Abstract
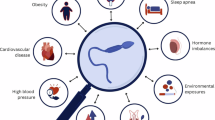
Declining fertility, overlooked mental health, and reduced life expectancy underscore the urgent need for renewed attention to men’s health. A semen analysis, traditionally used to assess fertility, holds untapped potential as a tool for promoting lifestyle changes and preventing chronic diseases in men. Spermatogenesis is highly sensitive to environmental and lifestyle factors and can be an early indicator of overall health. Disruptions in this process can signal underlying systemic issues and predict long-term health risks, including cardiovascular disease and metabolic disorders. An increasing number of men seek to engage in preconception care, as fertility is closely tied to a man’s sense of masculinity, identity and aspirations for fatherhood. In this context, a semen analysis can be a powerful motivator to encourage healthy behaviours and proactive health management. By incorporating semen analysis into primary care, health care providers can leverage men’s desire for fatherhood as an entry point to discuss broader health concerns, such as mental well-being, nutrition and physical activity. This approach would address immediate reproductive health, and also promote long-term wellness, helping to reduce the burden of chronic disease in men.
Key points
-
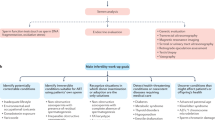
Semen analysis is a foundational but limited tool in assessing male fertility, which provides general insights into reproductive health through evaluation of sperm parameters (such as concentration, motility, morphology), but cannot definitively predict fertility.
-
Male fertility is highly influenced by environmental, lifestyle and medical factors, with evidence showing that both short-term and long-term interventions — such as nutritional supplementation and lifestyle changes — can lead to substantial improvements in semen quality and fertility potential.
-
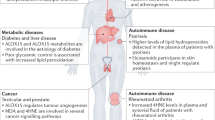
Semen analysis serves as a valuable indicator of overall male health, with abnormal results linked to increased risks of chronic disease, mortality and cancer.
-
Masculinity and fertility are closely connected, and leveraging this relationship through sensitive communication can motivate men to adopt healthier behaviours.
-
Semen analysis has the potential to be a proactive tool for assessing both fertility and general health in men, but improved health care communication, male-specific resources and primary care engagement are essential to fully realize these benefits.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Agarwal, A. et al. Male infertility. Lancet 397, 319–333 (2021).
Sharlip, I. D. et al. Best practice policies for male infertility. Fertil. Steril. 77, 873–882 (2002).
Fisher, J. R. & Hammarberg, K. Psychological and social aspects of infertility in men: an overview of the evidence and implications for psychologically informed clinical care and future research. Asian J. Androl. 14, 121–129 (2012).
Hammarberg, K., Collins, V., Holden, C., Young, K. & McLachlan, R. Men’s knowledge, attitudes and behaviours relating to fertility. Hum. Reprod. Update 23, 458–480 (2017).
Evens E. M. A global perspective on infertility an under recognized public health issue. Carolina Papers International Health No. 18 https://www.scribd.com/document/125595459/A-Global-Perspective-on-Infertility-an-Under-Recognized-Public-Health-Issue-original (2004).
Kimmins, S. et al. Frequency, morbidity and equity - the case for increased research on male fertility. Nat. Rev. Urol. 21, 102–124 (2024).
Levine, H. et al. Temporal trends in sperm count: a systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum. Reprod. Update 29, 157–176 (2023).
Levine, H. et al. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum. Reprod. Update 23, 646–659 (2017).
Cedars, M. I. et al. The sixth vital sign: what reproduction tells us about overall health. Proceedings from a NICHD/CDC workshop. Hum. Reprod. Open. 2017, hox008 (2017).
Choy, J. T. & Eisenberg, M. L. Male infertility as a window to health. Fertil. Steril. 110, 810–814 (2018).
Inhorn, M. C. & Patrizio, P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 21, 411–426 (2015).
Lee, T. Y. & Chu, T. Y. The Chinese experience of male infertility. West. J. Nurs. Res. 23, 714–725 (2001).
Obst, K. L., Oxlad, M., Turnbull, D. & McPherson, N. O. “No one asked me if I’m alright”: a mixed-methods study exploring information/support needs and challenges engaging men diagnosed with male-factor infertility. Am. J. Mens Health 17, 15579883231209210 (2023).
Sejbaek, C. S. et al. Depression among men in ART treatment: a register-based national cohort study. Hum. Reprod. Open. 2020, hoaa019 (2020).
Fisher, J. R., Baker, G. H. & Hammarberg, K. Long-term health, well-being, life satisfaction, and attitudes toward parenthood in men diagnosed as infertile: challenges to gender stereotypes and implications for practice. Fertil. Steril. 94, 574–580 (2010).
Greil, A. L., Slauson-Blevins, K. & McQuillan, J. The experience of infertility: a review of recent literature. Sociol. Health Illn. 32, 140–162 (2010).
Institute for Health Metrics and Evaluation. Global burden of disease (GBD): institute for health metrics and evaluation. Institute for Health Metrics and Evaluation https://www.healthdata.org/research-analysis/gbd-data?trk=public_post-text (2021).
