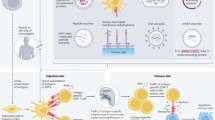
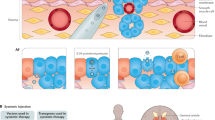
Abstract
Prostate cancer is the second most commonly diagnosed cancer and the fifth leading cause of death among men worldwide. Androgen deprivation therapy is a common prostate cancer treatment, but its efficacy is often hindered by the development of resistance, which results in reducing survival benefits. Immunotherapy showed great promise in treating solid tumours; however, clinically significant improvements have not been demonstrated for patients with prostate cancer, highlighting specific drawbacks of this therapeutic modality. Hence, exploring novel strategies to synergistically enhance the efficacy of prostate cancer immunotherapy is imperative. Clinical investigations have focused on the combined use of targeted or gene therapy and immunotherapy for prostate cancer. Notably, tumour-specific antigens and inflammatory mediators are released from tumour cells after targeted or gene therapy, and the recruitment and infiltration of immune cells, including CD8+ T cells and natural killer cells activated by immunotherapy, are further augmented, markedly improving the efficacy and prognosis of prostate cancer. Thus, immunotherapy, targeted therapy and gene therapy could have reciprocal synergistic effects in prostate cancer in combination, resulting in a proposed synergistic model encompassing these three therapeutic modalities, presenting novel potential treatment strategies for prostate cancer.
Key points
-
Prostate cancer is generally considered to consist of an immunologically cold tumour with low immune-cell infiltration and low immunogenic response.
-
Immunotherapies for prostate cancer such as immune checkpoint blockade have limited antitumour activity, encouraging research into novel strategies to potentiate the efficacy of immunotherapy.
-
Targeted therapy is the mainstay treatment for patients with prostate cancer, but it sometimes fails to improve the clinical outcomes owing to drug resistance.
-
Gene therapy for prostate cancer demonstrates a generally good safety profile but limited efficacy, and so has potential for prostate cancer treatment, particularly in promoting the efficacy of immunotherapy.
-
A synergistic targeting model has been proposed focusing on potentiating the effect of immunotherapy through targeted or gene therapy. This model is a novel treatment strategy for overcoming drug resistance and immunosuppression for prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Bergengren, O. et al. 2022 update on prostate cancer epidemiology and risk factors — a systematic review. Eur. Urol. 84, 191–206 (2023).
Litwin, M. S. & Tan, H.-J. The diagnosis and treatment of prostate cancer. JAMA 317, 2532–2542 (2017).
Shafi, A. A., Yen, A. E. & Weigel, N. L. Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharmacol. Ther. 140, 223–238 (2013).
Tombal, B. Non-metastatic CRPC and asymptomatic metastatic CRPC: which treatment for which patient? Ann. Oncol. 23, x251–x258 (2012).
Zhang, Y. & Zhang, Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol. Immunol. 17, 807–821 (2020).
Antonarakis, E. S. et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J. Clin. Oncol. 38, 395–405 (2020).
Sharma, P. et al. Nivolumab plus ipilimumab for metastatic castration-resistant prostate cancer: preliminary analysis of patients in the checkmate 650 trial. Cancer Cell 38, 489–499.e3 (2020).
Graff, J. N. et al. A phase II single-arm study of pembrolizumab with enzalutamide in men with metastatic castration-resistant prostate cancer progressing on enzalutamide alone. J. Immunother. Cancer 8, e000642 (2020).
Lee, P. & Gujar, S. Potentiating prostate cancer immunotherapy with oncolytic viruses. Nat. Rev. Urol. 15, 235–250 (2018).
Zhang, J. et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature 553, 91–95 (2018).
Bhatia, V. et al. Targeting advanced prostate cancer with STEAP1 chimeric antigen receptor T cell and tumor-localized IL-12 immunotherapy. Nat. Commun. 14, 2041 (2023).
Pachynski, R. K. et al. IL-7 expands lymphocyte populations and enhances immune responses to sipuleucel-T in patients with metastatic castration-resistant prostate cancer (mCRPC). J. Immunother. Cancer 9, e002903 (2021).
Brown, J. S., Sundar, R. & Lopez, J. Combining DNA damaging therapeutics with immunotherapy: more haste, less speed. Br. J. Cancer 118, 312–324 (2018).
Goel, S. et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 548, 471–475 (2017).
Lin, Y. X. et al. Reactivation of the tumor suppressor PTEN by mRNA nanoparticles enhances antitumor immunity in preclinical models. Sci. Transl. Med. 13, eaba9772 (2021).
Morel, K. L. et al. EZH2 inhibition activates a dsRNA-STING-interferon stress axis that potentiates response to PD-1 checkpoint blockade in prostate cancer. Nat. Cancer 2, 444–456 (2021).
Qi, Z. et al. Overcoming resistance to immune checkpoint therapy in PTEN-null prostate cancer by intermittent anti-PI3Kα/β/δ treatment. Nat. Commun. 13, 182 (2022).
Patnaik, A. et al. Cabozantinib eradicates advanced murine prostate cancer by activating antitumor innate immunity. Cancer Discov. 7, 750–765 (2017).
Gujar, S. A., Pan, D. A., Marcato, P., Garant, K. A. & Lee, P. W. Oncolytic virus-initiated protective immunity against prostate cancer. Mol. Ther. 19, 797–804 (2011).
Ozga, A. J., Chow, M. T. & Luster, A. D. Chemokines and the immune response to cancer. Immunity 54, 859–874 (2021).
Cha, E. & Fong, L. Immunotherapy for prostate cancer: biology and therapeutic approaches. J. Clin. Oncol. 29, 3677–3685 (2011).
Esensten, J. H., Helou, Y. A., Chopra, G., Weiss, A. & Bluestone, J. A. CD28 costimulation: from mechanism to therapy. Immunity 44, 973–988 (2016).
He, Q., Jiang, X., Zhou, X. & Weng, J. Targeting cancers through TCR-peptide/MHC interactions. J. Hematol. Oncol. 12, 139 (2019).
Giles, J. R., Globig, A.-M., Kaech, S. M. & Wherry, E. J. CD8+ T. cells in the cancer-immunity cycle. Immunity 56, 2231–2253 (2023).
Galon, J. & Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug. Discov. 18, 197–218 (2019).
Majidpoor, J. & Mortezaee, K. The efficacy of PD-1/PD-L1 blockade in cold cancers and future perspectives. Clin. Immunol. 226, 108707 (2021).
Brea, L. & Yu, J. Tumor-intrinsic regulators of the immune-cold microenvironment of prostate cancer. Trends Endocrinol. Metab. https://doi.org/10.1016/j.tem.2024.12.003 (2025).
Zhang, H. et al. Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer. J. Exp. Clin. Cancer Res. 40, 184 (2021).
Topalian, S. L., Weiner, G. J. & Pardoll, D. M. Cancer immunotherapy comes of age. J. Clin. Oncol. 29, 4828–4836 (2011).
Thakur, A., Vaishampayan, U. & Lum, L. G. Immunotherapy and immune evasion in prostate cancer. Cancers 5, 569–590 (2013).
Rebuzzi, S. E. et al. Immune checkpoint inhibitors in advanced prostate cancer: current data and future perspectives. Cancers 14, 1245 (2022).
Wu, Y. M. et al. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell 173, 1770–1782.e1714 (2018).
Pound, C. R. et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281, 1591–1597 (1999).
Sridaran, D. et al. Prostate cancer immunotherapy: improving clinical outcomes with a multi-pronged approach. Cell Rep. Med. 4, 101199 (2023).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Hummel, H. D. et al. Pasotuxizumab, a BiTE(®) immune therapy for castration-resistant prostate cancer: phase I, dose-escalation study findings. Immunotherapy 13, 125–141 (2021).
Autio, K. A. et al. First-in-human, phase 1 study of PF-06753512, a vaccine-based immunotherapy regimen (VBIR), in non-metastatic hormone-sensitive biochemical recurrence and metastatic castration-resistant prostate cancer (mCRPC). J. Immunother. Cancer 11, e005702 (2023).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Zhao, B. et al. Immune checkpoint of B7-H3 in cancer: from immunology to clinical immunotherapy. J. Hematol. Oncol. 15, 153 (2022).
Kontos, F. et al. B7-H3: an attractive target for antibody-based immunotherapy. Clin. Cancer Res. 27, 1227–1235 (2021).
Zucali, P. A. et al. Targeting CD38 and PD-1 with isatuximab plus cemiplimab in patients with advanced solid malignancies: results from a phase I/II open-label, multicenter study. J. Immunother. Cancer 10, e003697 (2022).
