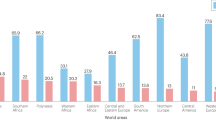
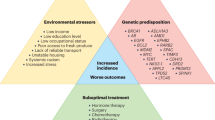
Abstract
In the USA, Black men are approximately twice as likely to be diagnosed with and to die of prostate cancer than white men. In the UK, despite Black men having vastly different ancestral contexts and health-care systems from Black men in the USA, the lifetime risk of being diagnosed with prostate cancer is two-to-three times higher among Black British men than among white British men and Black British men are twice as likely to die of prostate cancer as white British men. Examination of racial disparities in prostate cancer in the USA and UK highlights systemic, socio-economic and sociocultural factors that might contribute to these differences. Variation by ancestry could affect incidence and tumour genomics. Disparities in incidence might also be affected by screening guidelines and access to and uptake of screening. Disparities in treatment access, continuity of care and outcomes could contribute to survival differences. In both localized and metastatic settings, equal access could diminish the observed disparities in both the USA and the UK. An understanding of behavioural medicine, especially an appreciation of cultural beliefs about illness and treatment, could inform and improve the ways in which health systems can engage with and deliver care to patients in minoritized groups affected by prostate cancer. Methods of promoting equity include targeting systemic barriers including systemic racism, proportional recruitment of patients into clinical trials, diversifying the health-care workforce and facilitating care informed by cultural humility. Actively engaging patients and communities in research and intervention might enable the translation of research into increasingly equitable care for patients with prostate cancer in the UK, the USA and globally.
Key points
-
In the USA, Black men are more likely than white men to be diagnosed with prostate cancer, to be diagnosed at an younger age and with more advanced disease, and to die of prostate cancer. In the UK, the lifetime risk of being diagnosed with prostate cancer is two-to-three times higher in Black British men than in white British men; however, Black British men might be less likely to present with advanced disease than white British men.
-
Race is a social construct, but strong evidence supports elevated prostate cancer risk among men with African ancestry, including Caribbean men, Black British men, and Black men in the USA, and could be associated with known genomic variants. Differences in screening programmes, access and adherence might influence disparities in advanced disease at diagnosis, in the context of evolving public-health screening paradigms in the UK and the USA.
-
Racial variation in tumour genomic profiles has also been demonstrated; these differences might explain data suggesting that Black patients have improved response to radiotherapy, and could inform individualized treatment strategies and future research.
-
Evidence from both localized and metastatic disease in the UK and USA suggest that equal access to treatment reduces (and even eliminates) disparities in prostate cancer-specific mortality among patients who undergo treatment.
-
Health and sociocultural beliefs in the context of social determinants of health among these unique populations in the UK and the USA can influence access and adherence from screening to diagnosis to treatment.
-
Steps forward to promote equity include targeting systemic barriers including systemic racism, improving diversity in clinical research, promoting care informed by cultural humility and community-based participatory research, diversifying the health-care workforce, and improving access to clinical trials for minoritized groups. Actively engaging patients and communities in research and intervention might enable the translation of research findings into increasingly equitable care for patients with prostate cancer globally.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Rebbeck, T. R. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb. Perspect. Med. 8, a030387 (2018).
Flanagin, A., Frey, T. & Christiansen, S. L. AMA manual of style committee. updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA 326, 621–627 (2021).
Nyame, Y. A. et al. Deconstructing, addressing, and eliminating racial and ethnic inequities in prostate cancer care. Eur. Urol. 82, 341–351 (2022).
Daniszewski, J. The decision to capitalize Black. Associated Press https://blog.ap.org/announcements/the-decision-to-capitalize-black (2024).
Dee, E. C., Pierce, L. J., Winkfield, K. M. & Lam, M. B. In pursuit of equity in cancer care: moving beyond the affordable care act. Cancer 128, 3278–3283 (2022).
Zavala, V. A. et al. Cancer health disparities in racial/ethnic minorities in the United States. Br. J. Cancer 124, 315–332 (2021).
DeSantis, C. E., Miller, K. D., Goding Sauer, A., Jemal, A. & Siegel, R. L. Cancer statistics for African Americans, 2019. CA Cancer J. Clin. 69, 211–233 (2019).
Freedland, S. J. & Isaacs, W. B. Explaining racial differences in prostate cancer in the United States: sociology or biology? Prostate 62, 243–252 (2005).
Hougen, H. Y. et al. Disparities in diagnosis, treatment access, and time to treatment among Hispanic men with metastatic prostate cancer. JCO Oncol. Pract. 19, 645–653 (2023).
Dee, E. C. et al. Prostate cancer-specific mortality burden by risk group among men with localized disease: implications for research and clinical trial priorities. Prostate 80, 1128–1133 (2020).
Cuevas, A. G. et al. Placing prostate cancer disparities within a psychosocial context: challenges and opportunities for future research. Cancer Causes Control. 30, 443–456 (2019).
Major, J. M. et al. Socioeconomic status, healthcare density, and risk of prostate cancer among African American and Caucasian men in a large prospective study. Cancer Causes Control. 23, 1185–1191 (2012).
