tubular and spindle cell renal cell carcinoma: a review of clinicopathologic aspects. Diagn. Pathol. 10, 168 (2015).
Kondo, T. et al. Acquired cystic disease-associated renal cell carcinoma is the most common subtype in long-term dialyzed patients: central pathology results according to the 2016 WHO classification in a multi-institutional study. Pathol. Int. 68, 543–549 (2018).
Kondo, T. et al. Comparable survival outcome between acquired cystic disease associated renal cell carcinoma and clear cell carcinoma in patients with end-stage renal disease: a multi-institutional central pathology study. Pathology 53, 720–727 (2021).
Foshat, M. & Eyzaguirre, E. Acquired cystic disease-associated renal cell carcinoma: review of pathogenesis, morphology, ancillary tests, and clinical features. Arch. Pathol. Lab. Med. 141, 600–606 (2017).
Przybycin, C. G. et al. Acquired cystic disease-associated renal cell carcinoma (ACD-RCC): a multiinstitutional study of 40 cases with clinical follow-up. Am. J. Surg. Pathol. 42, 1156 (2018).
Polascik, T. J., Pound, C. R., Meng, M. V., Partin, A. W. & Marshall, F. F. Partial nephrectomy: technique complications and pathological findings. J. Urol. 154, 1312–1318 (1995).
Shah, P. H. et al. Partial nephrectomy is associated with higher risk of relapse compared with radical nephrectomy for clinical stage T1 renal cell carcinoma pathologically up staged to T3a. J. Urol. 198, 289–296 (2017).
Tsai, H.-Y., Lee, K.-H., Ng, K.-F., Kao, Y.-T. & Chuang, C.-K. Clinicopathologic analysis of renal epithelioid angiomyolipoma: consecutively excised 23 cases. Kaohsiung J. Med. Sci. 35, 33–38 (2019).
Nese, N. et al. Pure epithelioid PEComas (So-Called Epithelioid Angiomyolipoma) of the kidney: a clinicopathologic study of 41 cases detailed assessment of morphology and risk stratification. Am. J. Surg. Pathol. 35, 161–176 (2011).
He, W. et al. Epithelioid angiomyolipoma of the kidney: pathological features and clinical outcome in a series of consecutively resected tumors. Mod. Pathol. 26, 1355–1364 (2013).
Brimo, F. et al. Renal epithelioid angiomyolipoma with atypia: a series of 40 cases with emphasis on clinicopathologic prognostic indicators of malignancy. Am. J. Surg. Pathol. 34, 715–722 (2010).
Sharma, A. E., Parilla, M., Wanjari, P., Segal, J. P. & Antic, T. A tale of 2 morphologies: diagnostic pitfalls in TFEB-associated renal cell carcinomas, including a novel NEAT1-TFEB fusion. Int. J. Surg. Pathol. 29, 21–29 (2021).
Kenerson, H., Folpe, A. L., Takayama, T. K. & Yeung, R. S. Activation of the mTOR pathway in sporadic angiomyolipomas and other perivascular epithelioid cell neoplasms. Hum. Pathol. 38, 1361–1371 (2007).
Guo, G., Gu, L. & Zhang, X. Everolimus in invasive malignant renal epithelioid angiomyolipoma. Front. Oncol. 10, 610858 (2021).
Sanfilippo, R. et al. Role of chemotherapy, VEGFR inhibitors, and mTOR inhibitors in advanced perivascular epithelioid cell tumors (PEComas). Clin. Cancer Res. 25, 5295–5300 (2019).
Prendeville, S. et al. Accuracy of renal tumour biopsy for the diagnosis and subtyping of papillary renal cell carcinoma: analysis of paired biopsy and nephrectomy specimens with focus on discordant cases. J. Clin. Pathol. 72, 363–367 (2019).