Powles, T. et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: a randomized phase 3 trial. Nat. Med. 28, 144–153 (2022).
Brown, L. C. et al. A phase 2 trial of avelumab in men with aggressive-variant or neuroendocrine prostate cancer. Prostate Cancer Prostatic Dis. 25, 762–769 (2022).
Winquist, E. et al. Randomized phase II study of durvalumab with or without tremelimumab in patients with metastatic castration-resistant prostate cancer. Clin. Cancer Res. 31, 45–55 (2025).
Shenderov, E. et al. Neoadjuvant enoblituzumab in localized prostate cancer: a single-arm, phase 2 trial. Nat. Med. 29, 888–897 (2023).
Yu, E. Y. et al. Pembrolizumab plus olaparib in patients with metastatic castration-resistant prostate cancer: long-term results from the phase 1b/2 KEYNOTE-365 cohort A study. Eur. Urol. 83, 15–26 (2023).
Yu, E. Y. et al. Pembrolizumab plus docetaxel and prednisone in patients with metastatic castration-resistant prostate cancer: long-term results from the phase 1b/2 KEYNOTE-365 cohort B study. Eur. Urol. 82, 22–30 (2022).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Petrylak, D. P. et al. Safety and clinical activity of atezolizumab in patients with metastatic castration-resistant prostate cancer: a phase I study. Clin. Cancer Res. 27, 3360–3369 (2021).
Fumet, J. D. et al. Precision medicine phase II study evaluating the efficacy of a double immunotherapy by durvalumab and tremelimumab combined with olaparib in patients with solid cancers and carriers of homologous recombination repair genes mutation in response or stable after olaparib treatment. BMC Cancer 20, 748 (2020).
Boyiadzis, M. M. et al. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J. Immunother. Cancer 6, 35 (2018).
Rebello, R. J. et al. Prostate cancer. Nat. Rev. Dis. Prim. 7, 9 (2021).
Wolf, P., Alzubi, J., Gratzke, C. & Cathomen, T. The potential of CAR T cell therapy for prostate cancer. Nat. Rev. Urol. 18, 556–571 (2021).
Larson, R. C. & Maus, M. V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 21, 145–161 (2021).
Hou, A. J., Chen, L. C. & Chen, Y. Y. Navigating CAR-T cells through the solid-tumour microenvironment. Nat. Rev. Drug. Discov. 20, 531–550 (2021).
Liu, Y. et al. Strategies to enhance CAR-T persistence. Biomark. Res. 10, 86 (2022).
Dorff, T. B. et al. PSCA-CAR T cell therapy in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat. Med. 30, 1636–1644 (2024).
Narayan, V. et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat. Med. 28, 724–734 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03013712 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05732948 (2023).
Zanvit, P. et al. Antitumor activity of AZD0754, a dnTGFβRII-armored, STEAP2-targeted CAR-T cell therapy, in prostate cancer. J. Clin. Invest. 133, e169655 (2023).
Huehls, A. M., Coupet, T. A. & Sentman, C. L. Bispecific T-cell engagers for cancer immunotherapy. Immunol. Cell Biol. 93, 290–296 (2015).
Deegen, P. et al. The PSMA-targeting half-life extended BiTE therapy AMG 160 has potent antitumor activity in preclinical models of metastatic castration-resistant prostate cancer. Clin. Cancer Res. 27, 2928–2937 (2021).
Friedrich, M. et al. Regression of human prostate cancer xenografts in mice by AMG 212/BAY2010112, a novel PSMA/CD3-Bispecific BiTE antibody cross-reactive with non-human primate antigens. Mol. Cancer Ther. 11, 2664–2673 (2012).
Simão, D. C. et al. Bispecific T-cell engagers therapies in solid tumors: focusing on prostate cancer. Cancers 15, 1412 (2023).
Géraud, A. et al. Reactions and adverse events induced by T-cell engagers as anti-cancer immunotherapies, a comprehensive review. Eur. J. Cancer 205, 114075 (2024).
Kamat, N. V., Yu, E. Y. & Lee, J. K. BiTE-ing into prostate cancer with bispecific T-cell engagers. Clin. Cancer Res. 27, 2675–2677 (2021).
Dorff, T. et al. A phase I study of acapatamab, a half-life extended, PSMA-targeting bispecific T-cell engager for metastatic castration-resistant prostate cancer. Clin. Cancer Res. 30, 1488–1500 (2024).
Nolan-Stevaux, O. et al. AMG 509 (Xaluritamig), an anti-STEAP1 XmAb 2+1 T-cell redirecting immune therapy with avidity-dependent activity against prostate cancer. Cancer Discov. 14, 90–103 (2023).
Kelly, W. K. et al. Xaluritamig, a STEAP1 × CD3 XmAb 2+1 immune therapy for metastatic castration-resistant prostate cancer: results from dose exploration in a first-in-human study. Cancer Discov. 14, 76–89 (2023).
Vaishampayan, U. N. et al. Phase II trial of pembrolizumab and anti-CD3 x anti-HER2 bispecific antibody-armed activated T cells in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 29, 122–133 (2023).
Saxena, M., van der Burg, S. H., Melief, C. J. M. & Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 21, 360–378 (2021).
Hamdy, F. C. et al. Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N. Engl. J. Med. 388, 1547–1558 (2023).
Rastogi, I., Muralidhar, A. & McNeel, D. G. Vaccines as treatments for prostate cancer. Nat. Rev. Urol. 20, 544–559 (2023).
Higano, C. S. et al. Sipuleucel-T. Nat. Rev. Drug. Discov. 9, 513–514 (2010).
Sinha, M. et al. Pre-existing immune status associated with response to combination of sipuleucel-T and ipilimumab in patients with metastatic castration-resistant prostate cancer. J. Immunother. Cancer 9, e002254 (2021).
Dorff, T. et al. Phase Ib study of patients with metastatic castrate-resistant prostate cancer treated with different sequencing regimens of atezolizumab and sipuleucel-T. J. Immunother. Cancer 9, e002931 (2021).
Antonarakis, E. S. et al. Sequencing of sipuleucel-T and androgen deprivation therapy in men with hormone-sensitive biochemically recurrent prostate cancer: a phase II randomized trial. Clin. Cancer Res. 23, 2451–2459 (2017).
Marshall, C. H. et al. Randomized phase II trial of sipuleucel-T with or without radium-223 in men with bone-metastatic castration-resistant prostate cancer. Clin. Cancer Res. 27, 1623–1630 (2021).
Harrop, R., John, J. & Carroll, M. W. Recombinant viral vectors: cancer vaccines. Adv. Drug. Deliv. Rev. 58, 931–947 (2006).
Madan, R. A., Arlen, P. M., Mohebtash, M., Hodge, J. W. & Gulley, J. L. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert. Opin. Investig. Drugs 18, 1001–1011 (2009).
Arlen, P. M. et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J. Urol. 178, 1515–1520 (2007).
Parsons, J. K. et al. A phase 2, double-blind, randomized controlled trial of PROSTVAC in prostate cancer patients on active surveillance. Eur. Urol. Focus. 9, 447–454 (2023).
Gulley, J. L. et al. Phase III trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J. Clin. Oncol. 37, 1051–1061 (2019).
Bilusic, M. et al. Phase I study of a multitargeted recombinant Ad5 PSA/MUC-1/brachyury-based immunotherapy vaccine in patients with metastatic castration-resistant prostate cancer (mCRPC). J. Immunother. Cancer 9, e002374 (2021).
Cappuccini, F. et al. Safety and immunogenicity of novel 5T4 viral vectored vaccination regimens in early stage prostate cancer: a phase I clinical trial. J. Immunother. Cancer 8, e000928 (2020).
Poria, R. et al. Vaccine development: current trends and technologies. Life Sci. 336, 122331 (2024).
McNeel, D. G. et al. Phase II trial of a DNA vaccine encoding prostatic acid phosphatase (pTVG-HP [MVI-816]) in patients with progressive, nonmetastatic, castration-sensitive prostate cancer. J. Clin. Oncol. 37, 3507–3517 (2019).
Wargowski, E. et al. Prime-boost vaccination targeting prostatic acid phosphatase (PAP) in patients with metastatic castration-resistant prostate cancer (mCRPC) using Sipuleucel-T and a DNA vaccine. J. Immunother. Cancer 6, 21 (2018).
McNeel, D. G. et al. Phase 2 trial of T-cell activation using MVI-816 and pembrolizumab in patients with metastatic, castration-resistant prostate cancer (mCRPC). J. Immunother. Cancer 10, e004198 (2022).