Sohn, H. Racial and ethnic disparities in health insurance coverage: dynamics of gaining and losing coverage over the life-course. Popul. Res. Policy Rev. 36, 181–201 (2017).
Iyengar, S., Hall, I. J. & Sabatino, S. A. Racial/ethnic disparities in prostate cancer incidence, distant stage diagnosis, and mortality by U.S. census region and age-group, 2012–2015. Cancer Epidemiol. Biomark. Prev. 29, 1357–1364 (2020).
Swami, N. et al. Localized prostate cancer disparities in risk group at presentation and access to treatment for Hispanic men. Prostate Cancer Prostatic Dis. 26, 309–316 (2023).
Jones, A. L. & Chinegwundoh, F. Update on prostate cancer in black men within the UK. Ecancermedicalscience 8, 455 (2014).
Lloyd, T. et al. Lifetime risk of being diagnosed with, or dying from, prostate cancer by major ethnic group in England 2008–2010. BMC Med. 13, 171 (2015).
Fry, A., White, B., Nagarwalla, D., Shelton, J. & Jack, R. H. Relationship between ethnicity and stage at diagnosis in England: a national analysis of six cancer sites. BMJ Open. 13, e062079 (2023).
Bolnick, H. J. Designing a world-class health care system. North. Am. Actuarial J. 7, 1–23 (2003).
Ben-Shlomo, Y. et al. The risk of prostate cancer amongst black men in the United Kingdom: the PROCESS cohort study. Eur. Urol. 53, 99–105 (2008).
Metcalfe, C. et al. Pathways to diagnosis for Black men and White men found to have prostate cancer: the PROCESS cohort study. Br. J. Cancer 99, 1040–1045 (2008).
Culp, M. B., Soerjomataram, I., Efstathiou, J. A., Bray, F. & Jemal, A. Recent global patterns in prostate cancer incidence and mortality rates. Eur. Urol. 77, 38–52 (2020).
McGinley, K. F., Tay, K. J. & Moul, J. W. Prostate cancer in men of African origin. Nat. Rev. Urol. 13, 99–107 (2016).
Rayford, W. et al. Comparative analysis of 1152 African-American and European-American men with prostate cancer identifies distinct genomic and immunological differences. Commun. Biol. 4, 670 (2021).
Friedman, E. Money isn’t everything: nonfinancial barriers to access. JAMA 271, 1535–1538 (1994).
Schnittker, J. & Bhatt, M. The role of income and race/ethnicity in experiences with medical care in the United States and United Kingdom. Int. J. Health Serv. 38, 671–695 (2008).
Borrell L. N., et al. Race and genetic ancestry in medicine — a time for reckoning with racism. N. Engl. J. Med. 384, 474–480 (2021).
Barlow, M. et al. Ethnic differences in prostate-specific antigen levels in men without prostate cancer: a systematic review. Prostate Cancer Prostatic Dis. 26, 249–256 (2023).
Lacher, D. A., Thompson, T. D., Hughes, J. P. & Saraiya, M. Total, free, and percent free prostate-specific antigen levels among U.S. men, 2001–04. Adv. Data 379, 1–12 (2006).
DeAntoni, E. P. et al. Age- and race-specific reference ranges for prostate-specific antigen from a large community-based study. Urology 48, 234–239 (1996).
Espaldon, R. et al. Probability of an abnormal screening prostate-specific antigen result based on age, race, and prostate-specific antigen threshold. Urology 83, 599–605 (2014).
Lacher, D. A. & Hughes, J. P. Total, free, and complexed prostate-specific antigen levels among US men, 2007–2010. Clin. Chim. Acta 448, 220–227 (2015).
Weinrich, M. C. et al. Reference ranges for serum prostate-specific antigen in black and white men without cancer. Urology 52, 967–973 (1998).
Rhodes, T. et al. Benign prostate specific antigen distribution and associations with urological outcomes in community dwelling black and white men. J. Urol. 187, 87–91 (2012).
Crawford, E. D. et al. Prostate-specific antigen 1.5–4.0 ng/mL: a diagnostic challenge and danger zone. BJU Int. 108, 1743–1749 (2011).
Saraiya, M. et al. Total and percent free prostate-specific antigen levels among U.S. men, 2001–2002. Cancer Epidemiol. Biomark. Prev. 14, 2178–2182 (2005).
Sarma, A. V. et al. Racial differences in longitudinal changes in serum prostate-specific antigen levels: the Olmsted County study and the Flint men’s health study. Urology 83, 88–93 (2014).
Rhodes, T. et al. Distribution and associations of [-2]proenzyme-prostate specific antigen in community dwelling black and white men. J. Urol. 187, 92–96 (2012).
Down, L. et al. Association between patient ethnicity and prostate cancer diagnosis following a prostate-specific antigen test: a cohort study of 730,000 men in primary care in the UK. BMC Med. 22, 82 (2024).
Hassanipour-Azgomi, S. et al. Incidence and mortality of prostate cancer and their relationship with the Human Development Index worldwide. Prostate Int. 4, 118–124 (2016).
Brook, M. N. et al. Family history of prostate cancer and survival outcomes in the UK genetic prostate cancer study. Eur. Urol. 83, 257–266 (2023).