Xia, Q.-Y. et al. Clinicopathologic and molecular analysis of the TFEB fusion variant reveals new members of TFEB translocation renal cell carcinomas (RCCs): expanding the genomic spectrum. Am. J. Surg. Pathol. 44, 477–489 (2020).
Gupta, S. et al. TFEB expression profiling in renal cell carcinomas: clinicopathologic correlations. Am. J. Surg. Pathol. 43, 1445 (2019).
Peckova, K. et al. Aggressive and nonaggressive translocation t(6;11) renal cell carcinoma: comparative study of 6 cases and review of the literature. Ann. Diagn. Pathol. 18, 351–357 (2014).
Caliò, A. et al. TFEB rearranged renal cell carcinoma. A clinicopathologic and molecular study of 13 cases. Tumors harboring MALAT1-TFEB, ACTB-TFEB, and the novel NEAT1-TFEB translocations constantly express PDL1. Mod. Pathol. 34, 842–850 (2021).
Smith, N. E. et al. t(6;11) renal cell carcinoma (RCC) expanded immunohistochemical profile emphasizing novel RCC markers and report of 10 new genetically confirmed cases. Am. J. Surg. Pathol. 38, 604–614 (2014).
Liu, N. et al. Renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusions: clinical features, treatments and prognosis. PLoS ONE 11, e0166897 (2016).
Shen, Y., Liu, Z., Wei, Q. & Xue, W. Consensus on clinical diagnosis and treatment of fumarate hydratase-deficient renal cell carcinoma. Holist. Integr. Oncol. 3, 7 (2024).
Iannantuono, G. M., Riondino, S., Sganga, S., Roselli, M. & Torino, F. Activity of ALK inhibitors in renal cancer with ALK alterations: a systematic review. Int. J. Mol. Sci. 23, 3995 (2022).
Kauffman, E. C. et al. Molecular genetics and cellular characteristics of TFE3 and TFEB translocation renal cell carcinomas. Nat. Rev. Urol. 11, 465–475 (2014).
Argani, P., Matoso, A., Baraban, E. G., Epstein, J. I. & Antonescu, C. R. MED15::TFE3 renal cell carcinomas: report of two new cases and review of the literature confirming nearly universal multilocular cystic morphology. Int. J. Surg. Pathol. 31, 409–414 (2023).
Argani, P. et al. TFE3-fusion variant analysis defines specific clinicopathologic associations among Xp11 translocation cancers. Am. J. Surg. Pathol. 40, 723–737 (2016).
Wang, X.-T. et al. SFPQ/PSF-TFE3 renal cell carcinoma: a clinicopathologic study emphasizing extended morphology and reviewing the differences between SFPQ-TFE3 RCC and the corresponding mesenchymal neoplasm despite an identical gene fusion. Hum. Pathol. 63, 190–200 (2017).
Xia, Q. et al. Xp11.2 translocation renal cell carcinoma with NONO-TFE3 gene fusion: morphology, prognosis, and potential pitfall in detecting TFE3 gene rearrangement. Mod. Pathol. 30, 416–426 (2017).
Caliò, A. et al. TFE3 and TFEB-rearranged renal cell carcinomas: an immunohistochemical panel to differentiate from common renal cell neoplasms. Virchows Arch. 481, 877–891 (2022).
Williamson, S. R. et al. Report from the International Society of Urological Pathology (ISUP) consultation conference on molecular pathology of urogenital cancers. III. molecular pathology of kidney cancer. Am. J. Surg. Pathol. 44, e47–e65 (2020).
Caliò, A. et al. Comprehensive analysis of 34 MiT family translocation renal cell carcinomas and review of the literature: investigating prognostic markers and therapy targets. Pathology 52, 297–309 (2020).
Dong, X. et al. Clinicopathological features and prognosis of TFE3-positive renal cell carcinoma. Front. Oncol. 12, 1017425 (2022).
Skala, S. L. et al. Detection of 6 TFEB-amplified renal cell carcinomas and 25 renal cell carcinomas with MITF translocations: systematic morphologic analysis of 85 cases evaluated by clinical TFE3 and TFEB FISH assays. Mod. Pathol. 31, 179–197 (2018).