Latif, T. et al. Semen quality as a predictor of subsequent morbidity: a Danish cohort study of 4,712 men with long-term follow-up. Am. J. Epidemiol. 186, 910–917 (2017).
Inhorn, M. C. Global infertility and the globalization of new reproductive technologies: illustrations from Egypt. Soc. Sci. Med. 56, 1837–1851 (2003).
Dyer, S. J., Abrahams, N., Mokoena, N. E. & van der Spuy, Z. M. ‘You are a man because you have children’: experiences, reproductive health knowledge and treatment-seeking behaviour among men suffering from couple infertility in South Africa. Hum. Reprod. 19, 960–967 (2004).
Björndahl, L. & Kirkman Brown, J. The sixth edition of the WHO laboratory manual for the examination and processing of human semen: ensuring quality and standardization in basic examination of human ejaculates. Fertil. Steril. 117, 246–251 (2022).
Wang, C. & Swerdloff, R. S. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil. Steril. 102, 1502–1507 (2014).
Pasqualotto, F. F. et al. High percentage of abnormal semen parameters in a prevasectomy population. Fertil. Steril. 85, 954–960 (2006).
Bonde, J. P. et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet 352, 1172–1177 (1998).
Guzick, D. S. et al. Sperm morphology, motility, and concentration in fertile and infertile men. N. Engl. J. Med. 345, 1388–1393 (2001).
Esteves, S. C. Evolution of the World Health Organization semen analysis manual: where are we? Nat. Rev. Urol. 19, 439–446 (2022).
Zavos, P. M. & Goodpasture, J. C. Clinical improvements of specific seminal deficiencies via intercourse with a seminal collection device versus masturbation. Fertil. Steril. 51, 190–193 (1989).
Pound, N., Javed, M. H., Ruberto, C., Shaikh, M. A. & Del Valle, A. P. Duration of sexual arousal predicts semen parameters for masturbatory ejaculates. Physiol. Behav. 76, 685–689 (2002).
World Health Organization. WHO laboratory manual for the examination and processing of human semen 5th edn (World Health Organization, 2010).
Slama, R. et al. Time to pregnancy and semen parameters: a cross-sectional study among fertile couples from four European cities. Hum. Reprod. 17, 503–515 (2002).
Ayala, C., Steinberger, E. & Smith, D. P. The influence of semen analysis parameters on the fertility potential of infertile couples. J. Androl. 17, 718–725 (1996).
Zinaman, M. J., Brown, C. C., Selevan, S. G. & Clegg, E. D. Semen quality and human fertility: a prospective study with healthy couples. J. Androl. 21, 145–153 (2000).
Keihani, S. et al. Semen parameter thresholds and time-to-conception in subfertile couples: how high is high enough? Hum. Reprod. 36, 2121–2133 (2021).
Robinson, L. et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum. Reprod. 27, 2908–2917 (2012).
Cissen, M. et al. Measuring sperm DNA fragmentation and clinical outcomes of medically assisted reproduction: a systematic review and meta-analysis. PLoS ONE 11, e0165125 (2016).
Simon, L., Zini, A., Dyachenko, A., Ciampi, A. & Carrell, D. T. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J. Androl. 19, 80–90 (2017).
Tan, J., Taskin, O., Albert, A. & Bedaiwy, M. A. Association between sperm DNA fragmentation and idiopathic recurrent pregnancy loss: a systematic review and meta-analysis. Reprod. Biomed. Online 38, 951–960 (2019).
Agarwal, A. et al. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl. Androl. Urol. 5, 935–950 (2016).
Andrabi, S. W. et al. Fragmentation: causes, evaluation and management in male infertility. JBRA Assist. Reprod. 28, 306–319 (2024).
Newman, H., Catt, S., Vining, B., Vollenhoven, B. & Horta, F. DNA repair and response to sperm DNA damage in oocytes and embryos, and the potential consequences in ART: a systematic review. Mol. Hum. Reprod. 28, gaab071 (2022).
Peel, A., Saini, A., Deluao, J. C. & McPherson, N. O. Sperm DNA damage: the possible link between obesity and male infertility, an update of the current literature. Andrology 11, 1635–1652 (2023).
Aitken, R. J. Impact of oxidative stress on male and female germ cells: implications for fertility. Reproduction 159, R189–R201 (2020).
Jones, R., Mann, T. & Sherins, R. Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil. Steril. 31, 531–537 (1979).
Alvarez, J. G., Touchstone, J. C., Blasco, L. & Storey, B. T. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J. Androl. 8, 338–348 (1987).
Aitken, R. J. & Clarkson, J. S. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J. Reprod. Fertil. 81, 459–469 (1987).
Bisht, S. & Dada, R. Oxidative stress: major executioner in disease pathology, role in sperm DNA damage and preventive strategies. Front. Biosci. 9, 420–447 (2017).
O’Flaherty, C. & Matsushita-Fournier, D. Reactive oxygen species and protein modifications in spermatozoa. Biol. Reprod. 97, 577–585 (2017).