Qu, L. G., Brand, N. R., Chao, A. & Ilbawi, A. M. Interventions addressing barriers to delayed cancer diagnosis in low‐ and middle‐income countries: a systematic review. Oncologist 25, e1382–e1395 (2020).
Schröder, F. H. et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 384, 2027–2035 (2014).
Chou, R. et al. Screening for prostate cancer: a review of the evidence for the U.S. preventive services task force. Ann. Intern. Med. 155, 762–771 (2011).
Barocas, D. A. et al. Effect of the USPSTF grade D recommendation against screening for prostate cancer on incident prostate cancer diagnoses in the United States. J. Urol. 194, 1587–1593 (2015).
Burgess, L. et al. Association of the USPSTF grade D recommendation against prostate-specific antigen screening with prostate cancer-specific mortality. JAMA Netw. Open. 5, e2211869 (2022).
Leapman, M. S. et al. Changes in prostate-specific antigen testing relative to the revised US preventive services task force recommendation on prostate cancer screening. JAMA Oncol. 8, 41–47 (2022).
Kim, I. E. et al. Abrogation of survival disparity between Black and White individuals after the USPSTF’s 2012 prostate-specific antigen-based prostate cancer screening recommendation. Cancer 126, 5114–5123 (2020).
Johnson, J. R. et al. The complex interplay of modifiable risk factors affecting prostate cancer disparities in African American men. Nat. Rev. Urol. 21, 422–432 (2024).
Garraway, I. P. et al. Prostate cancer foundation screening guidelines for black men in the United States. NEJM Evid. 3, EVIDoa2300289 (2024).
Office for Health Improvement & Disparities. PSA testing and prostate cancer: advice for men without symptoms of prostate disease aged 50 and over. GOV.UK https://www.gov.uk/government/publications/prostate-specific-antigen-testing-description-in-brief/psa-testing-and-prostate-cancer-advice-for-men-without-symptoms-of-prostate-disease-aged-50-and-over (2022).
Bradley, S. H., Funston, G., Jones, D. & Watson, J. Diagnosing prostate cancer in asymptomatic patients. BMJ 377, e071076 (2022).
Melia, J., Moss, S. & Johns, L. Contributors in the participating laboratories. Rates of prostate-specific antigen testing in general practice in England and Wales in asymptomatic and symptomatic patients: a cross-sectional study. BJU Int. 94, 51–56 (2004).
Williams, N. et al. Prostate-specific antigen testing rates remain low in UK general practice: a cross-sectional study in six English cities. BJU Int. 108, 1402–1408 (2011).
Burns, R., Walsh, B., O’Neill, S. & O’Neill, C. An examination of variations in the uptake of prostate cancer screening within and between the countries of the EU-27. Health Policy 108, 268–276 (2012).
Nderitu, P. et al. Prostate-specific antigen testing in inner London general practices: are those at higher risk most likely to get tested? BMJ Open. 6, e011356 (2016).
Robb, K. et al. Ethnic disparities in knowledge of cancer screening programmes in the UK. J. Med. Screen. 17, 125–131 (2010).
Martins, T., Ukoumunne, O. C., Banks, J., Raine, R. & Hamilton, W. Ethnic differences in patients’ preferences for prostate cancer investigation: a vignette-based survey in primary care. Br. J. Gen. Pract. 65, e161–e170 (2015).
Volunteer Voices. ‘I have prostate cancer. By signing up to our future health I’m hoping I can help my sons’ chances of beating the disease’. Our Future Health https://ourfuturehealth.org.uk/news/i-have-prostate-cancer-by-signing-up-to-our-future-health-im-hoping-i-can-help-my-sons-chances-of-beating-the-disease/ (2023).
Fontanarosa, P. B. & Bauchner, H. Race, ancestry, and medical research. JAMA 320, 1539–1540 (2018).
Rebello, R. J. et al. Prostate cancer. Nat. Rev. Dis. Primers 7, 1–27 (2021).
Wong, M. C. S. et al. Global incidence and mortality for prostate cancer: analysis of temporal patterns and trends in 36 Countries. Eur. Urol. 70, 862–874 (2016).
Fedewa, S. A. & Jemal, A. Prostate cancer disease severity and country of origin among black men in the United States. Prostate Cancer Prostatic Dis. 16, 176–180 (2013).
Coard, K. C. M. & Skeete, D. H. A. A 6-year analysis of the clinicopathological profile of patients with prostate cancer at the University Hospital of the West Indies, Jamaica. BJU Int. 103, 1482–1486 (2009).
Yarney, J., Vanderpuye, V. & Mensah, J. Clinicopathologic features and determinants of Gleason score of prostate cancer in Ghanaian men. Urol. Oncol. 31, 325–330 (2013).
Obiorah, C. C. & Nwosu, S. O. A histopathological study of carcinoma of the prostate in Port Harcourt, Nigeria. Niger. J. Clin. Pract. 14, 363–367 (2011).
Kheirandish, P. & Chinegwundoh, F. Ethnic differences in prostate cancer. Br. J. Cancer 105, 481–485 (2011).
Freedman, M. L. et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc. Natl Acad. Sci. USA 103, 14068–14073 (2006).
