Abstract
A psychotherapeutic regimen that uses alternating bilateral sensory stimulation (ABS) has been used to treat post-traumatic stress disorder. However, the neural basis that underlies the long-lasting effect of this treatment—described as eye movement desensitization and reprocessing—has not been identified. Here we describe a neuronal pathway driven by the superior colliculus (SC) that mediates persistent attenuation of fear. We successfully induced a lasting reduction in fear in mice by pairing visual ABS with conditioned stimuli during fear extinction. Among the types of visual stimulation tested, ABS provided the strongest fear-reducing effect and yielded sustained increases in the activities of the SC and mediodorsal thalamus (MD). Optogenetic manipulation revealed that the SC–MD circuit was necessary and sufficient to prevent the return of fear. ABS suppressed the activity of fear-encoding cells and stabilized inhibitory neurotransmission in the basolateral amygdala through a feedforward inhibitory circuit from the MD. Together, these results reveal the neural circuit that underlies an effective strategy for sustainably attenuating traumatic memories.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data used in this study are available from the corresponding author upon reasonable request.
References
Quirk, G. J. et al. Erasing fear memories with extinction training. J. Neurosci. 30, 14993–14997 (2010).
Sandkühler, J. & Lee, J. How to erase memory traces of pain and fear. Trends Neurosci. 36, 343–352 (2013).
Goode, T. D. & Maren, S. Animal models of fear relapse. ILAR J. 55, 246–258 (2014).
Nader, K., Schafe, G. E. & Le Doux, J. E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406, 722–726 (2000).
Shema, R., Sacktor, T. C. & Dudai, Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKMζ. Science 317, 951–953 (2007).
Han, J.-H. et al. Selective erasure of a fear memory. Science 323, 1492–1496 (2009).
Karpova, N. N. et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science 334, 1731–1734 (2011).
Shapiro, F. Eye Movement Desensitization and Reprocessing (EMDR): Basic Principles, Protocols, and Procedures 2nd edn (Guilford, New York, 2001).
Resick, P. A. & Schnicke, M. K. Cognitive processing therapy for sexual assault victims. J. Consult. Clin. Psychol. 60, 748–756 (1992).
Badura-Brack, A. S. et al. Effect of attention training on attention bias variability and PTSD symptoms: randomized controlled trials in Israeli and U.S. combat veterans. Am. J. Psychiatry 172, 1233–1241 (2015).
Wurtz, H. et al. Preventing long-lasting fear recovery using bilateral alternating sensory stimulation: a translational study. Neuroscience 321, 222–235 (2016).
Sommer, M. A. & Wurtz, R. H. Brain circuits for the internal monitoring of movements. Annu. Rev. Neurosci. 31, 317–338 (2008).
Krauzlis, R. J., Lovejoy, L. P. & Zénon, A. Superior colliculus and visual spatial attention. Annu. Rev. Neurosci. 36, 165–182 (2013).
Wilson, S. A., Becker, L. A. & Tinker, R. H. Fifteen-month follow-up of eye movement desensitization and reprocessing (EMDR) treatment for posttraumatic stress disorder and psychological trauma. J. Consult. Clin. Psychol. 65, 1047–1056 (1997).
Edmond, T. & Rubin, A. Assessing the long-term effects of EMDR: results from an 18-month follow-up study with adult female survivors of CSA. J. Child Sex. Abuse 13, 69–86 (2004).
Stubblefield, E. A., Costabile, J. D. & Felsen, G. Optogenetic investigation of the role of the superior colliculus in orienting movements. Behav. Brain Res. 255, 55–63 (2013).
White, B. J., Kan, J. Y., Levy, R., Itti, L. & Munoz, D. P. Superior colliculus encodes visual saliency before the primary visual cortex. Proc. Natl Acad. Sci. USA 114, 9451–9456 (2017).
Sommer, M. A. & Wurtz, R. H. What the brain stem tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. J. Neurophysiol. 91, 1381–1402 (2004).
Sommer, M. A. & Wurtz, R. H. Influence of the thalamus on spatial visual processing in frontal cortex. Nature 444, 374–377 (2006).
Oyoshi, T., Nishijo, H., Asakura, T., Takamura, Y. & Ono, T. Emotional and behavioral correlates of mediodorsal thalamic neurons during associative learning in rats. J. Neurosci. 16, 5812–5829 (1996).