Castleton, P. et al. MiOXSYS® and OxiSperm® II assays appear to provide no clinical utility for determining oxidative stress in human sperm-results from repeated semen collections. Andrology 11, 1566–1578 (2023).
Heller, C. G. & Clermont, Y. Spermatogenesis in man: an estimate of its duration. Science 140, 184–186 (1963).
Lyons, H. E. et al. The influence of lifestyle and biological factors on semen variability. J. Assist. Reprod. Genet. 41, 1097–1109 (2024).
Li, Y. X. et al. Association between body mass index and semen quality: a systematic review and meta-analysis. Int. J. Obes. 48, 1383–1401 (2024).
Service, C. A., Puri, D., Al Azzawi, S., Hsieh, T. C. & Patel, D. P. The impact of obesity and metabolic health on male fertility: a systematic review. Fertil. Steril. 120, 1098–1111 (2023).
Palmer, N. O., Bakos, H. W., Fullston, T. & Lane, M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis 2, 253–263 (2012).
Leisegang, K., Sengupta, P., Agarwal, A. & Henkel, R. Obesity and male infertility: mechanisms and management. Andrologia 53, e13617 (2021).
Neto, F. T., Bach, P. V., Najari, B. B., Li, P. S. & Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 59, 10–26 (2016).
Schneider, G., Kirschner, M. A., Berkowitz, R. & Ertel, N. H. Increased estrogen production in obese men. J. Clin. Endocrinol. Metab. 48, 633–638 (1979).
de Boer, H., Verschoor, L., Ruinemans-Koerts, J. & Jansen, M. Letrozole normalizes serum testosterone in severely obese men with hypogonadotropic hypogonadism. Diabetes Obes. Metab. 7, 211–215 (2005).
Escobar-Morreale, H. F., Santacruz, E., Luque-Ramírez, M. & Botella Carretero, J. I. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum. Reprod. Update 23, 390–408 (2017).
De Silva, N. L. et al. Male hypogonadism: pathogenesis, diagnosis, and management. Lancet Diabetes Endocrinol. 12, 761–774 (2024).
Yeh, S. et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc. Natl Acad. Sci. USA 99, 13498–13503 (2002).
Smith, L. B. & Walker, W. H. The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol. 30, 2–13 (2014).
Gutorova, N. V., Kleshchyov, M. A., Tipisova, E. V. & Osadchuk, L. V. Effects of overweight and obesity on the spermogram values and levels of reproductive hormones in the male population of the European north of Russia. Bull. Exp. Biol. Med. 157, 95–98 (2014).
Jensen, T. K. et al. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil. Steril. 82, 863–870 (2004).
Liu, Y. & Ding, Z. Obesity, a serious etiologic factor for male subfertility in modern society. Reproduction 154, R123–R131 (2017).
Diamanti-Kandarakis, E. et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev. 30, 293–342 (2009).
Kumar, N. & Singh, A. K. Impact of environmental factors on human semen quality and male fertility: a narrative review. Environ. Sci. Eur. 34, 1–13 (2022).
Ge, R. S., Chen, G. R., Tanrikut, C. & Hardy, M. P. Phthalate ester toxicity in Leydig cells: developmental timing and dosage considerations. Reprod. Toxicol. 23, 366–373 (2007).
Garza, S. et al. Mitochondrial dynamics, Leydig cell function, and age-related testosterone deficiency. FASEB J. 36, e22637 (2022).
Ješeta, M. et al. Overview of the mechanisms of action of selected bisphenols and perfluoroalkyl chemicals on the male reproductive axes. Front. Genet. 12, 692897 (2021).
Knez, J., Kranvogl, R., Breznik, B. P., Vončina, E. & Vlaisavljević, V. Are urinary bisphenol A levels in men related to semen quality and embryo development after medically assisted reproduction? Fertil. Steril. 101, 215–221.e5 (2014).
Radwan, M. et al. Urinary bisphenol A levels and male fertility. Am. J. Mens Health 12, 2144–2151 (2018).
Adoamnei, E. et al. Urinary bisphenol A concentrations are associated with reproductive parameters in young men. Env. Res. 161, 122–128 (2018).
Lü, L. et al. Exposure interferes with reproductive hormones and decreases sperm counts: a systematic review and meta-analysis of epidemiological studies. Toxics 12, 294 (2024).
Goldstone, A. E., Chen, Z., Perry, M. J., Kannan, K. & Louis, G. M. Urinary bisphenol A and semen quality, the LIFE study. Reprod. Toxicol. 51, 7–13 (2015).
Jeseta, M. et al. Cross sectional study on exposure to BPA and its analogues and semen parameters in Czech men. Env. Pollut. 345, 123445 (2024).
Mruk, D. D. & Cheng, C. Y. The mammalian blood-testis barrier: its biology and regulation. Endocr. Rev. 36, 564–591 (2015).
Siu, E. R. et al. An occludin-focal adhesion kinase protein complex at the blood-testis barrier: a study using the cadmium model. Endocrinology 150, 3336–3344 (2009).