McNeel, D. G. et al. Phase 2 trial of a DNA vaccine (pTVG-HP) and nivolumab in patients with castration-sensitive non-metastatic (M0) prostate cancer. J. Immunother. Cancer 11, e008067 (2023).
Kyriakopoulos, C. E. et al. Multicenter phase I trial of a DNA vaccine encoding the androgen receptor ligand-binding domain (pTVG-AR, MVI-118) in patients with metastatic prostate cancer. Clin. Cancer Res. 26, 5162–5171 (2020).
Lorentzen, C. L., Haanen, J. B., Met, Ö. & Svane, I. M. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 23, e450–e458 (2022).
Kübler, H. et al. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study. J. Immunother. Cancer 3, 26 (2015).
Stenzl, A. et al. Results of the randomized, placebo-controlled phase I/IIB trial of CV9104, an mRNA based cancer immunotherapy, in patients with metastatic castration-resistant prostate cancer (mCRPC). Ann. Oncol. 28, v408–v409 (2017).
Zheng, X. et al. Tumor-antigens and immune landscapes identification for prostate adenocarcinoma mRNA vaccine. Mol. Cancer 20, 160 (2021).
Liu, W. et al. Peptide-based therapeutic cancer vaccine: current trends in clinical application. Cell Prolif. 54, e13025 (2021).
Lilleby, W., Seierstad, T., Inderberg, E. M. & Hole, K. H. Impact of human telomerase reverse transcriptase peptide vaccine combined with androgen deprivation therapy and radiotherapy in de novo metastatic prostate cancer: long-term clinical monitoring. Int. J. Cancer 152, 2166–2173 (2023).
Lilleby, W. et al. Phase I/IIa clinical trial of a novel hTERT peptide vaccine in men with metastatic hormone-naive prostate cancer. Cancer Immunol. Immunother. 66, 891–901 (2017).
Yoshimura, K. et al. A phase 2 randomized controlled trial of personalized peptide vaccine immunotherapy with low-dose dexamethasone versus dexamethasone alone in chemotherapy-naive castration-resistant prostate cancer. Eur. Urol. 70, 35–41 (2016).
Noguchi, M. et al. A randomized phase III trial of personalized peptide vaccination for castration-resistant prostate cancer progressing after docetaxel. Oncol. Rep. 45, 159–168 (2021).
Saad, F. et al. Emerging therapeutic targets for patients with advanced prostate cancer. Cancer Treat. Rev. 76, 1–9 (2019).
Vogelzang, N. J. et al. Efficacy and safety of autologous dendritic cell-based immunotherapy, docetaxel, and prednisone vs placebo in patients with metastatic castration-resistant prostate cancer: the VIABLE phase 3 randomized clinical trial. JAMA Oncol. 8, 546–552 (2022).
Podrazil, M. et al. Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget 6, 18192–18205 (2015).
Ficazzola, M. A. & Taneja, S. S. Prospects for gene therapy in human prostate cancer. Mol. Med. Today 4, 494–504 (1998).
Aurilio, G. et al. Androgen receptor signaling pathway in prostate cancer: from genetics to clinical applications. Cells 9, 2653 (2020).
Freedland, S. J. et al. Improved outcomes with enzalutamide in biochemically recurrent prostate cancer. N. Engl. J. Med. 389, 1453–1465 (2023).
Chi, K. N. et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 381, 13–24 (2019).
Fizazi, K. et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 377, 352–360 (2017).
Abida, W. et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J. Clin. Oncol. 38, 3763–3772 (2020).
de Bono, J. et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 382, 2091–2102 (2020).
Wang, R. & Liu, X. Epigenetic regulation of prostate cancer. Genes. Dis. 7, 606–613 (2020).
Kumaraswamy, A. et al. Recent advances in epigenetic biomarkers and epigenetic targeting in prostate cancer. Eur. Urol. 80, 71–81 (2021).
Yuan, K. et al. Selective inhibition of CDK4/6: a safe and effective strategy for developing anticancer drugs. Acta Pharm. Sin. B 11, 30–54 (2021).
Tewari, D., Patni, P., Bishayee, A., Sah, A. N. & Bishayee, A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: a novel therapeutic strategy. Semin. Cancer Biol. 80, 1–17 (2022).
Murillo-Garzón, V. & Kypta, R. WNT signalling in prostate cancer. Nat. Rev. Urol. 14, 683–696 (2017).
Du, Z. & Lovly, C. M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 17, 58 (2018).
Mirzaei, S. et al. Transforming growth factor-beta (TGF-β) in prostate cancer: a dual function mediator? Int. J. Biol. Macromolecules 206, 435–452 (2022).
Gharibpoor, F., Kamali Zonouzi, S., Razi, S. & Rezaei, N. AMPK’s double-faced role in advanced stages of prostate cancer. Clin. Transl. Oncol. 24, 2064–2073 (2022).
Corn, P. G. et al. A phase II study of cabozantinib and androgen ablation in patients with hormone-naïve metastatic prostate cancer. Clin. Cancer Res. 26, 990–999 (2020).
Song, B. et al. Targeting FOXA1-mediated repression of TGF-β signaling suppresses castration-resistant prostate cancer progression. J. Clin. Invest. 129, 569–582 (2019).
Culig, Z. & Santer, F. R. Androgen receptor signaling in prostate cancer. Cancer Metastasis Rev. 33, 413–427 (2014).
Fontana, F. et al. Gonadotropin-releasing hormone receptors in prostate cancer: molecular aspects and biological functions. Int. J. Mol. Sci. 21, 9511 (2020).
Chertin, B. et al. An implant releasing the gonadotropin hormone-releasing hormone agonist histrelin maintains medical castration for up to 30 months in metastatic prostate cancer. J. Urol. 163, 838–844 (2000).
Pettersson, B., Varenhorst, E., Petas, A. & Sandow, J. Duration of testosterone suppression after a 9.45 mg implant of the GnRH-analogue buserelin in patients with localised carcinoma of the prostate a 12-month follow-up study. Eur. Urol. 50, 483–489 (2006).
Bolla, M. et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N. Engl. J. Med. 337, 295–300 (1997).
Crawford, E. D. et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N. Engl. J. Med. 321, 419–424 (1989).
Kuhn, J. M. et al. A randomized comparison of the clinical and hormonal effects of two GnRH agonists in patients with prostate cancer. Eur. Urol. 32, 397–403 (1997).
Van Poppel, H. et al. Degarelix: a novel gonadotropin-releasing hormone (GnRH) receptor blocker-results from a 1-yr, multicentre, randomised, phase 2 dosage-finding study in the treatment of prostate cancer. Eur. Urol. 54, 805–813 (2008).
Shore, N. D. et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N. Engl. J. Med. 382, 2187–2196 (2020).
Tan, M. H., Li, J., Xu, H. E., Melcher, K. & Yong, E. L. Androgen receptor: structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 36, 3–23 (2015).
Møller, S., Iversen, P. & Franzmann, M.-B. Flutamide-induced liver failure. J. Hepatol. 10, 346–349 (1990).
Scher, H. I. & Kelly, W. K. Flutamide withdrawal syndrome: its impact on clinical trials in hormone-refractory prostate cancer. J. Clin. Oncol. 11, 1566–1572 (1993).
Penson, D. F. et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial. J. Clin. Oncol. 34, 2098–2106 (2016).
Desai, K., McManus, J. M. & Sharifi, N. Hormonal therapy for prostate cancer. Endocr. Rev. 42, 354–373 (2021).
Chen, Y., Zhou, Q., Hankey, W., Fang, X. & Yuan, F. Second generation androgen receptor antagonists and challenges in prostate cancer treatment. Cell Death Dis. 13, 632 (2022).
Armstrong, A. J. et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J. Clin. Oncol. 37, 2974–2986 (2019).
Hussain, M. et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 378, 2465–2474 (2018).
Sternberg, C. N. et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 382, 2197–2206 (2020).
Fizazi, K. et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N. Engl. J. Med. 383, 1040–1049 (2020).
Smith, M. R. et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N. Engl. J. Med. 378, 1408–1418 (2018).
Smith, M. R. et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N. Engl. J. Med. 386, 1132–1142 (2022).
Fizazi, K. et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 380, 1235–1246 (2019).
Sweeney, C. J. et al. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 24, 323–334 (2023).
Joseph, J. D. et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 3, 1020–1029 (2013).
Borgmann, H. et al. Moving towards precision urologic oncology: targeting enzalutamide-resistant prostate cancer and mutated forms of the androgen receptor using the novel inhibitor darolutamide (ODM-201). Eur. Urol. 73, 4–8 (2018).