Haiman, C. A. et al. Multiple regions within 8q24 independently affect risk for prostate cancer. Nat. Genet. 39, 638–644 (2007).
Amundadottir, L. T. et al. A common variant associated with prostate cancer in European and African populations. Nat. Genet. 38, 652–658 (2006).
Lachance, J. et al. Genetic hitchhiking and population bottlenecks contribute to prostate cancer disparities in men of African descent. Cancer Res. 78, 2432–2443 (2018).
Taioli, E. et al. Polymorphisms in CYP17 and CYP3A4 and prostate cancer in men of African descent. Prostate 73, 668–676 (2013).
Hjelmborg, J. B. et al. The heritability of prostate cancer in the Nordic Twin Study of Cancer. Cancer Epidemiol. Biomark. Prev. 23, 2303–2310 (2014).
Wang, A. et al. Characterizing prostate cancer risk through multi-ancestry genome-wide discovery of 187 novel risk variants. Nat. Genet. 55, 2065–2074 (2023).
Conti, D. V. et al. Trans-ancestry genome-wide association meta-analysis of prostate cancer identifies new susceptibility loci and informs genetic risk prediction. Nat. Genet. 53, 65–75 (2021).
Schumacher, F. R. et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat. Genet. 50, 928–936 (2018).
Dadaev, T. et al. Fine-mapping of prostate cancer susceptibility loci in a large meta-analysis identifies candidate causal variants. Nat. Commun. 9, 2256 (2018).
Mahal, B. A. et al. Racial differences in genomic profiling of prostate cancer. N. Engl. J. Med. 383, 1083–1085 (2020).
Mahal, B. A. et al. Prostate cancer genomic-risk differences between African-American and white men across Gleason scores. Eur. Urol. 75, 1038–1040 (2019).
Halabi, S. et al. Overall survival of black and white men with metastatic castration-resistant prostate cancer treated with docetaxel. J. Clin. Oncol. 37, 403–410 (2019).
Yamoah, K. et al. Racial and ethnic disparities in prostate cancer outcomes in the veterans affairs health care system. JAMA Netw. Open. 5, e2144027 (2022).
Spratt, D. E. & Osborne, J. R. Disparities in castration-resistant prostate cancer trials. J. Clin. Oncol. 33, 1101–1103 (2015).
Rencsok, E. M. et al. Diversity of enrollment in prostate cancer clinical trials: current status and future directions. Cancer Epidemiol. Biomark. Prev. 29, 1374–1380 (2020).
Lewis, D. D. & Cropp, C. D. The impact of African ancestry on prostate cancer disparities in the era of precision medicine. Genes 11, 1471 (2020).
Maskarinec, G. & Noh, J. J. The effect of migration on cancer incidence among Japanese in Hawaii. Ethn. Dis. 14, 431–439 (2004).
Rodriguez, C. et al. Meat consumption among Black and White men and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol. Biomark. Prev. 15, 211–216 (2006).
Wilson, K. M. et al. Meat, fish, poultry, and egg intake at diagnosis and risk of prostate cancer progression. Cancer Prev. Res. 9, 933–941 (2016).
Barrington, W. E. et al. Difference in association of obesity with prostate cancer risk between US African American and non-Hispanic white men in the selenium and vitamin E cancer prevention trial (SELECT). JAMA Oncol. 1, 342–349 (2015).
Amling, C. L. et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J. Clin. Oncol. 22, 439–445 (2004).
Lofton, H., Ard, J. D., Hunt, R. R. & Knight, M. G. Obesity among African American people in the United States: a review. Obesity 31, 306–315 (2023).
Bailey, Z. D. et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet 389, 1453–1463 (2017).
Zare, H., Aazami, A., Shalby, N., Gilmore, D. R. & Thorpe, R. J. Measuring racial differences in obesity risk factors in non-Hispanic black and white men aged 20 years or older. Am. J. Mens. Health 17, 15579883231205845 (2023).
Office for Health Improvement & Disparities. Obesity Profile: short statistical commentary May 2023. GOV.UK https://www.gov.uk/government/statistics/obesity-profile-update-may-2023/obesity-profile-short-statistical-commentary-may-2023 (2023).
Gómez-Acebo, I. et al. Risk model for prostate cancer using environmental and genetic factors in the Spanish Multi-Case-Control (MCC) study. Sci. Rep. 7, 8994 (2017).
Nelson, W. G. et al. Health inequity drives disease biology to create disparities in prostate cancer outcomes. J. Clin. Invest. 132, e155031 (2022).
Bosland, M. C. et al. Prevalence of prostate cancer at autopsy in Nigeria — a preliminary report. Prostate 81, 553–559 (2021).
Jahn, J. L., Giovannucci, E. L. & Stampfer, M. J. The high prevalence of undiagnosed prostate cancer at autopsy: implications for epidemiology and treatment of prostate cancer in the prostate-specific antigen-era. Int. J. Cancer 137, 2795–2802 (2015).
Johnstone, S. E. & Baylin, S. B. Stress and the epigenetic landscape: a link to the pathobiology of human diseases? Nat. Rev. Genet. 11, 806–812 (2010).