Herry, C. & Garcia, R. Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J. Neurosci. 22, 577–583 (2002).
Lee, S. & Shin, H.-S. The role of mediodorsal thalamic nucleus in fear extinction. J. Anal. Sci. Technol. 7, 13 (2016).
Milad, M. R. & Quirk, G. J. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420, 70–74 (2002).
Amano, T., Unal, C. T. & Paré, D. Synaptic correlates of fear extinction in the amygdala. Nat. Neurosci. 13, 489–494 (2010).
Lee, S. et al. Bidirectional modulation of fear extinction by mediodorsal thalamic firing in mice. Nat. Neurosci. 15, 308–314 (2011).
Cheong, E. et al. Tuning thalamic firing modes via simultaneous modulation of T- and L-type Ca2+ channels controls pain sensory gating in the thalamus. J. Neurosci. 28, 13331–13340 (2008).
Herry, C. et al. Switching on and off fear by distinct neuronal circuits. Nature 454, 600–606 (2008).
Senn, V. et al. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 81, 428–437 (2014).
Lin, H.-C., Mao, S.-C. & Gean, P.-W. Block of γ-aminobutyric acid-A receptor insertion in the amygdala impairs extinction of conditioned fear. Biol. Psychiatry 66, 665–673 (2009).
Mátyás, F., Lee, J., Shin, H.-S. & Acsády, L. The fear circuit of the mouse forebrain: connections between the mediodorsal thalamus, frontal cortices and basolateral amygdala. Eur. J. Neurosci. 39, 1810–1823 (2014).
Nakazawa, K. et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297, 211–218 (2002).
Delevich, K., Tucciarone, J., Huang, Z. J. & Li, B. The mediodorsal thalamus drives feedforward inhibition in the anterior cingulate cortex via parvalbumin interneurons. J. Neurosci. 35, 5743–5753 (2015).
Lee, C. W. & Cuijpers, P. A meta-analysis of the contribution of eye movements in processing emotional memories. J. Behav. Ther. Exp. Psychiatry 44, 231–239 (2013).
Mello, P. G., Silva, G. R., Donat, J. C. & Kristensen, C. H. An update on the efficacy of cognitive-behavioral therapy, cognitive therapy, and exposure therapy for posttraumatic stress disorder. Int. J. Psychiatry Med. 46, 339–357 (2013).
Chen, L., Zhang, G., Hu, M. & Liang, X. Eye movement desensitization and reprocessing versus cognitive-behavioral therapy for adult posttraumatic stress disorder: systematic review and meta-analysis. J. Nerv. Ment. Dis. 203, 443–451 (2015).
Haagen, J. F. G., Smid, G. E., Knipscheer, J. W. & Kleber, R. J. The efficacy of recommended treatments for veterans with PTSD: a metaregression analysis. Clin. Psychol. Rev. 40, 184–194 (2015).
McHaffie, J. G. & Stein, B. E. Eye movements evoked by electrical stimulation in the superior colliculus of rats and hamsters. Brain Res. 247, 243–253 (1982).
Gandhi, N. J. & Katnani, H. A. Motor functions of the superior colliculus. Annu. Rev. Neurosci. 34, 205–231 (2011).
Ignashchenkova, A., Dicke, P. W., Haarmeier, T. & Thier, P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat. Neurosci. 7, 56–64 (2004).
Wei, P. et al. Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat. Commun. 6, 6756 (2015).
Evans, D. A. et al. A synaptic threshold mechanism for computing escape decisions. Nature 558, 590–594 (2018).
Cohen, J. D. & Castro-Alamancos, M. A. Early sensory pathways for detection of fearful conditioned stimuli: tectal and thalamic relays. J. Neurosci. 27, 7762–7776 (2007).
Kaczkurkin, A. N. & Foa, E. B. Cognitive-behavioral therapy for anxiety disorders: an update on the empirical evidence. Dialogues Clin. Neurosci. 17, 337–346 (2015).
LeDoux, J. E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000).
Kim, D. et al. Phospholipase C isozymes selectively couple to specific neurotransmitter receptors. Nature 389, 290–293 (1997).
Kadir, S. N., Goodman, D. F. M. & Harris, K. D. High-dimensional cluster analysis with the masked EM algorithm. Neural Comput. 26, 2379–2394 (2014).
Schmitzer-Torbert, N., Jackson, J., Henze, D., Harris, K. & Redish, A. D. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131, 1–11 (2005).