Argani, P., Zhang, L., Reuter, V. E., Tickoo, S. K. & Antonescu, C. R. RBM10-TFE3 renal cell carcinoma: a potential diagnostic pitfall due to cryptic intrachromosomal Xp11.2 inversion resulting in false-negative TFE3 FISH. Am. J. Surg. Pathol. 41, 655–662 (2017).
Classe, M. et al. Incidence, clinicopathological features and fusion transcript landscape of translocation renal cell carcinomas. Histopathology 70, 1089–1097 (2017).
Ellis, C. L. et al. Clinical heterogeneity of Xp11 translocation renal cell carcinoma: impact of fusion subtype, age, and stage. Mod. Pathol. 27, 875–886 (2014).
Bakouny, Z. et al. Integrative clinical and molecular characterization of translocation renal cell carcinoma. Cell Rep. 38, 110190 (2022).
Sun, G. et al. Integrated exome and RNA sequencing of TFE3-translocation renal cell carcinoma. Nat. Commun. 12, 5262 (2021).
Trpkov, K. et al. Fumarate hydratase-deficient renal cell carcinoma is strongly correlated with fumarate hydratase mutation and hereditary leiomyomatosis and renal cell carcinoma syndrome. Am. J. Surg. Pathol. 40, 865 (2016).
Galea, L. A. et al. ALK-rearranged renal cell carcinoma with TPM3::ALK gene fusion and review of the literature. Virchows Arch. Int. J. Pathol. 482, 625–633 (2022).
Leibovich, B. C. et al. Predicting oncologic outcomes in renal cell carcinoma after surgery. Eur. Urol. 73, 772–780 (2018).
Moch, H. The WHO/ISUP grading system for renal carcinoma [German]. Pathol 37, 355–360 (2016).
Delahunt, B., Eble, J. N., Egevad, L. & Samaratunga, H. Grading of renal cell carcinoma. Histopathology 74, 4–17 (2019).
Volpe, A. et al. Contemporary results of percutaneous biopsy of 100 small renal masses: a single center experience. J. Urol. 180, 2333–2337 (2008).
Amaral, B. S. et al. Renal tumor biopsy: rationale to avoid surgery in small renal masses. Curr. Urol. Rep. 22, 46 (2021).
Avulova, S. et al. Grading chromophobe renal cell carcinoma: evidence for a four-tiered classification incorporating coagulative tumor necrosis. Eur. Urol. 79, 225–231 (2021).
Baiocco, J. A. & Metwalli, A. R. Multiplex partial nephrectomy, repeat partial nephrectomy, and salvage partial nephrectomy remain the primary treatment in multifocal and hereditary kidney cancer. Front. Oncol. 7, 244 (2017).
Schmidt, L. et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat. Genet. 16, 68–73 (1997).
Carlo, M. I. et al. Familial kidney cancer: implications of new syndromes and molecular insights. Eur. Urol. 76, 754–764 (2019).
Argani, P. & Mehra, R. Renal cell carcinoma associated with tuberous sclerosis complex (TSC)/mammalian target of rapamycin (MTOR) genetic alterations. Mod. Pathol. 35, 296–297 (2022).
Swartz, M. A. et al. Renal medullary carcinoma: clinical, pathologic, immunohistochemical, and genetic analysis with pathogenetic implications. Urology 60, 1083–1089 (2002).
Smith, N. E. et al. VCL-ALK renal cell carcinoma in children with sickle-cell trait: the eighth sickle-cell nephropathy? Am. J. Surg. Pathol. 38, 858–863 (2014).
Shuch, B. et al. Defining early-onset kidney cancer: implications for germline and somatic mutation testing and clinical management. J. Clin. Oncol. 32, 431–437 (2014).