Chung, N. P. & Cheng, C. Y. Is cadmium chloride-induced inter-Sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology 142, 1878–1888 (2001).
el-Sabeawy, F. et al. Treatment of rats during pubertal development with 2,3,7,8-tetrachlorodibenzo-p-dioxin alters both signaling kinase activities and epidermal growth factor receptor binding in the testis and the motility and acrosomal reaction of sperm. Toxicol. Appl. Pharmacol. 150, 427–442 (1998).
World Health Organization. Dioxins. WHO https://www.who.int/news-room/fact-sheets/detail/dioxins-and-their-effects-on-human-health (2023).
Xiao, X. et al. Differential effects of c-Src and c-Yes on the endocytic vesicle-mediated trafficking events at the Sertoli cell blood-testis barrier: an in vitro study. Am. J. Physiol. Endocrinol. Metab. 307, E553–E562 (2014).
Xiao, X., Mruk, D. D., Cheng, F. L. & Cheng, C. Y. C-Src and c-Yes are two unlikely partners of spermatogenesis and their roles in blood-testis barrier dynamics. Adv. Exp. Med. Biol. 763, 295–317 (2012).
Mocarelli, P. et al. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ. Health Perspect. 116, 70–77 (2008).
Quintanilla-Vega, B. et al. Lead interaction with human protamine (HP2) as a mechanism of male reproductive toxicity. Chem. Res. Toxicol. 13, 594–600 (2000).
Ratcliffe, J. M. et al. Semen quality in papaya workers with long term exposure to ethylene dibromide. Br. J. Ind. Med. 44, 317–326 (1987).
Schrader, S. M., Turner, T. W. & Ratcliffe, J. M. The effects of ethylene dibromide on semen quality: a comparison of short-term and chronic exposure. Reprod. Toxicol. 2, 191–198 (1988).
Amir, D. The spermicidal effect of ethylene dibromide in bulls and rams. Mol. Reprod. Dev. 28, 99–109 (1991).
Meistrich, M. L. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil. Steril. 100, 1180–1186 (2013).
Duffin, K. et al. Impacts of cancer therapy on male fertility: past and present. Mol. Aspects Med. 100, 101308 (2024).
Vakalopoulos, I., Dimou, P., Anagnostou, I. & Zeginiadou, T. Impact of cancer and cancer treatment on male fertility. Hormones 14, 579–589 (2015).
Aitken, R. J., Smith, T. B., Jobling, M. S., Baker, M. A. & De Iuliis, G. N. Oxidative stress and male reproductive health. Asian J. Androl. 16, 31–38 (2014).
Wright, C., Milne, S. & Leeson, H. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod. Biomed. Online 28, 684–703 (2014).
Aitken, R. J., Jones, K. T. & Robertson, S. A. Reactive oxygen species and sperm function-in sickness and in health. J. Androl. 33, 1096–1106 (2012).
Aitken, R. J., Drevet, J. R., Moazamian, A. & Gharagozloo, P. Male infertility and oxidative stress: a focus on the underlying mechanisms. Antioxidants 11, 306 (2022).
Aitken, R. J., Wingate, J. K., De Iuliis, G. N., Koppers, A. J. & McLaughlin, E. A. Cis-unsaturated fatty acids stimulate reactive oxygen species generation and lipid peroxidation in human spermatozoa. J. Clin. Endocrinol. Metab. 91, 4154–4163 (2006).
Aitken, R. J. et al. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J. Biol. Chem. 287, 33048–33060 (2012).
Calamera, J., Buffone, M., Ollero, M., Alvarez, J. & Doncel, G. F. Superoxide dismutase content and fatty acid composition in subsets of human spermatozoa from normozoospermic, asthenozoospermic, and polyzoospermic semen samples. Mol. Reprod. Dev. 66, 422–430 (2003).
Aitken, R. J., Clarkson, J. S. & Fishel, S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol. Reprod. 41, 183–197 (1989).
Sanocka, D. & Kurpisz, M. Reactive oxygen species and sperm cells. Reprod. Biol. Endocrinol. 2, 12 (2004).
Aitken, R. J., Gibb, Z., Baker, M. A., Drevet, J. & Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 28, 1–10 (2016).
Aboulmaouahib, S. et al. Impact of alcohol and cigarette smoking consumption in male fertility potential: looks at lipid peroxidation, enzymatic antioxidant activities and sperm DNA damage. Andrologia 50, e12926 (2018).
He, Y. et al. Ketamine inhibits human sperm function by Ca2+-related mechanism. Biochem. Biophys. Res. Commun. 478, 501–506 (2016).
Safarinejad, M. R. et al. The effects of opiate consumption on serum reproductive hormone levels, sperm parameters, seminal plasma antioxidant capacity and sperm DNA integrity. Reprod. Toxicol. 36, 18–23 (2013).
Amor, H., Hammadeh, M. E., Mohd, I. & Jankowski, P. M. Impact of heavy alcohol consumption and cigarette smoking on sperm DNA integrity. Andrologia 54, e14434 (2022).