Gu, W. et al. Rezvilutamide versus bicalutamide in combination with androgen-deprivation therapy in patients with high-volume, metastatic, hormone-sensitive prostate cancer (CHART): a randomised, open-label, phase 3 trial. Lancet Oncol. 23, 1249–1260 (2022).
Békés, M., Langley, D. R. & Crews, C. M. PROTAC targeted protein degraders: the past is prologue. Nat. Rev. Drug. Discov. 21, 181–200 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05177042 (2025).
Bryce, A. & Ryan, C. J. Development and clinical utility of abiraterone acetate as an androgen synthesis inhibitor. Clin. Pharmacol. Ther. 91, 101–108 (2012).
Fizazi, K. et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 20, 686–700 (2019).
James, N. D. et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N. Engl. J. Med. 377, 338–351 (2017).
Mostaghel, E. A. et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin. Cancer Res. 17, 5913–5925 (2011).
Attard, G., Reid, A. H., Olmos, D. & de Bono, J. S. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res. 69, 4937–4940 (2009).
Agarwal, N. et al. Orteronel for metastatic hormone-sensitive prostate cancer: a multicenter, randomized, open-label phase III trial (SWOG-1216). J. Clin. Oncol. 40, 3301–3309 (2022).
Taplin, M. E. et al. Androgen receptor modulation optimized for response-splice variant: a phase 3, randomized trial of galeterone versus enzalutamide in androgen receptor splice variant-7-expressing metastatic castration-resistant prostate cancer. Eur. Urol. 76, 843–851 (2019).
Culig, Z. Androgen receptor coactivators in regulation of growth and differentiation in prostate cancer. J. Cell Physiol. 231, 270–274 (2016).
Culig, Z. & Puhr, M. Androgen receptor-interacting proteins in prostate cancer development and therapy resistance. Am. J. Pathol. 194, 324–334 (2024).
Nguyen, D. P., Li, J. & Tewari, A. K. Inflammation and prostate cancer: the role of interleukin 6 (IL-6). BJU Int. 113, 986–992 (2014).
Lee, E. et al. GREB1 amplifies androgen receptor output in human prostate cancer and contributes to antiandrogen resistance. Elife 8, e41913 (2019).
Yuan, F. et al. Molecular determinants for enzalutamide-induced transcription in prostate cancer. Nucleic Acids Res. 47, 10104–10114 (2019).
Abida, W. et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis. Oncol. 2017, PO.17.00029 (2017).
Teyssonneau, D. et al. Prostate cancer and PARP inhibitors: progress and challenges. J. Hematol. Oncol. 14, 51 (2021).
Mateo, J. et al. A decade of clinical development of PARP inhibitors in perspective. Ann. Oncol. 30, 1437–1447 (2019).
Taylor, A. K., Kosoff, D., Emamekhoo, H., Lang, J. M. & Kyriakopoulos, C. E. PARP inhibitors in metastatic prostate cancer. Front. Oncol. 13, 1159557 (2023).
Mateo, J. et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl. J. Med. 373, 1697–1708 (2015).
Fizazi, K. et al. Rucaparib or physician’s choice in metastatic prostate cancer. N. Engl. J. Med. 388, 719–732 (2023).
Mosca, L., Ilari, A., Fazi, F., Assaraf, Y. G. & Colotti, G. Taxanes in cancer treatment: activity, chemoresistance and its overcoming. Drug Resist. Updates 54, 100742 (2021).
Agarwal, N. et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. Lancet 402, 291–303 (2023).
Chi, K. N. et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J. Clin. Oncol. 41, 3339–3351 (2023).
Hussain, M. et al. Targeting androgen receptor and DNA repair in metastatic castration-resistant prostate cancer: results from NCI 9012. J. Clin. Oncol. 36, 991–999 (2018).
Plant-Fox, A. S., O’Halloran, K. & Goldman, S. Pediatric brain tumors: the era of molecular diagnostics, targeted and immune-based therapeutics, and a focus on long term neurologic sequelae. Curr. Probl. Cancer 45, 100777 (2021).
Seed, G. et al. Elucidating acquired PARP inhibitor resistance in advanced prostate cancer. Cancer Cell 42, 2113–2123.e2114 (2024).
Cahuzac, M., Péant, B., Mes-Masson, A. M. & Saad, F. Development of olaparib-resistance prostate cancer cell lines to identify mechanisms associated with acquired resistance. Cancers 14, 3877 (2022).
Cai, M. et al. Current therapy and drug resistance in metastatic castration-resistant prostate cancer. Drug. Resist. Updat. 68, 100962 (2023).
Dawson, M. A. & Kouzarides, T. Cancer epigenetics: from mechanism to therapy. Cell 150, 12–27 (2012).
Nishiyama, A. & Nakanishi, M. Navigating the DNA methylation landscape of cancer. Trends Genet. 37, 1012–1027 (2021).
Li, L. C., Okino, S. T. & Dahiya, R. DNA methylation in prostate cancer. Biochim. Biophys. Acta 1704, 87–102 (2004).
Song, Y., Wu, F. & Wu, J. Targeting histone methylation for cancer therapy: enzymes, inhibitors, biological activity and perspectives. J. Hematol. Oncol. 9, 49 (2016).
Crea, F. et al. The emerging role of histone lysine demethylases in prostate cancer. Mol. Cancer 11, 52 (2012).
Jerónimo, C. et al. Epigenetics in prostate cancer: biologic and clinical relevance. Eur. Urol. 60, 753–766 (2011).
Kim, K. H. & Roberts, C. W. Targeting EZH2 in cancer. Nat. Med. 22, 128–134 (2016).
Li, M. et al. LSD1 inhibition disrupts super-enhancer-driven oncogenic transcriptional programs in castration-resistant prostate cancer. Cancer Res. 83, 1684–1698 (2023).
Audia, J. E. & Campbell, R. M. Histone modifications and cancer. Cold Spring Harb. Perspect. Biol. 8, a019521 (2016).
Di Cerbo, V. & Schneider, R. Cancers with wrong HATs: the impact of acetylation. Brief. Funct. Genomics 12, 231–243 (2013).
Waddell, A. R., Huang, H. & Liao, D. CBP/p300: critical co-activators for nuclear steroid hormone receptors and emerging therapeutic targets in prostate and breast cancers. Cancers 13, 2872 (2021).
Abbas, A. & Gupta, S. The role of histone deacetylases in prostate cancer. Epigenetics 3, 300–309 (2008).
Dai, X. et al. Prostate cancer-associated SPOP mutations confer resistance to BET inhibitors through stabilization of BRD4. Nat. Med. 23, 1063–1071 (2017).
Filippakopoulos, P. & Knapp, S. Targeting bromodomains: epigenetic readers of lysine acetylation. Nat. Rev. Drug Discov. 13, 337–356 (2014).
Singal, R. et al. Phase I/II study of azacitidine, docetaxel, and prednisone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel-based therapy. Clin. Genitourin. Cancer 13, 22–31 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05037500 (2025).
Zhang, L. et al. Lenalidomide improves the antitumor activity of CAR-T cells directed toward the intracellular Wilms Tumor 1 antigen. Hematology 26, 818–826 (2021).
Liu, Y. et al. Modeling lung adenocarcinoma metastases using patient-derived organoids. Cell Rep. Med. 5, 101777 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04846478 (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04388852 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03460977 (2025).
Li, C. et al. PiggyBac-generated CAR19-T cells plus lenalidomide cause durable complete remission of triple-hit refractory/relapsed DLBCL: a case report. Front. Immunol. 12, 599493 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04628988 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03568656 (2024).
Rimando, J. C. et al. Flotetuzumab and other T-cell immunotherapies upregulate MHC class II expression on acute myeloid leukemia cells. Blood 141, 1718–1723 (2023).
Lasko, L. M. et al. Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours. Nature 550, 128–132 (2017).
Rana, Z., Diermeier, S., Hanif, M. & Rosengren, R. J. Understanding failure and improving treatment using HDAC inhibitors for prostate cancer. Biomedicines 8, 22 (2020).
Schneider, B. J. et al. Phase I study of vorinostat (suberoylanilide hydroxamic acid, NSC 701852) in combination with docetaxel in patients with advanced and relapsed solid malignancies. Invest. New Drugs 30, 249–257 (2012).
Rathkopf, D. E. et al. A phase 2 study of intravenous panobinostat in patients with castration-resistant prostate cancer. Cancer Chemother. Pharmacol. 72, 537–544 (2013).
Lin, J. et al. Phase I study of entinostat in combination with enzalutamide for treatment of patients with metastatic castration-resistant prostate cancer. Oncologist 26, e2136–e2142 (2021).