An, B., Hong, I. & Choi, S. Long-term neural correlates of reversible fear learning in the lateral amygdala. J. Neurosci. 32, 16845–16856 (2012).
Acknowledgements
We thank Y.-S. Kim for providing the PLCß4 knockdown virus, G. Buzsáki for advising us on silicon probe recording in freely moving mice, and J. J. Shin for discussions on slice recordings. This work was supported by IBS grant IBS-R001-D1.
Reviewer information
Nature thanks J. Johansen, G. Quirk and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
J. Baek, S.L. and H.-S.S. designed the experiments and wrote the manuscript. J. Baek performed in vitro and in vivo electrophysiology and optogenetic experiments. S.L. performed behavioural experiments. S.-W.K. contributed to genetic studies. M.K. and Y.Y. contributed to histological work. T.C. performed in vitro electrophysiology. K.K.K. and J. Byun contributed to in vitro electrophysiology analysis. J. Byun performed blinded counting. S.J.K. aided in the interpretation of data and contributed to editing the manuscript. J.J. and H.-S.S. supervised the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Effect of ABS pairing on fear extinction of strong fear memory and effect on memory reactivation and reconsolidation.
a, One day after fear conditioning (0.7 mA foot shock), visual stimulation was presented during fear extinction (n = 7 mice for each group). Mixed-design ANOVA for extinction: F4,30 = 78.62, P = 1.85 × 10−15 for group effect. One-way ANOVA for recall test: F4,30 = 53.95, P = 2.81 × 10−13. b, Effects of ABS pairing on fear relapse (n = 7 mice for each group). Two-way ANOVA: F1,36 = 138.521, P = 6.73 × 10−14 for group effect. Post hoc multiple comparison with Bonferroni correction; ***P < 0.001. Asterisks above bars indicate significant difference in comparison to recall. c, Effects of ABS pairing during memory reactivation (CS, n = 8; ABS + CS, n = 8 mice). Student’s t-test, two-sided: t(14) = −3.9058, P = 0.001584 for memory reactivation; t(14) = 0.2411, P = 0.813 for PR-LTM; **P < 0.01. Data shown as mean ± s.e.m. See Supplementary Table 1 for statistical details.
Extended Data Fig. 2 Single-unit recording of SC.
a, Coronal sections showing the positions of the silicon probes (left) and tetrodes (right). SGS, stratum griseum superficiale; SGI, stratum griseum intermediale; SGP, stratum griseum profundum. b, Schematic of 64-channel silicon probes used for SC recordings. c, Example waveforms of recorded neurons from a single shank. d, Probe tracks (left) and tetrode tip locations (right). e, Example single-unit responses of the SC to sensory stimulation (500-ms bins; pie charts, n = 109 cells). Sensory stimulation blocks were pseudo-randomly presented. f, Averaged SC responses during 5 s after stimulus onset (n = 109 cells). Mixed-design ANOVA: F3,324 = 15.4, P = 2.17 × 10−19 for stimulation effect. g, h, Positive responses of SC neurons from CS group (g; n = 33 cells) and ABS + CS group (h; n = 62 cells) during fear extinction. i, Averaged positive responses across extinction trials (early, second-to-fifth trials; mid, sixth-to-tenth trials; late, eleventh-to-fifteenth trials; samples from g, h). Mixed-design ANOVA: F1,93 = 7.621, P = 0.00695 for group effect. j, k, Negative responses of SC neurons from CS group (j; n = 10 cells) and ABS + CS group (k; n = 8 cells) during fear extinction. l, Averaged negative responses across extinction trials (samples from j, k). Mixed-design ANOVA: F1,16 = 0.71, P = 0.412 for group effect. Mean ± s.e.m.; post hoc multiple comparison with Bonferroni correction; *P < 0.05. See Supplementary Table 1 for statistical details.