Nazmara, Z. et al. Correlation between protamine-2 and miRNA-122 in sperm from heroin-addicted men: a case-control study. Urol. J. 17, 638–644 (2020).
Imhof ML, J. Lipovac, M. Chedraui, P. Riedl, C. Improvement of sperm quality after micronutrient supplementation. ESPEN J. 7, e50–e53 (2012).
Nguyen, N. D., Le, M. T., Tran, N. Q. T., Nguyen, Q. H. V. & Cao, T. N. Micronutrient supplements as antioxidants in improving sperm quality and reducing DNA fragmentation. Basic. Clin. Androl. 33, 23 (2023).
Lipovac, M., Nairz, V., Aschauer, J. & Riedl, C. The effect of micronutrient supplementation on spermatozoa DNA integrity in subfertile men and subsequent pregnancy rate. Gynecol. Endocrinol. 37, 711–715 (2021).
Komiya, A. et al. Results of lifestyle modification promotion and reproductive/general health check for male partners of couples seeking conception. Heliyon 9, e15203 (2023).
Eisenberg, M. L. et al. Semen quality, infertility and mortality in the USA. Hum. Reprod. 29, 1567–1574 (2014).
Del Giudice, F. et al. The association between mortality and male infertility: systematic review and meta-analysis. Urology 154, 148–157 (2021).
Glazer, C. H. et al. Male factor infertility and risk of death: a nationwide record-linkage study. Hum. Reprod. 34, 2266–2273 (2019).
Shiraishi, K. & Matsuyama, H. Effects of medical comorbidity on male infertility and comorbidity treatment on spermatogenesis. Fertil. Steril. 110, 1006–11.e2 (2018).
Ventimiglia, E. et al. Infertility as a proxy of general male health: results of a cross-sectional survey. Fertil. Steril. 104, 48–55 (2015).
Hales C. M., Carroll M. D., Fryar C. D., Ogden C. L. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 1–8 (2020).
Hales, C. N. Metabolic consequences of intrauterine growth retardation. Acta Paediatr. 86, 184–187 (1997).
Yeh, T. L. et al. The relationship between metabolically healthy obesity and the risk of cardiovascular disease: a systematic review and meta-analysis. J. Clin. Med. 8, 1228 (2019).
Salas-Huetos, A. et al. Male adiposity, sperm parameters and reproductive hormones: an updated systematic review and collaborative meta-analysis. Obes. Rev. 22, e13082 (2021).
Sepidarkish, M. et al. The effect of body mass index on sperm DNA fragmentation: a systematic review and meta-analysis. Int. J. Obes. 44, 549–558 (2020).
Lotti, F., Marchiani, S., Corona, G. & Maggi, M. Metabolic syndrome and reproduction. Int. J. Mol. Sci. 22, 1988 (2021).
Ehala-Aleksejev, K. & Punab, M. The effect of metabolic syndrome on male reproductive health: a cross-sectional study in a group of fertile men and male partners of infertile couples. PLoS ONE 13, e0194395 (2018).
Dupont, C. et al. Metabolic syndrome and smoking are independent risk factors of male idiopathic infertility. Basic. Clin. Androl. 29, 9 (2019).
Zhao, L. & Pang, A. Effects of metabolic syndrome on semen quality and circulating sex hormones: a systematic review and meta-analysis. Front. Endocrinol. 11, 428 (2020).
Pergialiotis, V. et al. Diabetes mellitus and functional sperm characteristics: a meta-analysis of observational studies. J. Diabetes Complications 30, 1167–1176 (2016).
Torres, M. et al. Male fertility is reduced by chronic intermittent hypoxia mimicking sleep apnea in mice. Sleep 37, 1757–1765 (2014).
McLachlan, R. I. Approach to the patient with oligozoospermia. J. Clin. Endocrinol. Metab. 98, 873–880 (2013).
Lundy, S. D. & Vij, S. C. Male infertility in renal failure and transplantation. Transl. Androl. Urol. 8, 173–181 (2019).
Zhang, Q. F. et al. Does COVID-19 affect sperm quality in males? the answer may be yes, but only temporarily. Virol. J. 21, 24 (2024).
Basaria, S. Male hypogonadism. Lancet 383, 1250–1263 (2014).
Bojesen, A., Juul, S. & Gravholt, C. H. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J. Clin. Endocrinol. Metab. 88, 622–626 (2003).
Herlihy, A. S., Halliday, J. L., Cock, M. L. & McLachlan, R. I. The prevalence and diagnosis rates of Klinefelter syndrome: an Australian comparison. Med. J. Aust. 194, 24–28 (2011).
Deebel, N. A., Bradshaw, A. W. & Sadri-Ardekani, H. Infertility considerations in Klinefelter syndrome: from origin to management. Best. Pract. Res. Clin. Endocrinol. Metab. 34, 101480 (2020).
Kanakis, G. A. & Nieschlag, E. Klinefelter syndrome: more than hypogonadism. Metabolism 86, 135–144 (2018).