Motzer, R. J. et al. NCCN guidelines insights: kidney cancer, version 1.2021: featured updates to the NCCN guidelines. J. Natl Compr. Canc. Netw. 18, 1160–1170 (2020).
Drobner, J., Portal, D., Runcie, K., Yang, Y. & Singer, E. A. Systemic treatment for advanced and metastatic non-clear cell renal cell carcinoma: examining modern therapeutic strategies for a notoriously challenging malignancy. J. Kidney Cancer VHL 10, 37–60 (2023).
Williams, J. H., Frazier, H. A., Gawith, K. E., Laskin, W. B. & Christenson, P. J. Transitional cell carcinoma of the kidney with tumor thrombus into the vena cava. Urology 48, 932–935 (1996).
Li, M. et al. Transitional cell carcinoma with extension of the renal vein and IVC tumor thrombus: report of three cases and literature review. World J. Surg. Oncol. 14, 309 (2016).
Tong, G.-X. et al. Expression of PAX8 in normal and neoplastic renal tissues: an immunohistochemical study. Mod. Pathol. 22, 1218–1227 (2009).
Shuch, B., Bratslavsky, G., Linehan, W. M. & Srinivasan, R. Sarcomatoid renal cell carcinoma: a comprehensive review of the biology and current treatment strategies. Oncologist 17, 46 (2012).
Damayanti, N. P. et al. Therapeutic targeting of TFE3/IRS-1/PI3K/mTOR axis in translocation renal cell carcinoma. Clin. Cancer Res. 24, 5977–5989 (2018).
Lang, M. et al. High-throughput and targeted drug screens identify pharmacological candidates against MiT-translocation renal cell carcinoma. J. Exp. Clin. Cancer Res. 42, 99 (2023).
Suarez, C. et al. Update in collecting duct carcinoma: current aspects of the clinical and molecular characterization of an orphan disease. Front. Oncol. 12, 970199 (2022).
Siadat, F. & Trpkov, K. ESC, ALK, HOT and LOT: three letter acronyms of emerging renal entities knocking on the door of the WHO classification. Cancers 12, 168 (2020).
Dawood, A. et al. Case report: disease progression of renal cell carcinoma containing a novel putative pathogenic KAT6A::NRG1 fusion on Ipilimumab-Nivolumab immunotherapy. A case study and review of the literature. Front. Oncol. 13, 1111706 (2023).
Hora, M. et al. Re: Tumour seeding in the tract of percutaneous renal tumour biopsy: a report on seven cases from a UK tertiary referral centre. Eur. Urol. 76, e96 (2019).
Zhou, Y., Murugan, P., Li, F. & Bu, L. Needle tract seeding in renal tumor biopsies: experience from a single institution. Diagn. Pathol. 16, 43 (2021).
Taneja, K. & Williamson, S. R. Updates in pathologic staging and histologic grading of renal cell carcinoma. Surg. Pathol. Clin. 11, 797–812 (2018).
Salmasi, A. et al. Association between renal mass biopsy and upstaging to perinephric fat involvement in a contemporary cohort of patients with clinical T1a renal cell carcinoma. Urol. Oncol. 36, 527.e13–527.e19 (2018).
Zhang, L. et al. Tumor necrosis as a prognostic variable for the clinical outcome in patients with renal cell carcinoma: a systematic review and meta-analysis. BMC Cancer 18, 870 (2018).
Staehler, M. et al. Long-term follow-up in patients undergoing renal mass biopsy: seeding is not anecdotal. Clin. Genitourin. Cancer 22, 189–192 (2024).
Deng, J. et al. A comparison of the prognosis of papillary and clear cell renal cell carcinoma: evidence from a meta-analysis. Medicine 98, e16309 (2019).
Petersson, F. et al. A distinctive translocation carcinoma of the kidney; ‘rosette forming,’ t(6;11), HMB45-positive renal tumor: a histomorphologic, immunohistochemical, ultrastructural, and molecular genetic study of 4 cases. Hum. Pathol. 43, 726–736 (2012).