Extended Data Fig. 3 Freezing behaviour and correlation with SC activity during fear extinction.
a, b, Fear extinction (a) and subsequent retention tests (b) with SC single-unit recordings (CS, n = 10; ABS + CS, n = 8 mice). Mixed-design ANOVA for extinction: F1,16 = 29.73, P = 5.32 × 10−5 for group effect. Mixed-design ANOVA for retention tests: F1,16 = 32.65, P = 3.2 × 10−5 for group effect. Mean ± s.e.m.; post hoc multiple comparison with Bonferroni correction; *P < 0.05, **P < 0.01, ***P < 0.001. Asterisks above bars indicate significant difference in comparison to recall. c–f, Pearson’s correlation analyses of SC positive responses (CS, n = 9; ABS + CS, n = 8 mice) during fear extinction with freezing during late extinction trials (c; a block of the last three extinction trials), recall test (d), spontaneous recovery test (e) or renewal test (f). g–j, Pearson’s correlation analyses of SC negative responses (CS, n = 5; ABS + CS, n = 5 mice) during fear extinction with freezing during late extinction trials (g), recall test (h), spontaneous recovery test (i) or renewal test (j). See Supplementary Table 1 for statistical details.
Extended Data Fig. 4 Single-unit recording of MD.
a, Coronal section showing the position of the recording sites (red arrow). HB, habenular nucleus; PVT, paraventricular thalamic nucleus. b, c, An example spike sorting result from a single tetrode. b, Example feature plot showing clusters of candidate spikes; c, average waveforms of isolated units from the tetrode. d, Tetrode tip locations in MD. e, f, Positive responses of MD neurons in CS group (e; n = 49 cells) and ABS + CS group (f; n = 63 cells) g, Averaged positive responses across extinction trials (early, second-to-fifth trials; mid, sixth-to-tenth trials; late, eleventh-to-fifteenth trials; samples from e, f). Mixed-design ANOVA: F1,110 = 17.83, P = 4.99 × 10−5 for group effect. h, i, Negative responses of MD neurons in CS group (h; n = 31 cells) and ABS + CS group (i; n = 44 cells) during fear extinction. j, Averaged negative responses of the MD across extinction trials (samples from h, i). Mixed-design ANOVA: F1,73 = 1.762, P = 0.188 for group effect. Mean ± s.e.m.; post hoc multiple comparison with Bonferroni correction; ***P < 0.001. See Supplementary Table 1 for statistical details.
Extended Data Fig. 5 Freezing behaviour and correlation with MD activity during fear extinction.
a, b, Fear extinction (a) and subsequent retention tests (b) with MD single-unit recordings (CS, n = 6; ABS + CS, n = 8 mice). Mixed-design ANOVA for extinction: F1,12 = 13.85, P = 0.000292 for group effect. Mixed-design ANOVA for retention tests: F1,12 = 33.1, P = 9.11 × 10−5 for group effect. Mean ± s.e.m.; post hoc multiple comparison with Bonferroni correction; **P < 0.01, ***P < 0.001. c–f, Pearson’s correlation analyses of MD positive responses (CS, n = 6; ABS + CS, n = 8 mice) during fear extinction with freezing during late extinction trials (c, a block of the last three extinction trials), recall test (d), spontaneous recovery test (e) or renewal test (f). g–j, Pearson’s correlation analyses of MD negative responses (CS, n = 4; ABS + CS, n = 8 mice) during fear extinction with freezing during late extinction trials (g), recall test (h), spontaneous recovery test (i) or renewal test (j). See Supplementary Table 1 for statistical details.
Extended Data Fig. 6 Plcb4 deletion disturbing MD activity blocks the effects of ABS paired extinction.
a, Effects of the Plcb4 knockout (KO) on ABS paired extinction (wild-type (WT) CS, n = 5; WT ABS + CS, n = 5; KO CS n = 5; KO ABS + CS n = 7 mice). Mixed-design ANOVA for fear extinction: F3,18 = 57.56, P = 2.01 × 10−9 for group effect. One-way ANOVA for recall test: F3,18 = 35.24, P = 9.6 × 10−8. b, Effects of Plcb4 knockdown in MD on ABS paired extinction (shControl CS, n = 4; shControl ABS + CS, n = 7; shPlcb4 CS, n = 4; shPlcb4 ABS + CS, n = 5 mice). Mixed-design ANOVA for fear extinction: F3,16 = 19.25, P = 1.47 × 10−5 for group effect. One-way ANOVA for recall test: F3,16 = 26.18, P = 2.07 × 10−6. Mean ± s.e.m; ***P < 0.001. See Supplementary Table 1 for statistical details. c–j, Knockdown of Plcb4 in the MD by injection of shRNA lentiviral vector. Double fluorescence labelling of PLCβ4 expression with DAPI counterstain in the MD of shControl-injected mice (c–f) and shPlcb4-injected mice (g–j). Histology was confirmed for all mice in b after behavioural experiments. d–f, h–j, Higher magnification images corresponding to the rectangles in c, g, respectively. Scale bars, 1,000 μm (c, g); 100 μm (d–f, h–j).