Eigl, B. J. et al. A phase II study of the HDAC inhibitor SB939 in patients with castration resistant prostate cancer: NCIC clinical trials group study IND195. Invest. New Drugs 33, 969–976 (2015).
Aggarwal, R. R. et al. A phase Ib/IIa study of the pan-BET inhibitor ZEN-3694 in combination with enzalutamide in patients with metastatic castration-resistant prostate cancer. Clin. Cancer Res. 26, 5338–5347 (2020).
Cousin, S. et al. Safety, pharmacokinetic, pharmacodynamic and clinical activity of molibresib for the treatment of nuclear protein in testis carcinoma and other cancers: results of a phase I/II open-label, dose escalation study. Int. J. Cancer 150, 993–1006 (2022).
Lewin, J. et al. Phase Ib trial with birabresib, a small-molecule inhibitor of bromodomain and extraterminal proteins, in patients with selected advanced solid tumors. J. Clin. Oncol. 36, 3007–3014 (2018).
Piha-Paul, S. A. et al. First-in-human study of mivebresib (ABBV-075), an oral pan-inhibitor of bromodomain and extra terminal proteins, in patients with relapsed/refractory solid tumors. Clin. Cancer Res. 25, 6309–6319 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04556617 (2025).
Barbieri, C. E. et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat. Genet. 44, 685–689 (2012).
Evan, G. I. & Vousden, K. H. Proliferation, cell cycle and apoptosis in cancer. Nature 411, 342–348 (2001).
Kase, A. M., Copland Iii, J. A. & Tan, W. Novel therapeutic strategies for CDK4/6 inhibitors in metastatic castrate-resistant prostate cancer. Onco Targets Ther. 13, 10499–10513 (2020).
Bertoli, C., Skotheim, J. M. & de Bruin, R. A. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 14, 518–528 (2013).
Han, W. et al. Exploiting the tumor-suppressive activity of the androgen receptor by CDK4/6 inhibition in castration-resistant prostate cancer. Mol. Ther. 30, 1628–1644 (2022).
de Kouchkovsky, I. et al. A phase Ib/II study of the CDK4/6 inhibitor ribociclib in combination with docetaxel plus prednisone in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 28, 1531–1539 (2022).
Palmbos, P. L. et al. A randomized phase II study of androgen deprivation therapy with or without palbociclib in rb-positive metastatic hormone-sensitive prostate cancer. Clin. Cancer Res. 27, 3017–3027 (2021).
Finn, R. S. et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 375, 1925–1936 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04408924 (2024).
Miller, T. W., Rexer, B. N., Garrett, J. T. & Arteaga, C. L. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 13, 224 (2011).
Shorning, B. Y., Dass, M. S., Smalley, M. J. & Pearson, H. B. The PI3K-AKT-mTOR pathway and prostate cancer: at the crossroads of AR, MAPK, and WNT signaling. Int. J. Mol. Sci. 21, 4507 (2020).
Pungsrinont, T., Kallenbach, J. & Baniahmad, A. Role of PI3K-AKT-mTOR pathway as a pro-survival signaling and resistance-mediating mechanism to therapy of prostate cancer. Int. J. Mol. Sci. 22, 11088 (2021).
Murugan, A. K. mTOR: role in cancer, metastasis and drug resistance. Semin. Cancer Biol. 59, 92–111 (2019).
Toren, P. & Zoubeidi, A. Targeting the PI3K/Akt pathway in prostate cancer: challenges and opportunities (review). Int. J. Oncol. 45, 1793–1801 (2014).
Robinson, D. et al. Integrative clinical genomics of advanced prostate cancer. Cell 161, 1215–1228 (2015).
Armstrong, A. J. et al. Phase II trial of the PI3 kinase inhibitor buparlisib (BKM-120) with or without enzalutamide in men with metastatic castration resistant prostate cancer. Eur. J. Cancer 81, 228–236 (2017).
Sarker, D. et al. A phase I, open-label, dose-finding study of GSK2636771, a PI3Kβ inhibitor, administered with enzalutamide in patients with metastatic castration-resistant prostate cancer. Clin. Cancer Res. 27, 5248–5257 (2021).
Choudhury, A. D. et al. A phase I study investigating AZD8186, a potent and selective inhibitor of PI3Kβ/δ, in patients with advanced solid tumors. Clin. Cancer Res. 28, 2257–2269 (2022).
Manning, B. D. & Toker, A. AKT/PKB signaling: navigating the network. Cell 169, 381–405 (2017).
Tortorella, E., Giantulli, S., Sciarra, A. & Silvestri, I. AR and PI3K/AKT in prostate cancer: a tale of two interconnected pathways. Int. J. Mol. Sci. 24, 2046 (2023).
Raith, F., O’Donovan, D. H., Lemos, C., Politz, O. & Haendler, B. Addressing the reciprocal crosstalk between the AR and the PI3K/AKT/mTOR signaling pathways for prostate cancer treatment. Int. J. Mol. Sci. 24, 2289 (2023).
Gupta, S. et al. A phase I trial of combined ridaforolimus and MK-2206 in patients with advanced malignancies. Clin. Cancer Res. 21, 5235–5244 (2015).
Crabb, S. J. et al. Overall survival update for patients with metastatic castration-resistant prostate cancer treated with capivasertib and docetaxel in the phase 2 ProCAID clinical trial. Eur. Urol. 82, 512–515 (2022).
Sweeney, C. et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet 398, 131–142 (2021).
Jhanwar-Uniyal, M. et al. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv. Biol. Regul. 72, 51–62 (2019).
Kremer, C. L. et al. Expression of mTOR signaling pathway markers in prostate cancer progression. Prostate 66, 1203–1212 (2006).
Templeton, A. J. et al. Phase 2 trial of single-agent everolimus in chemotherapy-naive patients with castration-resistant prostate cancer (SAKK 08/08). Eur. Urol. 64, 150–158 (2013).
Chow, H. et al. A phase 2 clinical trial of everolimus plus bicalutamide for castration-resistant prostate cancer. Cancer 122, 1897–1904 (2016).
George, D. J. et al. Phase 2 clinical trial of TORC1 inhibition with everolimus in men with metastatic castration-resistant prostate cancer. Urol. Oncol. 38, 79.e15–79.e22 (2020).
Armstrong, A. J. et al. A phase II trial of temsirolimus in men with castration-resistant metastatic prostate cancer. Clin. Genitourin. Cancer 11, 397–406 (2013).
Barata, P. C. et al. Phase I/II study evaluating the safety and clinical efficacy of temsirolimus and bevacizumab in patients with chemotherapy refractory metastatic castration-resistant prostate cancer. Invest. N. Drugs 37, 331–337 (2019).
Graham, L. et al. A phase II study of the dual mTOR inhibitor MLN0128 in patients with metastatic castration resistant prostate cancer. Invest. N. Drugs 36, 458–467 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02064608 (2019).
Sweeney, C. J. et al. Phase Ib/II study of enzalutamide with samotolisib (LY3023414) or placebo in patients with metastatic castration-resistant prostate cancer. Clin. Cancer Res. 28, 2237–2247 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01717898 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01485861 (2023).
Liu, J. et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal. Transduct. Target Ther. 7, 13 (2022).
Kypta, R. M. & Waxman, J. Wnt/β-catenin signalling in prostate cancer. Nat. Rev. Urol. 9, 418–428 (2012).
Anastas, J. N. & Moon, R. T. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 13, 11–26 (2013).
Horvath, L. G. et al. Secreted frizzled-related protein 4 inhibits proliferation and metastatic potential in prostate cancer. Prostate 67, 1081–1090 (2007).
Zhang, Z. et al. Inhibition of the Wnt/β-catenin pathway overcomes resistance to enzalutamide in castration-resistant prostate cancer. Cancer Res. 78, 3147–3162 (2018).
Patel, R. et al. Activation of β-catenin cooperates with loss of Pten to drive AR-independent castration-resistant prostate cancer. Cancer Res. 80, 576–590 (2020).
Koushyar, S., Grant, G. H. & Uysal-Onganer, P. The interaction of Wnt-11 and signalling cascades in prostate cancer. Tumour Biol. 37, 13049–13057 (2016).
Sha, J. et al. PRKAR2B promotes prostate cancer metastasis by activating Wnt/β-catenin and inducing epithelial-mesenchymal transition. J. Cell Biochem. 119, 7319–7327 (2018).
Fang, Q. et al. β-ionone inhibits epithelial-mesenchymal transition (EMT) in prostate cancer cells by negatively regulating the Wnt/β-catenin pathway. Front. Biosci. 27, 335 (2022).
Kaplan, Z., Zielske, S. P., Ibrahim, K. G. & Cackowski, F. C. Wnt and β-catenin signaling in the bone metastasis of prostate cancer. Life 11, 1099 (2021).