Pei, J. et al. NEAT1-TFE3 and KAT6A-TFE3 renal cell carcinomas, new members of MiT family translocation renal cell carcinoma. Mod. Pathol. 32, 710–716 (2019).
Wang, X. et al. RNA sequencing of Xp11 translocation-associated cancers reveals novel gene fusions and distinctive clinicopathologic correlations. Mod. Pathol. 31, 1346–1360 (2018).
Argani, P. et al. Primary renal neoplasms with the ASPL-TFE3 gene fusion of alveolar soft part sarcoma: a distinctive tumor entity previously included among renal cell carcinomas of children and adolescents. Am. J. Pathol. 159, 179–192 (2001).
Guo, W., Zhu, Y., Pu, X., Guo, H. & Gan, W. Clinical and pathological heterogeneity of four common fusion subtypes in Xp11.2 translocation renal cell carcinoma. Front. Oncol. 13, 1116648 (2023).
Song, Y. et al. Xp11 translocation renal cell carcinoma with morphological features mimicking multilocular cystic renal neoplasm of low malignant potential: a series of six cases with molecular analysis. J. Clin. Pathol. 74, 171–176 (2021).
Ye, H. et al. A rare partner of TFE3 in the Xp11 translocation renal cell carcinoma: clinicopathological analyses and detection of MED15-TFE3 fusion. Biomed. Res. Int. 2019, 5974089 (2019).
Zhan, H.-Q., Chen, H., Wang, C.-F. & Zhu, X.-Z. A case of PSF-TFE3 gene fusion in Xp11.2 renal cell carcinoma with melanotic features. Hum. Pathol. 46, 476–481 (2015).
Rao, Q. et al. PSF/SFPQ is a very common gene fusion partner in tfe3 rearrangement–associated perivascular epithelioid cell tumors (PEComas) and melanotic Xp11 translocation renal cancers: clinicopathologic, immunohistochemical, and molecular characteristics suggesting classification as a distinct entity. Am. J. Surg. Pathol. 39, 1181 (2015).
Pivovarcikova, K. et al. TFE3-fusion variant analysis defines specific clinicopathologic associations among Xp11 translocation cancers. Am. J. Surg. Pathol. 41, 138 (2017).
Xia, Q. et al. Xp11 translocation renal cell carcinomas (RCCs) with RBM10-TFE3 gene fusion demonstrating melanotic features and overlapping morphology with t(6;11) RCC: interest and diagnostic pitfall in detecting a paracentric inversion of: TFE3. Am. J. Surg. Pathol. 41, 663 (2017).
Fukuda, H. et al. A novel partner of TFE3 in the Xp11 translocation renal cell carcinoma: clinicopathological analyses and detection of EWSR1-TFE3 fusion. Virchows Arch. 474, 389–393 (2019).
Argani, P. et al. A novel CLTC-TFE3 gene fusion in pediatric renal adenocarcinoma with t(X;17)(p11.2;q23). Oncogene 22, 5374–5378 (2003).
Author information
Authors and Affiliations
Contributions
H.M. researched data for the article. S.E.S. and A.B. contributed substantially to discussion of the content. S.E.S. and H.M. wrote the article. H.M., M.A.T.D., M.W., E.B., R.B., P.P., F.M., M.G.B.T. and A.B. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Brian Lane, who co-reviewed with Dennis Boynton, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mansour, H., Tran-Dang, MA., Walkden, M. et al. Renal mass biopsy — a practical and clinicopathologically relevant approach to diagnosis. Nat Rev Urol 22, 8–25 (2025). https://doi.org/10.1038/s41585-024-00897-5
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41585-024-00897-5
This article is cited by
-
Noninvasive renal mass diagnostic models based on radiomic features and clinical data: a multi-center study
Abdominal Radiology (2026)
-
Renal mass biopsies: time to tailor the approach
European Radiology (2025)