Vick, A. D. & Burris, H. H. Epigenetics and health disparities. Curr. Epidemiol. Rep. 4, 31–37 (2017).
De Marzo, A. M. et al. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 7, 256–269 (2007).
Awasthi, S. et al. Comparative genomics reveals distinct immune-oncologic pathways in African American men with prostate cancer. Clin. Cancer Res. 27, 320–329 (2021).
Powell, I. J. et al. Genes associated with prostate cancer are differentially expressed in African American and European American men. Cancer Epidemiol. Biomark. Prev. 22, 891–897 (2013).
Butler, E. N., Kelly, S. P., Coupland, V. H., Rosenberg, P. S. & Cook, M. B. Fatal prostate cancer incidence trends in the United States and England by race, stage, and treatment. Br. J. Cancer 123, 487–494 (2020).
Dee, E. C. et al. Disparities in refusal of locoregional treatment for prostate adenocarcinoma. JCO Oncol. Pract. 17, e1489–e1501 (2021).
Jain, B. et al. Racial disparities in treatment delay among younger men with prostate cancer. Prostate Cancer Prostatic Dis. 25, 590–592 (2022).
Dee, E. C. et al. Factors influencing noncompletion of radiation therapy among men with localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 109, 1279–1285 (2021).
Dee, E. C. et al. Shorter radiation regimens and treatment noncompletion among patients with breast and prostate cancer in the United States: an analysis of racial disparities in access and quality. JCO Oncol. Pract. 19, e197–e212 (2023).
Chhatre, S., Malkowicz, S. B. & Jayadevappa, R. Continuity of care in acute survivorship phase, and short and long-term outcomes in prostate cancer patients. Prostate 81, 1310–1319 (2021).
Ellis, L. et al. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J. Clin. Oncol. 36, 25–33 (2018).
Wadhwa, A. et al. Neighborhood deprivation, race and ethnicity, and prostate cancer outcomes across California health care systems. JAMA Netw. Open. 7, e242852 (2024).
Evans, S. et al. Clinical presentation and initial management of Black men and White men with prostate cancer in the United Kingdom: the PROCESS cohort study. Br. J. Cancer 102, 249–254 (2010).
Kum, F. et al. Presentation, follow-up, and outcomes among African/Afro-Caribbean men on active surveillance for prostate cancer: experiences of a high-volume UK centre. Prostate Cancer Prostatic Dis. 24, 549–557 (2021).
Dess, R. T. et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 5, 975–983 (2019).
Riviere, P. et al. Survival of African American and non-Hispanic white men with prostate cancer in an equal-access health care system. Cancer 126, 1683–1690 (2020).
Oehrlein, N. et al. Race-specific prostate cancer outcomes in a cohort of military health care beneficiaries undergoing surgery: 1990–2017. Cancer Med. 11, 4354–4365 (2022).
Kishan, A. U. et al. Association of black race with improved outcomes following definitive radiotherapy with androgen deprivation therapy for high-risk prostate cancer: a meta-analysis of eight randomized trials. J. Clin. Oncol. 38, 327–327 (2020).
Mahal, B. A., Berman, R. A., Taplin, M. E. & Huang, F. W. Prostate cancer-specific mortality across Gleason scores in black vs nonblack men. JAMA 320, 2479–2481 (2018).
Deka, R. et al. Association between African American race and clinical outcomes in men treated for low-risk prostate cancer with active surveillance. JAMA 324, 1747–1754 (2020).
Leapman, M. S. et al. Mediators of racial disparity in the use of prostate magnetic resonance imaging among patients with prostate cancer. JAMA Oncol. 8, 687–696 (2022).
Vince, R. A. et al. Evaluation of social determinants of health and prostate cancer outcomes among black and white patients: a systematic review and meta-analysis. JAMA Netw. Open. 6, e2250416 (2023).
Thompson, I. et al. Association of African-American ethnic background with survival in men with metastatic prostate cancer. J. Natl Cancer Inst. 93, 219–225 (2001).
Tangen, C. M. et al. Improved overall survival trends of men with newly diagnosed M1 prostate cancer: a SWOG phase III trial experience (S8494, S8894 and S9346). J. Urol. 188, 1164–1169 (2012).
Freedland, S. J. et al. The impact of race on survival in metastatic prostate cancer: a systematic literature review. Prostate Cancer Prostatic Dis. 26, 461–474 (2023).
Muralidhar, V. et al. Association between black race and improved survival following sipuleucel-T immunotherapy in metastatic castrate-resistant prostate cancer: implications for immune biology and integration of radiation therapy with immunotherapy. Int. J. Radiat. Oncol. Biol. Phys. 108, e901 (2020).
Sartor, O. et al. Survival of African-American and Caucasian men after sipuleucel-T immunotherapy: outcomes from the PROCEED registry. Prostate Cancer Prostatic Dis. 23, 517–526 (2020).
Zhao, H. et al. Racial discrepancies in overall survival among men treated with 223radium. J. Urol. 203, 331–337 (2020).
Marar, M. et al. Outcomes among African American and non-Hispanic white men with metastatic castration-resistant prostate cancer with first-line abiraterone. JAMA Netw. Open. 5, e2142093 (2022).