Clemente-Suárez, V. J., Beltrán-Velasco, A. I., Redondo-Flórez, L., Martín-Rodríguez, A. & Tornero-Aguilera, J. F. Global impacts of western diet and its effects on metabolism and health: a narrative review. Nutrients 15, 2749 (2023).
Naidu, R. et al. Chemical pollution: a growing peril and potential catastrophic risk to humanity. Env. Int. 156, 106616 (2021).
World Health Organization. Overweight prevalence among children under 5 years of age (%weight-for-height >+2 SD). WHO https://www.who.int/data/gho/data/indicators/indicator-details/GHO/gho-jme-country-children-aged-5-years-overweight-(-weight-for-height-2-sd) (2025).
World Health Organisation. Global status report on noncommunicable diseases 2010. (World Health Organisation, 2011).
Heindel, J. J., Lustig, R. H., Howard, S. & Corkey, B. E. Obesogens: a unifying theory for the global rise in obesity. Int. J. Obes. 48, 449–460 (2024).
Cancer Australia. Cancer incidence. Cancer Australia https://ncci.canceraustralia.gov.au/diagnosis/cancer-incidence/cancer-incidence (2024).
van Oostrom, S. H. et al. Time trends in prevalence of chronic diseases and multimorbidity not only due to aging: data from general practices and health surveys. PLoS ONE 11, e0160264 (2016).
Ward, B. W. & Schiller, J. S. Prevalence of multiple chronic conditions among US adults: estimates from the National Health Interview Survey, 2010. Prev. Chronic Dis. 10, E65 (2013).
Cristodoro, M., Zambella, E., Fietta, I., Inversetti, A. & Di Simone, N. Dietary patterns and fertility. Biology 13, 131 (2024).
Salas-Huetos, A., Babio, N., Carrell, D. T., Bulló, M. & Salas-Salvadó, J. Adherence to the Mediterranean diet is positively associated with sperm motility: a cross-sectional analysis. Sci. Rep. 9, 3389 (2019).
Ricci, E. et al. Semen quality and alcohol intake: a systematic review and meta-analysis. Reprod. Biomed. Online 34, 38–47 (2017).
Gaskins, A. J., Colaci, D. S., Mendiola, J., Swan, S. H. & Chavarro, J. E. Dietary patterns and semen quality in young men. Hum. Reprod. 27, 2899–2907 (2012).
Caruso, P. et al. Effects of Mediterranean diet on semen parameters in healthy young adults: a randomized controlled trial. Minerva Endocrinol. 45, 280–287 (2020).
Montano, L. et al. Effects of a lifestyle change intervention on semen quality in healthy young men living in highly polluted areas in Italy: the FASt randomized controlled trial. Eur. Urol. Focus. 8, 351–359 (2022).
Walsh, T. J., Croughan, M. S., Schembri, M., Chan, J. M. & Turek, P. J. Increased risk of testicular germ cell cancer among infertile men. Arch. Intern. Med. 169, 351–356 (2009).
Walsh, T. J. et al. Increased risk of high-grade prostate cancer among infertile men. Cancer 116, 2140–2147 (2010).
Eisenberg, M. L., Li, S., Brooks, J. D., Cullen, M. R. & Baker, L. C. Increased risk of cancer in infertile men: analysis of U.S. claims data. J. Urol. 193, 1596–1601 (2015).
Hanson, H. A. et al. Subfertility increases risk of testicular cancer: evidence from population-based semen samples. Fertil. Steril. 105, 322–8.e1 (2016).
Schultz, N., Hamra, F. K. & Garbers, D. L. A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc. Natl Acad. Sci. USA 100, 12201–12206 (2003).
Mukherjee, S., Ridgeway, A. D. & Lamb, D. J. DNA mismatch repair and infertility. Curr. Opin. Urol. 20, 525–532 (2010).
Belladelli, F., Basran, S. & Eisenberg, M. L. Male fertility and physical exercise. World J. Mens Health 41, 482–488 (2023).
Gaskins, A. J. et al. Paternal physical and sedentary activities in relation to semen quality and reproductive outcomes among couples from a fertility center. Hum. Reprod. 29, 2575–2582 (2014).
American Heart Association. Lifestyle changes to prevent a heart attack. American Heart Association https://www.heart.org/en/health-topics/heart-attack/life-after-a-heart-attack/lifestyle-changes-for-heart-attack-prevention (2025).
Mayo Clinic. Strategies to prevent heart disease. Mayo Clinic https://www.mayoclinic.org/diseases-conditions/heart-disease/in-depth/heart-disease-prevention/art-20046502 (2023).
Kim, H. L. et al. Lifestyle modification in the management of metabolic syndrome: statement from Korean society of cardiometabolic syndrome (KSCMS). Korean Circ. J. 52, 93–109 (2022).
Oh, S., Kim, E. & Shoda, J. Editorial: lifestyle modification strategies as first line of chronic disease management. Front. Physiol. 14, 1204581 (2023).
Greaves, C. J. et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public. Health 11, 119 (2011).