Kelsey, R. Foxy–5 in prostate cancer model. Nat. Rev. Urol. 14, 638–638 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02020291 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02655952 (2018).
Wozney, J. L. & Antonarakis, E. S. Growth factor and signaling pathways and their relevance to prostate cancer therapeutics. Cancer Metastasis Rev. 33, 581–594 (2014).
George, D. J. Receptor tyrosine kinases as rational targets for prostate cancer treatment: platelet-derived growth factor receptor and imatinib mesylate. Urology 60, 115–121 (2002).
Varkaris, A., Katsiampoura, A. D., Araujo, J. C., Gallick, G. E. & Corn, P. G. Src signaling pathways in prostate cancer. Cancer Metastasis Rev. 33, 595–606 (2014).
Mahajan, K. & Mahajan, N. P. Shepherding AKT and androgen receptor by Ack1 tyrosine kinase. J. Cell Physiol. 224, 327–333 (2010).
Gelman, I. H. Androgen receptor activation in castration-recurrent prostate cancer: the role of Src-family and Ack1 tyrosine kinases. Int. J. Biol. Sci. 10, 620–626 (2014).
Smith, M. et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J. Clin. Oncol. 34, 3005–3013 (2016).
Liow, E. et al. Phase 2 study of neoadjuvant FGFR inhibition and androgen deprivation therapy prior to prostatectomy. Clin. Genitourin. Cancer 20, 452–458 (2022).
Cathomas, R. et al. Efficacy of cetuximab in metastatic castration-resistant prostate cancer might depend on EGFR and PTEN expression: results from a phase II trial (SAKK 08/07). Clin. Cancer Res. 18, 6049–6057 (2012).
Choi, Y. J. et al. Phase II study of dovitinib in patients with castration-resistant prostate cancer (KCSG-GU11-05). Cancer Res. Treat. 50, 1252–1259 (2018).
McHugh, D. J. et al. A phase I trial of IGF-1R inhibitor cixutumumab and mTOR inhibitor temsirolimus in metastatic castration-resistant prostate cancer. Clin. Genitourin. Cancer 18, 171–178.e172 (2020).
Sun, D. Y., Wu, J. Q., He, Z. H., He, M. F. & Sun, H. B. Cancer-associated fibroblast regulate proliferation and migration of prostate cancer cells through TGF-β signaling pathway. Life Sci. 235, 116791 (2019).
Jiao, C. et al. TGF-β signaling regulates SPOP expression and promotes prostate cancer cell stemness. Aging 12, 7747–7760 (2020).
Fournier, P. G. et al. The TGF-β signaling regulator PMEPA1 suppresses prostate cancer metastases to bone. Cancer Cell 27, 809–821 (2015).
Akhurst, R. J. & Hata, A. Targeting the TGFβ signalling pathway in disease. Nat. Rev. Drug. Discov. 11, 790–811 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02452008 (2024).
Kong, D. et al. Procoxacin bidirectionally inhibits osteoblastic and osteoclastic activity in bone and suppresses bone metastasis of prostate cancer. J. Exp. Clin. Cancer Res. 42, 45 (2023).
Chen, C. et al. Metformin exerts anti-AR-negative prostate cancer activity via AMPK/autophagy signaling pathway. Cancer Cell Int. 21, 404 (2021).
Clements, A. et al. Metformin in prostate cancer: two for the price of one. Ann. Oncol. 22, 2556–2560 (2011).
Rothermundt, C. et al. Metformin in chemotherapy-naive castration-resistant prostate cancer: a multicenter phase 2 trial (SAKK 08/09). Eur. Urol. 66, 468–474 (2014).
Wilson, B. E. et al. Effects of metformin and statins on outcomes in men with castration-resistant metastatic prostate cancer: secondary analysis of COU-AA-301 and COU-AA-302. Eur. J. Cancer 170, 296–304 (2022).
Xu, H. et al. ADP-dependent glucokinase controls metabolic fitness in prostate cancer progression. Mil. Med. Res. 10, 64 (2023).
Pan, H., Zhu, Y., Wei, W., Shao, S. & Rui, X. Transcription factor FoxM1 is the downstream target of c-Myc and contributes to the development of prostate cancer. World J. Surg. Oncol. 16, 59 (2018).
Lin, J. Z. et al. FOXM1 contributes to docetaxel resistance in castration-resistant prostate cancer by inducing AMPK/mTOR-mediated autophagy. Cancer Lett. 469, 481–489 (2020).
You, S. et al. Integrated classification of prostate cancer reveals a novel luminal subtype with poor outcome. Cancer Res. 76, 4948–4958 (2016).
Xu, H. et al. SR9009 inhibits lethal prostate cancer subtype 1 by regulating the LXRα/FOXM1 pathway independently of REV-ERBs. Cell Death Dis. 13, 949 (2022).
Gómez-Navarro, J., Curiel, D. T. & Douglas, J. T. Gene therapy for cancer. Eur. J. Cancer 35, 2039–2057 (1999).
Gutierrez, A. A., Lemoine, N. R. & Sikora, K. Gene therapy for cancer. Lancet 339, 715–721 (1992).
Wadhwa, P. D. et al. Cancer gene therapy: scientific basis. Annu. Rev. Med. 53, 437–452 (2002).
Nasu, Y. et al. Suicide gene therapy with adenoviral delivery of HSV-tK gene for patients with local recurrence of prostate cancer after hormonal therapy. Mol. Ther. 15, 834–840 (2007).
Cai, Z. et al. Targeting strategies of adenovirus-mediated gene therapy and virotherapy for prostate cancer (Review). Mol. Med. Rep. 16, 6443–6458 (2017).
Altwaijry, N., Somani, S. & Dufès, C. Targeted nonviral gene therapy in prostate cancer. Int. J. Nanomed. 13, 5753–5767 (2018).
Thomas, C. E., Ehrhardt, A. & Kay, M. A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 4, 346–358 (2003).
Santana-Armas, M. L. & Tros de Ilarduya, C. Strategies for cancer gene-delivery improvement by non-viral vectors. Int. J. Pharm. 596, 120291 (2021).
Joyce, J. A. & Fearon, D. T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80 (2015).
Liu, D., Wang, L. & Guo, Y. Advances in and prospects of immunotherapy for prostate cancer. Cancer Lett. 601, 217155 (2024).
Harrington, K. J., Spitzweg, C., Bateman, A. R., Morris, J. C. & Vile, R. G. Gene therapy for prostate cancer: current status and future prospects. J. Urol. 166, 1220–1233 (2001).
Brill, T. H. et al. Allogeneic retrovirally transduced, IL-2- and IFN-γ-secreting cancer cell vaccine in patients with hormone refractory prostate cancer — a phase I clinical trial. J. Gene Med. 9, 547–560 (2007).
Agha-Mohammadi, S. & Lotze, M. T. Immunomodulation of cancer: potential use of selectively replicating agents. J. Clin. Invest. 105, 1173–1176 (2000).
Gregg, J. R. & Thompson, T. C. Considering the potential for gene-based therapy in prostate cancer. Nat. Rev. Urol. 18, 170–184 (2021).
Belldegrun, A. et al. Interleukin 2 gene therapy for prostate cancer: phase I clinical trial and basic biology. Hum. Gene Ther. 12, 883–892 (2001).
Hull, G. W. et al. Prostate cancer gene therapy: comparison of adenovirus-mediated expression of interleukin 12 with interleukin 12 plus B7-1 for in situ gene therapy and gene-modified, cell-based vaccines. Clin. Cancer Res. 6, 4101–4109 (2000).
Simons, J. W. & Sacks, N. Granulocyte-macrophage colony-stimulating factor−transduced allogeneic cancer cellular immunotherapy: the GVAX® vaccine for prostate cancer. Urol. Oncol.: Semin. Original Invest. 24, 419–424 (2006).
Higano, C. S. et al. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer 113, 975–984 (2008).
Vuky, J. et al. Phase II trial of neoadjuvant docetaxel and CG1940/CG8711 followed by radical prostatectomy in patients with high-risk clinically localized prostate cancer. Oncologist 18, 687–688 (2013).
Wang, G., Zhao, D., Spring, D. J. & DePinho, R. A. Genetics and biology of prostate cancer. Genes. Dev. 32, 1105–1140 (2018).
Clayman, G. L. et al. Adenovirus-mediated p53 gene transfer in patients with advanced recurrent head and neck squamous cell carcinoma. J. Clin. Oncol. 16, 2221–2232 (1998).