Ng, K., Wilson, P., Mutsvangwa, K., Hounsome, L. & Shamash, J. Overall survival of black and white men with metastatic castration-resistant prostate cancer (mCRPC): a 20-year retrospective analysis in the largest healthcare trust in England. Prostate Cancer Prostatic Dis. 24, 718–724 (2021).
Warner, E. T. et al. Racial and ethnic differences in breast cancer survival: mediating effect of tumor characteristics and sociodemographic and treatment factors. J. Clin. Oncol. 33, 2254–2261 (2015).
Pilon, D. et al. Medication adherence among patients with advanced prostate cancer using oral therapies. Future Oncol. 18, 231–243 (2022).
Stewart, S. J. F., Moon, Z. & Horne, R. Medication nonadherence: health impact, prevalence, correlates and interventions. Psychol. Health 38, 726–765 (2023).
Mohamad Al-Ali, B., Kramer, G., Madersbacher, S. & Berger, I. Abiraterone for castration-resistant prostate cancer: adherence, survival and hospitalization. Wien. Klin. Wochenschr. 129, 380–384 (2017).
Freedland, S. J. et al. Medication patterns of abiraterone acetate plus prednisone or enzalutamide and PSA progression in veterans with metastatic castration-resistant prostate cancer. Curr. Med. Res. Opin. 37, 635–642 (2021).
Horne R., Cooper V., Wileman V., Chan A. Supporting adherence to medicines for long-term conditions. Eur. Psychol. 24, 82–96 (2019).
Horne, R. et al. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the necessity-concerns framework. PLoS ONE 8, e80633 (2013).
Foot, H., La Caze, A., Gujral, G. & Cottrell, N. The necessity-concerns framework predicts adherence to medication in multiple illness conditions: a meta-analysis. Patient Educ. Couns. 99, 706–717 (2016).
Coughlin, S. S., Vernon, M., Klaassen, Z., Tingen, M. S. & Cortes, J. E. Knowledge of prostate cancer among African American men: a systematic review. Prostate 81, 202–213 (2021).
Pedersen, V. H., Armes, J. & Ream, E. Perceptions of prostate cancer in Black African and Black Caribbean men: a systematic review of the literature. Psychooncology 21, 457–468 (2012).
Mulugeta, B., Williamson, S., Monks, R., Hack, T. & Beaver, K. Cancer through black eyes — the views of UK based black men towards cancer: a constructivist grounded theory study. Eur. J. Oncol. Nurs. 29, 8–16 (2017).
Austin, K. L. et al. Perceived barriers to flexible sigmoidoscopy screening for colorectal cancer among UK ethnic minority groups: a qualitative study. J. Med. Screen. 16, 174–179 (2009).
Brown, T. et al. Fear, family and the placing of emotion: black women’s responses to a breast cancer awareness intervention. Soc. Sci. Med. 195, 90–96 (2017).
Shungu, N. & Sterba, K. R. Barriers and facilitators to informed decision-making about prostate cancer screening among black men. J. Am. Board. Fam. Med. 34, 925–936 (2021).
Bamidele, O. O. et al. Barriers and facilitators to accessing and utilising post-treatment psychosocial support by Black men treated for prostate cancer — a systematic review and qualitative synthesis. Support. Care Cancer 30, 3665–3690 (2022).
Haynes-Maslow, L., Allicock, M. & Johnson, L. S. Cancer support needs for African American breast cancer survivors and caregivers. J. Cancer Educ. 31, 166–171 (2016).
Okoro, F. O., Song, L., Auten, B., Whitaker-Brown, C. & Cornelius, J. African-American survivors of prostate cancer: a meta-synthesis of qualitative studies. J. Cancer Surviv. 15, 40–53 (2021).
Neves, N. M., Queiroz, L. A., Cuck, G., Dzik, C. & Pereira, F. M. T. Prostate cancer and spirituality: a systematic review. J. Relig. Health https://doi.org/10.1007/s10943-023-01845-0 (2023).
King-Okoye, M., Arber, A. & Faithfull, S. Routes to diagnosis for men with prostate cancer: men’s cultural beliefs about how changes to their bodies and symptoms influence help-seeking actions. A narrative review of the literature. Eur. J. Oncol. Nurs. 30, 48–58 (2017).
Salsman, J. M., Fitchett, G., Merluzzi, T. V., Sherman, A. C. & Park, C. L. Religion, spirituality, and health outcomes in cancer: a case for a meta-analytic investigation. Cancer 121, 3754–3759 (2015).
Wagland, R. et al. Adjustment strategies amongst black African and black Caribbean men following treatment for prostate cancer: findings from the Life After Prostate Cancer Diagnosis (LAPCD) study. Eur. J. Cancer Care 29, e13183 (2020).
White, M. & Verhoef, M. Cancer as part of the journey: the role of spirituality in the decision to decline conventional prostate cancer treatment and to use complementary and alternative medicine. Integr. Cancer Ther. 5, 117–122 (2006).
Ahmedani, B. K., Peterson, E. L., Wells, K. E., Rand, C. S. & Williams, L. K. Asthma medication adherence: the role of God and other health locus of control factors. Ann. Allergy Asthma Immunol. 110, 75–79.e2 (2013).