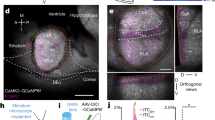
Extended Data Fig. 7 Verification of viral expression and functional connectivity of the SC–MD pathway.
a, Retrograde tracer CTB (green) was injected into the MD. Only 6.12% (37/600) of CTB-positive neurons were GABA-positive and only 4.38% (37/844) of GABA-positive neurons were CTB-positive. Experiments were repeated with three mice (two slices per mouse) with similar results, and combined cell numbers are presented. White arrow indicates a CTB-positive GABAergic neuron in the SC. Scale bar, 20 μm. b, Illustration of viral injections in SC and fibre placement in MD. c, Coronal section showing a neuron expressing eNpHR3.0–eYFP in SC. Viral expression was confirmed in 20 mice after behavioural experiments (Fig. 2g–i). d, Coronal section showing fibres expressing eNpHR3.0–eYFP in MD. Viral expression was confirmed in 20 mice after behavioural experiments (Fig. 2g–i). e, Optical fibre placements for SC–MD silencing experiments. f, ChR2–YFP virus injection in SC and slicing position for whole-cell recording of MD neurons (blue dashed line). g, A sample trace of action potentials recorded from MD neurons in slice culture in response to ChR2 stimulation of the SC–MD pathway. h, Optical fibre placements for SC–MD photostimulation experiments.
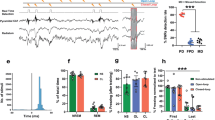
Extended Data Fig. 8 Single-unit recording of BLA neurons and their classification.
a, Coronal section (left) and illustration (right) showing the position of the recording site. LA, lateral nucleus of the amygdala; BA, basal nucleus of the amygdala. b, An example spike sorting showing clusters of candidate spikes (left) and average waveforms of four isolated units (right) from a single tetrode. c, Heat map and classified BLA responses during extinction trials (1-s bins; χ2(2) = 16.204, P = 0.0003029 (CS, n = 190; ABS + CS n = 227 cells). d, e, Average positive responses (d; CS, n = 67; ABS + CS, n = 63 cells) and negative responses (e; CS, n = 36; ABS + CS, n = 84 cells) in the BLA during fear extinction (1-s bins). Mann–Whitney U-test, two-sided: P = 0.3736 for positive responses; P = 0.296 for negative responses. f, g, Pearson’s correlation analysis of BLA positive responses (f; CS, n = 8, ABS + CS, n = 6 mice) or negative responses (g; CS, n = 8, ABS + CS, n = 9 mice) during fear extinction with average freezing level during spontaneous recovery and renewal. h, Proportions of the classified BLA responses (χ2(3) = 2.0536, P = 0.5613). i–k, Averaged pip responses (20-ms bins) of classified fear cells (i; CS, n = 34; ABS + CS, n = 42 cells), resistant cells (j; CS, n = 21; ABS + CS, n = 16 cells) and extinction cells (k; CS, n = 24; ABS + CS, n = 30 cells) during the first extinction trial (left) and the last extinction trial (right). l–n, Time course of averaged pip responses (left) and trial responses (right) of fear cells (l; samples from i), resistant cells (m; samples from j) and extinction cells (n; samples from k) during fear extinction (early, second-to-fifth trials; mid, sixth-to-tenth trials; late, eleventh-to-fifteenth trials). Mixed-design ANOVA for pip responses: F1,74 = 0.513, P = 0.476 for group effect of fear cells; F1,35 = 2.859, P = 0.0998 for group effect of resistant cells; F1,52 = 0.345, P = 0.559 for group effect of extinction cells. Mixed-design ANOVA for trial responses: F1,74 = 4.775, P = 0.032 for group effect of fear cells; F1,35 = 4.846, P = 0.0344 for group effect of resistant cells; F1,52 = 0.638, P = 0.428 for group effect of extinction cells. Mean ± s.e.m.; post hoc multiple comparison with Bonferroni correction. See Supplementary Table 1 for statistical details.