Swisher, S. G. et al. Adenovirus-mediated p53 gene transfer in advanced non-small-cell lung cancer. J. Natl Cancer Inst. 91, 763–771 (1999).
Schuler, M. et al. Adenovirus-mediated wild-type p53 gene transfer in patients receiving chemotherapy for advanced non-small-cell lung cancer: results of a multicenter phase II study. J. Clin. Oncol. 19, 1750–1758 (2001).
Wolf, J. K. et al. A phase I study of Adp53 (INGN 201; ADVEXIN) for patients with platinum- and paclitaxel-resistant epithelial ovarian cancer. Gynecol. Oncol. 94, 442–448 (2004).
Guan, Y. S. et al. p53 gene therapy in combination with transcatheter arterial chemoembolization for HCC: one-year follow-up. World J. Gastroenterol. 17, 2143–2149 (2011).
Tamura, R. E., Lana, M. G., Costanzi-Strauss, E. & Strauss, B. E. Combination of cabazitaxel and p53 gene therapy abolishes prostate carcinoma tumor growth. Gene Ther. 27, 15–26 (2020).
Kunimura, N. et al. Combination of rAd-p53 in situ gene therapy and anti-PD-1 antibody immunotherapy induced anti-tumor activity in mouse syngeneic urogenital cancer models. Sci. Rep. 10, 17464 (2020).
Ai, J. et al. rAAV-delivered PTEN therapeutics for prostate cancer. Mol. Ther. Nucleic Acids 27, 122–132 (2022).
Deng, J. H., Deng, Q., Kuo, C. H., Delaney, S. W. & Ying, S. Y. MiRNA targets of prostate cancer. Methods Mol. Biol. 936, 357–369 (2013).
Vo, J. N. et al. The landscape of circular RNA in cancer. Cell 176, 869–881.e813 (2019).
Qiu, K., Zheng, Z. & Huang, Y. Long intergenic noncoding RNA 00844 promotes apoptosis and represses proliferation of prostate cancer cells through upregulating GSTP1 by recruiting EBF1. J. Cell Physiol. 235, 8472–8485 (2020).
Chalanqui, M. J., O’Doherty, M., Dunne, N. J. & McCarthy, H. O. MiRNA 34a: a therapeutic target for castration-resistant prostate cancer. Expert. Opin. Ther. Targets 20, 1075–1085 (2016).
Ai, J. et al. rAAV-based and intraprostatically delivered miR-34a therapeutics for efficient inhibition of prostate cancer progression. Gene Ther. 29, 418–424 (2022).
Huarte, M. et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142, 409–419 (2010).
Wang, X. et al. Long intragenic non-coding RNA lincRNA-p21 suppresses development of human prostate cancer. Cell Prolif. 50, e12318 (2017).
Attard, G. et al. Prostate cancer. Lancet 387, 70–82 (2016).
Xue, J., Chen, K., Hu, H. & Gopinath, S. C. B. Progress in gene therapy treatments for prostate cancer. Biotechnol. Appl. Biochem. 69, 1166–1175 (2022).
Pope, I. M., Poston, G. J. & Kinsella, A. R. The role of the bystander effect in suicide gene therapy. Eur. J. Cancer 33, 1005–1016 (1997).
Duarte, S., Carle, G., Faneca, H., Lima, M. C. P. D. & Pierrefite-Carle, V. Suicide gene therapy in cancer: where do we stand now? Cancer Lett. 324, 160–170 (2012).
Nyati, S. et al. A phase I clinical trial of oncolytic adenovirus mediated suicide and interleukin-12 gene therapy in patients with recurrent localized prostate adenocarcinoma. PLoS ONE 18, e0291315 (2023).
Herman, J. R. et al. In situ gene therapy for adenocarcinoma of the prostate: a phase I clinical trial. Hum. Gene Ther. 10, 1239–1249 (1999).
Yang, K., Feng, S. & Luo, Z. Oncolytic adenovirus, a new treatment strategy for prostate cancer. Biomedicines 10, 3262 (2022).
Wang, G., Liu, Y., Liu, S., Lin, Y. & Hu, C. Oncolyic virotherapy for prostate cancer: lighting a fire in winter. Int. J. Mol. Sci. 23, 12647 (2022).
Sweeney, K. & Halldén, G. Oncolytic adenovirus-mediated therapy for prostate cancer. Oncolytic Virother 5, 45–57 (2016).
Small, E. J. et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol. Ther. 14, 107–117 (2006).
Ryan, P. C. et al. Antitumor efficacy and tumor-selective replication with a single intravenous injection of OAS403, an oncolytic adenovirus dependent on two prevalent alterations in human cancer. Cancer Gene Ther. 11, 555–569 (2004).
Freytag, S. O. et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Res. 62, 4968–4976 (2002).
Barton, K. N. et al. Second-generation replication-competent oncolytic adenovirus armed with improved suicide genes and ADP gene demonstrates greater efficacy without increased toxicity. Mol. Ther. 13, 347–356 (2006).
Oyama, M. et al. Oncolytic viral therapy for human prostate cancer by conditionally replicating herpes simplex virus 1 vector G207. Jpn. J. Cancer Res. 91, 1339–1344 (2000).
Walker, J. R. et al. Local and systemic therapy of human prostate adenocarcinoma with the conditionally replicating herpes simplex virus vector G207. Hum. Gene Ther. 10, 2237–2243 (1999).
van de Merbel, A. F. et al. Reovirus mutant jin-3 exhibits lytic and immune-stimulatory effects in preclinical human prostate cancer models. Cancer Gene Ther. 29, 793–802 (2022).
Mao, L. J. et al. Oncolytic adenovirus harboring interleukin-24 improves chemotherapy for advanced prostate cancer. J. Cancer 9, 4391–4397 (2018).
Mellman, I., Chen, D. S., Powles, T. & Turley, S. J. The cancer-immunity cycle: indication, genotype, and immunotype. Immunity 56, 2188–2205 (2023).
Claps, M. et al. Immune-checkpoint inhibitors and metastatic prostate cancer therapy: learning by making mistakes. Cancer Treat. Rev. 88, 102057 (2020).
Schepisi, G. et al. CAR-T cell therapy: a potential new strategy against prostate cancer. J. Immunother. Cancer 7, 258 (2019).
Jiang, Y. et al. Prospect of prostate cancer treatment: armed CAR-T or combination therapy. Cancers 14, 967 (2022).
Armstrong, A. J. et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J. Clin. Oncol. 40, 1616–1622 (2022).
Buck, S. A. J., Koolen, S. L. W., Mathijssen, R. H. J., de Wit, R. & van Soest, R. J. Cross-resistance and drug sequence in prostate cancer. Drug. Resist. Updates 56, 100761 (2021).
Sun, X. et al. PANoptosis: mechanisms, biology, and role in disease. Immunol. Rev. 321, 246–262 (2024).
Ocansey, D. K. W. et al. Current evidence and therapeutic implication of PANoptosis in cancer. Theranostics 14, 640–661 (2024).
Yi, X. et al. Construction of PANoptosis signature: novel target discovery for prostate cancer immunotherapy. Mol. Ther. Nucleic Acids 33, 376–390 (2023).
Peyraud, F. & Italiano, A. Combined PARP inhibition and immune checkpoint therapy in solid tumors. Cancers 12, 1502 (2020).
Mao, W. et al. Immunogenicity of prostate cancer is augmented by BET bromodomain inhibition. J. Immunother. Cancer 7, 277 (2019).
Madan, R. A. et al. Clinical and immunologic impact of short-course enzalutamide alone and with immunotherapy in non-metastatic castration sensitive prostate cancer. J. Immunother. Cancer 9, e001556 (2021).
Tan, H. Y. et al. Targeting tumour microenvironment by tyrosine kinase inhibitor. Mol. Cancer 17, 43 (2018).
Chabanon, R. M. et al. PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J. Clin. Invest. 129, 1211–1228 (2019).
Yang, F. et al. Non-coding RNAs: emerging roles in the characterization of immune microenvironment and immunotherapy of prostate cancer. Biochem. Pharmacol. 214, 115669 (2023).
Dos Reis, F. D., Jerónimo, C. & Correia, M. P. Epigenetic modulation and prostate cancer: paving the way for NK cell anti-tumor immunity. Front. Immunol. 14, 1152572 (2023).
Kwon, E. D. et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 15, 700–712 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT06236139 (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01487863 (2019).
Bishop, J. L. et al. PD-L1 is highly expressed in enzalutamide resistant prostate cancer. Oncotarget 6, 234–242 (2015).
Guan, X. et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature 606, 791–796 (2022).
Pala, L., De Pas, T. & Conforti, F. Boosting anticancer immunotherapy through androgen receptor blockade. Cancer Cell 40, 455–457 (2022).