Thomas, V. N., Saleem, T. & Abraham, R. Barriers to effective uptake of cancer screening among Black and minority ethnic groups. Int. J. Palliat. Nurs. 11, 564–571 (2005).
Vrinten, C., Wardle, J. & Marlow, L. A. Cancer fear and fatalism among ethnic minority women in the United Kingdom. Br. J. Cancer 114, 597–604 (2016).
Koffman, J., Morgan, M., Edmonds, P., Speck, P. & Higginson, I. J. “I know he controls cancer”: the meanings of religion among Black Caribbean and White British patients with advanced cancer. Soc. Sci. Med. 67, 780–789 (2008).
Clarke, N. et al. Negative emotions and cancer fatalism are independently associated with uptake of Faecal Immunochemical Test-based colorectal cancer screening: results from a population-based study. Prev. Med. 145, 106430 (2021).
Lu, L., Liu, J. & Yuan, Y. C. Cultural differences in cancer information acquisition: cancer risk perceptions, fatalistic beliefs, and worry as predictors of cancer information seeking and avoidance in the U.S. and China. Health Commun. 37, 1442–1451 (2022).
Beeken, R. J., Simon, A. E., von Wagner, C., Whitaker, K. L. & Wardle, J. Cancer fatalism: deterring early presentation and increasing social inequalities? Cancer Epidemiol. Biomark. Prev. 20, 2127–2131 (2011).
Özer, Z., Turan, G. B. & Karaman, S. Effects of fatalism perception and attitudes towards complementary and alternative medicine on medication adherence in patients with epilepsy. Eur. J. Integr. Med. 61, 102271 (2023).
Walker, R. J. et al. Effect of diabetes fatalism on medication adherence and self-care behaviors in adults with diabetes. Gen. Hosp. Psychiatry 34, 598–603 (2012).
Bamidele O., et al. Life after prostate cancer: a systematic literature review and thematic synthesis of the post-treatment experiences of Black African and Black Caribbean men. Eur. J. Cancer Care 27, e12784 (2018).
DeWitt-Foy, M. E., Gam, K., Modlin, C., Kim, S. P. & Abouassaly, R. Race, decisional regret and prostate cancer beliefs: identifying targets to reduce racial disparities in prostate cancer. J. Urol. 205, 426–433 (2021).
Machirori, M., Patch, C. & Metcalfe, A. Study of the relationship between Black men, culture and prostate cancer beliefs. Cogent Med. 5, 1442636 (2018).
Rivas, C. et al. Ethnicity and the prostate cancer experience: a qualitative metasynthesis. Psychooncology 25, 1147–1156 (2016).
Rajbabu, K. et al. Racial origin is associated with poor awareness of prostate cancer in UK men, but can be increased by simple information. Prostate Cancer Prostatic Dis. 10, 256–260 (2007).
Allen, J. D., Kennedy, M., Wilson-Glover, A. & Gilligan, T. D. African-American men’s perceptions about prostate cancer: implications for designing educational interventions. Soc. Sci. Med. 64, 2189–2200 (2007).
Anderson, B. & Marshall-Lucette, S. African and Afro-Caribbean men’s experiences of prostate cancer. Br. J. Nurs. 22, 1296–1298, 1300–1302, 1304–1307 (2013).
Fry, S. L., Hopkinson, J. & Kelly, D. “We’re talking about black men here, there’s a difference”; cultural differences in socialised knowledge of prostate cancer risk: a qualitative research study. Eur. J. Oncol. Nurs. 56, 102080 (2022).
Nanton, V. & Dale, J. It don’t make sense to worry too much”: the experience of prostate cancer in African-Caribbean men in the UK. Eur. J. Cancer Care 20, 62–71 (2011).
Black British Voices. Black British Voices Project Report August 2023. The Black British Voices Research Project Team https://www.bbvp.org/_files/ugd/487862_ec6ef8c150d248e193aa1617d9f47875.pdf (2023).
Lillard, J. W., Moses, K. A., Mahal, B. A. & George, D. J. Racial disparities in Black men with prostate cancer: a literature review. Cancer 128, 3787–3795 (2022).
Balakrishnan, A. S. et al. Minority recruitment trends in phase III prostate cancer clinical trials (2003 to 2014): progress and critical areas for improvement. J. Urol. 201, 259–267 (2019).
Adams-Campbell, L. L. et al. Enrollment of African Americans onto clinical treatment trials: study design barriers. J. Clin. Oncol. 22, 730–734 (2004).
Ahaghotu, C., Tyler, R. & Sartor, O. African American participation in oncology clinical trials — focus on prostate cancer: implications, barriers, and potential solutions. Clin. Genitourin. Cancer 14, 105–116 (2016).
Esdaille, A. R., Ibilibor, C., Holmes, A., Palmer, N. R. & Murphy, A. B. Access and representation: a narrative review of the disparities in access to clinical trials and precision oncology in black men with prostate cancer. Urology 163, 90–98 (2022).
NHS workforce. GOV.UK https://www.ethnicity-facts-figures.service.gov.uk/workforce-and-business/workforce-diversity/nhs-workforce/latest/ (2023).