Rhodes, R. E., Janssen, I., Bredin, S. S. D., Warburton, D. E. R. & Bauman, A. Physical activity: health impact, prevalence, correlates and interventions. Psychol. Health 32, 942–975 (2017).
Cleland, V. et al. Effectiveness of interventions to promote physical activity and/or decrease sedentary behaviour among rural adults: a systematic review and meta-analysis. Obes. Rev. 18, 727–741 (2017).
Rippe, J. M. Lifestyle strategies for risk factor reduction, prevention, and treatment of cardiovascular disease. Am. J. Lifestyle Med. 13, 204–212 (2019).
Darkins, A., Kendall, S., Edmonson, E., Young, M. & Stressel, P. Reduced cost and mortality using home telehealth to promote self-management of complex chronic conditions: a retrospective matched cohort study of 4,999 veteran patients. Telemed. J. E Health 21, 70–76 (2015).
Darkins, A. et al. Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed. J. E Health 14, 1118–1126 (2008).
McCarthy D. Kaiser Permanente: bridging the quality divide with integrated practice, group accountability, and health information technology. The Commonwealth Fund https://www.commonwealthfund.org/publications/case-study/2009/jun/kaiser-permanente-bridging-quality-divide-integrated-practice (2009).
Rivera, A. & Scholar, J. Traditional masculinity: a review of toxicity rooted in social norms and gender socialization. ANS Adv. Nurs. Sci. 43, E1–E10 (2020).
Burton M. Negotiating masculinity: how infertility impacts hegemonic masculinity. LUJA 1, 49–57 (2014).
Pakpahan, C. et al. “Masculine?” A metasynthesis of qualitative studies on traditional masculinity on infertility. F1000Res 12, 252 (2023).
Trussell, J. C. et al. Association between testosterone, semen parameters, and live birth in men with unexplained infertility in an intrauterine insemination population. Fertil. Steril. 111, 1129–1134 (2019).
Cervi LK, D. Organising male infertility: masculinities and fertility treatment. Gend. Work. Organ. 29, 1113–1131 (2022).
Clarke, M. J., Marks, A. D. & Lykins, A. D. Effect of normative masculinity on males’ dysfunctional sexual beliefs, sexual attitudes, and perceptions of sexual functioning. J. Sex. Res. 52, 327–337 (2015).
Malik, S. H. & Coulson, N. The male experience of infertility: a thematic analysis of an online infertility support group bulletin board. J. Reprod. Infant Psychol. 26, 18–30 (2008).
Peronace, L. A., Boivin, J. & Schmidt, L. Patterns of suffering and social interactions in infertile men: 12 months after unsuccessful treatment. J. Psychosom. Obstet. Gynaecol. 28, 105–114 (2007).
Culley, L., Hudson, N. & Lohan, M. Where are all the men? The marginalization of men in social scientific research on infertility. Reprod. Biomed. Online 27, 225–235 (2013).
Wischmann, T. & Thorn, P. (Male) infertility: what does it mean to men? New evidence from quantitative and qualitative studies. Reprod. Biomed. Online 27, 236–243 (2013).
Mikkelsen, A. T., Madsen, S. A. & Humaidan, P. Psychological aspects of male fertility treatment. J. Adv. Nurs. 69, 1977–1986 (2013).
Arya, S. T. & Dibb, B. The experience of infertility treatment: the male perspective. Hum. Fertil. 19, 242–248 (2016).
Tabong, P. T. & Adongo, P. B. Understanding the social meaning of infertility and childbearing: a qualitative study of the perception of childbearing and childlessness in Northern Ghana. PLoS ONE 8, e54429 (2013).
Inhorn, M. C. Middle Eastern masculinities in the age of new reproductive technologies: male infertility and stigma in Egypt and Lebanon. Med. Anthropol. Q. 18, 162–182 (2004).
Serour, G. I. & Serour, A. G. The impact of religion and culture on medically assisted reproduction in the Middle East and Europe. Reprod. Biomed. Online 43, 421–433 (2021).
Nimbi, F. M., Tripodi, F., Rossi, R., Navarro-Cremades, F. & Simonelli, C. Male sexual desire: an overview of biological, psychological, sexual, relational, and cultural factors influencing desire. Sex. Med. Rev. 8, 59–91 (2020).
Coward, R. M. et al. Fertility related quality of life, gonadal function and erectile dysfunction in male partners of couples with unexplained infertility. J. Urol. 202, 379–384 (2019).
Ozkan, B., Orhan, E., Aktas, N. & Coskuner, E. R. Depression and sexual dysfunction in Turkish men diagnosed with infertility. Urology 85, 1389–1393 (2015).
Mahalik, J. R., Di Bianca, M. & Sepulveda, J. Examining father status and purpose to understand new days’ healthier lives. Psychol. Men. Masc. 21, 570–577 (2020).
Lewington, L., Sebar, B. & Lee, J. “Becoming the man you always wanted to be”: exploring the representation of health and masculinity in Men’s Health magazine. Health Promot. J. Austr. 29, 243–250 (2018).