Xu, P. et al. Androgen receptor blockade resistance with enzalutamide in prostate cancer results in immunosuppressive alterations in the tumor immune microenvironment. J. Immunother. Cancer 11, e006581 (2023).
Karzai, F. et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J. Immunother. Cancer 6, 141 (2018).
Antonarakis, E. S. et al. Pembrolizumab plus olaparib for patients with previously treated and biomarker-unselected metastatic castration-resistant prostate cancer: the randomized, open-label, phase III KEYLYNK-010 trial. J. Clin. Oncol. 41, 3839–3850 (2023).
Ghoneim, H. E. et al. De novo epigenetic programs inhibit PD-1 blockade-mediated T cell rejuvenation. Cell 170, 142–157.e119 (2017).
Kang, N. et al. EZH2 inhibition: a promising strategy to prevent cancer immune editing. Epigenomics 12, 1457–1476 (2020).
Murphy, S. et al. Overcome prostate cancer resistance to immune checkpoint therapy with ketogenic diet-induced epigenetic reprogramming. Preprint at bioRxiv https://doi.org/10.1101/2023.08.07.552383 (2023).
Yamada, Y. et al. Targeting DNA methylation and B7-H3 in RB1-deficient and neuroendocrine prostate cancer. Sci. Transl. Med. 15, eadf6732 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02998567 (2024).
Park, S. H. et al. Going beyond polycomb: EZH2 functions in prostate cancer. Oncogene 40, 5788–5798 (2021).
Gameiro, S. R., Malamas, A. S., Tsang, K. Y., Ferrone, S. & Hodge, J. W. Inhibitors of histone deacetylase 1 reverse the immune evasion phenotype to enhance T-cell mediated lysis of prostate and breast carcinoma cells. Oncotarget 7, 7390–7402 (2016).
Liu, J. et al. p300/CBP inhibition enhances the efficacy of programmed death-ligand 1 blockade treatment in prostate cancer. Oncogene 39, 3939–3951 (2020).
Su, W. et al. The polycomb repressor complex 1 drives double-negative prostate cancer metastasis by coordinating stemness and immune suppression. Cancer Cell 36, 139–155.e10 (2019).
Want, M. Y. et al. WHSC1/NSD2 regulates immune infiltration in prostate cancer. J. Immunother. Cancer 9, e001374 (2021).
Jain, N. et al. Disruption of SUV39H1-mediated H3K9 methylation sustains CAR T-cell function. Cancer Discov. 14, 142–157 (2024).
Gao, X., Leone, G. W. & Wang, H. Cyclin D-CDK4/6 functions in cancer. Adv. Cancer Res. 148, 147–169 (2020).
Liu, C. et al. The immunological role of CDK4/6 and potential mechanism exploration in ovarian cancer. Front. Immunol. 12, 799171 (2021).
Jin, X. et al. Phosphorylated RB promotes cancer immunity by inhibiting NF-κB activation and PD-L1 expression. Mol. Cell 73, 22–35.e26 (2019).
Deng, J. et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 8, 216–233 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04751929 (2025).
Dart, D. A., Uysal-Onganer, P. & Jiang, W. G. Prostate-specific PTen deletion in mice activates inflammatory microRNA expression pathways in the epithelium early in hyperplasia development. Oncogenesis 6, 400 (2017).
Crane, C. A. et al. PI(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene 28, 306–312 (2009).
Chaudagar, K. et al. Suppression of tumor cell lactate-generating signaling pathways eradicates murine PTEN/p53-deficient aggressive-variant prostate cancer via macrophage phagocytosis. Clin. Cancer Res. 29, 4930–4940 (2023).
Chaudagar, K. et al. Reversal of lactate and PD-1-mediated macrophage immunosuppression controls growth of PTEN/p53-deficient prostate cancer. Clin. Cancer Res. 29, 1952–1968 (2023).
Lu, X. et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 543, 728–732 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03673787 (2022).
Suryawanshi, A., Tadagavadi, R. K., Swafford, D. & Manicassamy, S. Modulation of inflammatory responses by Wnt/β-catenin signaling in dendritic cells: a novel immunotherapy target for autoimmunity and cancer. Front. Immunol. 7, 460 (2016).
Stakheev, D. et al. The WNT/β-catenin signaling inhibitor XAV939 enhances the elimination of LNCaP and PC-3 prostate cancer cells by prostate cancer patient lymphocytes in vitro. Sci. Rep. 9, 4761 (2019).
Agarwal, N. et al. Cabozantinib in combination with atezolizumab in patients with metastatic castration-resistant prostate cancer: results from an expansion cohort of a multicentre, open-label, phase 1b trial (COSMIC-021). Lancet Oncol. 23, 899–909 (2022).
Agarwal, N. et al. A phase III, randomized, open-label study (CONTACT-02) of cabozantinib plus atezolizumab versus second novel hormone therapy in patients with metastatic castration-resistant prostate cancer. Future Oncol. 18, 1185–1198 (2022).
Qiao, Y. et al. Autophagy inhibition by targeting PIKfyve potentiates response to immune checkpoint blockade in prostate cancer. Nat. Cancer 2, 978–993 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04159896 (2024).
Deng, G. et al. Ibrutinib inhibits BTK signaling in tumor-infiltrated B cells and amplifies antitumor immunity by PD-1 checkpoint blockade for metastatic prostate cancer. Cancers 15, 2356 (2023).
Mauri, C. & Bosma, A. Immune regulatory function of B cells. Annu. Rev. Immunol. 30, 221–241 (2012).
Stiff, A. et al. Myeloid-derived suppressor cells express Bruton’s tyrosine kinase and can be depleted in tumor-bearing hosts by ibrutinib treatment. Cancer Res. 76, 2125–2136 (2016).
Sridaran, D. et al. Inhibiting ACK1-mediated phosphorylation of C-terminal Src kinase counteracts prostate cancer immune checkpoint blockade resistance. Nat. Commun. 13, 6929 (2022).
Guan, W. et al. Inhibition of TAMs improves the response to docetaxel in castration-resistant prostate cancer. Endocr. Relat. Cancer 26, 131–140 (2019).
Miller, A. M., Ozenci, V., Kiessling, R. & Pisa, P. Immune monitoring in a phase 1 trial of a PSA DNA vaccine in patients with hormone-refractory prostate cancer. J. Immunother. 28, 389–395 (2005).
Pavlenko, M. et al. A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br. J. Cancer 91, 688–694 (2004).
Junghans, R. P. et al. Phase I trial of anti-PSMA designer CAR-T cells in prostate cancer: possible role for interacting interleukin 2-T cell pharmacodynamics as a determinant of clinical response. Prostate 76, 1257–1270 (2016).
Yanagisawa, N. et al. Cytopathic effects and local immune responses in repeated neoadjuvant HSV-tk + ganciclovir gene therapy for prostate cancer. Asian J. Urol. 8, 280–288 (2021).
Zafar, S. et al. Oncolytic adenovirus type 3 coding for CD40L facilitates dendritic cell therapy of prostate cancer in humanized mice and patient samples. Hum. Gene Ther. 32, 192–202 (2021).
Li, X. et al. CXCL10-armed oncolytic adenovirus promotes tumor-infiltrating T-cell chemotaxis to enhance anti-PD-1 therapy. Oncoimmunology 11, 2118210 (2022).
Ju, F. et al. Oncolytic virus expressing PD-1 inhibitors activates a collaborative intratumoral immune response to control tumor and synergizes with CTLA-4 or TIM-3 blockade. J. Immunother. Cancer 10, e004762 (2022).
Fang, L. et al. Recombinant oncolytic adenovirus armed with CCL5, IL-12, and IFN-γ promotes CAR-T infiltration and proliferation in vivo to eradicate local and distal tumors. Cell Death Discov. 9, 328 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03514836 (2021).
Shoushtari, A. N. et al. Pilot study of ONCOS-102 and pembrolizumab: remodeling of the tumor microenvironment and clinical outcomes in anti-PD-1-resistant advanced melanoma. Clin. Cancer Res. 29, 100–109 (2023).
Acknowledgements
This study was supported by the National Key R&D Program of China (2023YFC3403200), the National Natural Science Foundation of China (82472774 and 81702536). We thank all the colleagues from the Department of Urology, Institute of Urology, West China Hospital, Sichuan University, for supporting our work.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Susan Slovin, Zoran Culig and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Han, Z. & Ai, J. Synergistic targeting strategies for prostate cancer. Nat Rev Urol 22, 645–671 (2025). https://doi.org/10.1038/s41585-025-01042-6
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41585-025-01042-6