Kamran, S. C., Winkfield, K. M., Reede, J. Y. & Vapiwala, N. Intersectional analysis of U.S. medical faculty diversity over four decades. N. Engl. J. Med. 386, 1363–1371 (2022).
Evans, K. R., Lewis, M. J. & Hudson, S. V. The role of health literacy on African American and Hispanic/Latino perspectives on cancer clinical trials. J. Cancer Educ. 27, 299–305 (2012).
Butler, S. S. et al. Racial disparities in patient-reported measures of physician cultural competency among cancer survivors in the United States. JAMA Oncol. 6, 152–154 (2020).
Loeb, S. et al. The effect of racial concordance on patient trust in online videos about prostate cancer: a randomized clinical trial. JAMA Netw. Open. 6, e2324395 (2023).
Cleland, J. A. The qualitative orientation in medical education research. Korean J. Med. Educ. 29, 61–71 (2017).
Dee, E. C. et al. Leveraging national and global political determinants of health to promote equity in cancer care. J. Natl Cancer Inst. 115, 1157–1163 (2023).
George, D. J. et al. A prospective trial of abiraterone acetate plus prednisone in Black and White men with metastatic castrate-resistant prostate cancer. Cancer 127, 2954–2965 (2021).
Woods, V. D., Montgomery, S. B. & Herring, R. P. Recruiting Black/African American men for research on prostate cancer prevention. Cancer 100, 1017–1025 (2004).
Winkfield, K. M., Flowers, C. R. & Mitchell, E. P. Making the case for improving oncology workforce diversity. Am. Soc. Clin. Oncol. Educ. Book. 37, 18–22 (2017).
Wang, W. J., Ramsey, S. D., Bennette, C. S. & Bansal, A. Racial disparities in access to prostate cancer clinical trials: a county-level analysis. JNCI Cancer Spectr. 6, pkab093 (2021).
Acknowledgements
E.C.D. is funded in part through the Prostate Cancer Foundation Young Investigator Award and through the Cancer Center Support Grant from the National Cancer Institute (P30 CA008748). K.N. has received personal fees from Pfizer, GSK and TESARO, Boehringer Ingelheim, travel grants from Conquer Cancer Foundation and research funding from Cancer Research UK. P.L.N. is funded in part through the National Institutes of Health (R01-CA240582). E.C.D., R.T., K.N., G.A.-M., Z.M., K.M., G.F., L.T.A.M., E.P., J.S. and R.H. are part of a collaborative effort (TRANSFORM) aimed at mitigating disparities in prostate cancer in the UK, funded by Prostate Cancer Research.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
K.N. has received personal fees from Pfizer, GSK and TESARO, Boehringer Ingelheim, travel grants from Conquer Cancer Foundation and research funding from Cancer Research UK. V.M. is employed by Northwest Permanente. B.A.M. receives funding from the Prostate Cancer Foundation (PCF), the American Society for Radiation Oncology (ASTRO), the Department of Defense, and the Sylvester Comprehensive Cancer Center. E.P. reports receiving consulting fees from Janssen and Merck Sharp & Dohme, financial support from Bayer, and non-financial support from Amgen and Astellas. D.E.S. reports personal fees from Janssen, Blue Earth, AstraZeneca and Boston Scientific outside the submitted work. P.L.N. reported receiving grants and personal fees from Bayer, Janssen and Astellas and personal fees from Boston Scientific, Dendreon, Ferring, COTA, Blue Earth Diagnostics, Myovant Sciences and Augmenix outside the submitted work. R.H. reports grants/research support from AstraZeneca, National Institute for Health Research (NIHR) and Asthma UK (AUKCAR); honoraria/consultation fees from AbbVie, Amgen, Astellas, AstraZeneca, Biogen, Erasmus, Idec, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Sharp Dohme, Novartis, Pfizer, Roche, Shire Pharmaceuticals and TEVA; and is a founder and shareholder of a UCL Business company (Spoonful of Sugar) providing consultancy on supporting patients with medicine- and treatment-related behaviours to healthcare policymakers, providers and industry. Z.M. reports paid work for UCL Business Company Spoonful of Sugar. All other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Clayton Yates, Stefan Ambs, Yaw Nyame and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Cancer Research UK screening for prostate cancer: https://www.cancerresearchuk.org/about-cancer/prostate-cancer/gettingdiagnosed/screening
Our Future Health study: https://ourfuturehealth.org.uk/
Prostate Cancer UK risk checker: https://prostatecanceruk.org/risk-checker
RESPOND study: https://www.respondstudy.org/
UK Cancer Patient Experience Survey: https://www.ncpes.co.uk/latest-national-results/
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dee, E.C., Todd, R., Ng, K. et al. Racial disparities in prostate cancer in the UK and the USA: similarities, differences and steps forwards. Nat Rev Urol 22, 223–234 (2025). https://doi.org/10.1038/s41585-024-00948-x
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41585-024-00948-x
This article is cited by
-
Rising metastatic prostate cancer rates but narrowing racial gap
BMC Medicine (2025)
-
Beyond Black and White: Cancer Disparities Within Racial Groups
Journal of General Internal Medicine (2025)