De Jonge, C. J. et al. Current global status of male reproductive health. Hum. Reprod. Open. 2024, hoae017 (2024).
De Jonge, C. & Barratt, C. L. R. The present crisis in male reproductive health: an urgent need for a political, social, and research roadmap. Andrology 7, 762–768 (2019).
Torkel, S. et al. Barriers and enablers to a healthy lifestyle in people with infertility: a mixed-methods systematic review. Hum. Reprod. Update 30, 569–583 (2024).
Healthy male. A case for change. Healthy Male https://healthymale.org.au/plus-paternal/a-case-for-change (2024).
Vargas, C., Whelan, J., Brimblecombe, J. & Allender, S. Co-creation, co-design, co-production for public health — a perspective on definition and distinctions. Public Health Res Pract. 32, 3222211 (2022).
Slattery, P., Saeri, A. K. & Bragge, P. Research co-design in health: a rapid overview of reviews. Health Res. Policy Syst. 18, 17 (2020).
Sanz, M. F., Acha, B. V. & García, M. F. Co-design for people-centred care digital solutions: a literature review. Int. J. Integr. Care 21, 16 (2021).
Ng, S. K., Martin, S. A., Adams, R. J., O’Loughlin, P. & Wittert, G. A. The effect of multimorbidity patterns and the impact of comorbid anxiety and depression on primary health service use: the men androgen inflammation lifestyle environment and stress (MAILES) study. Am. J. Mens. Health 14, 1557988320959993 (2020).
Mursa, R., Patterson, C. & Halcomb, E. Men’s help-seeking and engagement with general practice: an integrative review. J. Adv. Nurs. 78, 1938–1953 (2022).
McGraw, J., White, K. M. & Russell-Bennett, R. Masculinity and men’s health service use across four social generations: findings from Australia’s Ten to Men study. SSM Popul. Health 15, 100838 (2021).
Håkonsen, L. B. et al. Does weight loss improve semen quality and reproductive hormones? results from a cohort of severely obese men. Reprod. Health 8, 24 (2011).
Faure, C. et al. In subfertile couple, abdominal fat loss in men is associated with improvement of sperm quality and pregnancy: a case-series. PLoS ONE 9, e86300 (2014).
Rafiee, B., Morowvat, M. H. & Rahimi-Ghalati, N. Comparing the effectiveness of dietary vitamin C and exercise interventions on fertility parameters in normal obese men. Urol. J. 13, 2635–2639 (2016).
Rosety, M. et al. Exercise improved semen quality and reproductive hormone levels in sedentary obese adults. Nutr. Hosp. 34, 603–607 (2017).
Mir J., Franken D., Andrabi S. W., Ashraf M., Rao K. Impact of weight loss on sperm DNA integrity in obese men. Andrologia 50, e12957 (2018).
Jaffar, M. & Ashraf, M. Does weight loss improve fertility with respect to semen parameters — results from a large cohort study. Int. J. Infertil. Fetal Med. 8, 12–17 (2017).
Bisht, S. et al. Sperm methylome alterations following yoga-based lifestyle intervention in patients of primary male infertility: a pilot study. Andrologia 52, e13551 (2020).
Mombeyni, A., Shakerian, S., Habibi, A. & Ghanbarzadeh, M. The effect of 12 weeks of concurrent training on hypothalamic-pituitary-gonadal axis hormones and semen fertility indices of sedentary obese men. Med. Sport 74, 269–283 (2021).
Andersen, E. et al. Sperm count is increased by diet-induced weight loss and maintained by exercise or GLP-1 analogue treatment: a randomized controlled trial. Hum. Reprod. 37, 1414–1422 (2022).
Humaidan, P. et al. The combined effect of lifestyle intervention and antioxidant therapy on sperm DNA fragmentation and seminal oxidative stress in IVF patients: a pilot study. Int. Braz. J. Urol. 48, 131–156 (2022).
Ismail, A., Abdelghany, A. & Atef, H. Response of testosterone and semen parameters to a 14-week aerobic training in sedentary obese men with hyperglycaemia. Physiother. Q. 31, 28–33 (2023).
Sharma, A. et al. Improvements in sperm motility following low- or high-intensity dietary interventions in men with obesity. J. Clin. Endocrinol. Metab. 109, 449–460 (2024).
Author information
Authors and Affiliations
Contributions
H.E.L., A.P. and N.O.M. researched data for the article. H.E.L., A.P., V.N. and N.O.M. contributed substantially to discussion of the content. H.E.L., A.P., M.G., J.D., V.N. and N.M.P. wrote the article. H.E.L., A.P., M.G., O.O. and N.M.P. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Ralf Henkel, Sandro Esteves and Luca Boeri for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lyons, H.E., Peel, A., Gonzalez, M. et al. Unlocking the power of semen analysis in primary health care — a path to men’s health and lifestyle transformation. Nat Rev Urol 22, 687–702 (2025). https://doi.org/10.1038/s41585-025-01047-1
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41585-025-01047-1