Extended Data Fig. 9 Freezing behaviour and correlation with BLA activity during fear extinction.
a, b, Fear extinction (a) and subsequent retention tests (b) with BLA single-unit recordings (CS, n = 8; ABS + CS, n = 9 mice). Mixed-design ANOVA for extinction: F1,15 = 19.46, P = 0.000505 for group effect. Mixed-design ANOVA for retention tests: F1,15 = 27.29, P = 0.000103 for group effect. Mean ± s.e.m.; post hoc multiple comparison with Bonferroni correction; **P < 0.01, ***P < 0.001. c–f, Pearson’s correlation analyses of fear-cell trial responses (CS, n = 8; ABS + CS, n = 9 mice) with freezing during late extinction trials (c; a block of the last three extinction trials), recall test (d), spontaneous recovery test (e) or renewal test (f). g–j, Pearson’s correlation analyses of resistant-cell trial responses (CS, n = 7; ABS + CS, n = 7 mice) with freezing during late extinction trials (g), recall test (h), spontaneous recovery test (i) or renewal test (j). k–n, Pearson’s correlation analyses of extinction-cell trial responses (CS, n = 8; ABS + CS, n = 7 mice) with freezing during late extinction trials (k), recall test (l), spontaneous recovery test (m) or renewal test (n). See Supplementary Table 1 for statistical details.
Extended Data Fig. 10 The MD drives feedforward inhibition in the BLA.
a, Fear extinction training for ex vivo mIPSC recordings in the BLA (conditioned (cond), n = 3; 1 d CS, n = 2; 1 d ABS + CS, n = 3; 7 d CS, n = 3; 7 d ABS + CS, n = 3 mice). Statistical analysis was not performed because of the small sample size. b, Optical fibre placements for MD–BLA silencing experiments. c, Viral injections used to visualize the MD–BLA projection. The results (d, e) were replicated with seven mice including five mice obtained after whole-cell recording (h). d, Coronal section under excitation with low laser power optimized for visualizing fluorescence in MD area. e, Coronal section under excitation with high laser power optimized for visualizing fluorescence in the BLA complex. CeA, central amygdala. f, Viral injection (top) and whole-cell recording (bottom) for the feedforward inhibition test. g, Sample traces evoked by photostimulation of MD fibres. h, Averaged latencies of EPSCs (B6/J, n = 7; Grik4-cre, n = 8 cells) and IPSCs (B6/J, n = 11; Grik4-cre, n = 6 cells) from the laser onset to 10% rise time. i, j, Light-evoked outward currents recorded at +10 mV were blocked by bicuculline (i) or CNQX and d-AP5 (j), indicating that recorded currents represent feedforward inhibition. k, Fear extinction training for ex vivo recording of MD–BLA synaptic transmission (CS, n = 3; ABS + CS, n = 3 mice). Mixed-design ANOVA: F1,4 = 7.305, P = 0.0539 for group effect. Data shown as mean ± s.e.m. See Supplementary Table 1 for statistical details.
Supplementary information
Supplementary Table
This table contains detailed statistical results.
Video 1: Fear extinction with ABS-paired CS reduces freezing behaviour.
The CS group (conventional extinction group) was presented only with the auditory CS during the whole extinction trials (a,b). The first extinction trial of the ABS-paired group (c) was presented only with the auditory CS. Then, the alternating bilateral sensory stimulation (ABS) was paired with the CS beginning at the second extinction trials (d).
Rights and permissions
About this article
Cite this article
Baek, J., Lee, S., Cho, T. et al. Neural circuits underlying a psychotherapeutic regimen for fear disorders. Nature 566, 339–343 (2019). https://doi.org/10.1038/s41586-019-0931-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-019-0931-y
This article is cited by
-
Eye Movement Desensitization and Reprocessing in der Behandlung psychosomatischer Störungsbilder
Die Psychotherapie (2025)
-
„Eye movement desensitization and reprocessing“ (EMDR) in der Schmerztherapie
Der Schmerz (2025)
-
How Fear Memory is Updated: From Reconsolidation to Extinction?
Neuroscience Bulletin (2025)
-
The impact of trauma core dimensions on anxiety and depression: a latent regression model through the Post-Traumatic Symptom Questionnaire (PTSQ)
Scientific Reports (2024)
-
Memory Trace for Fear Extinction: Fragile yet Reinforceable
Neuroscience Bulletin (2024)

Jeansok Kim
Anthropomorphizing and Neurobiologizing a Potential Treatment For Fear-related Disorders in Humans. A Commentary on Baek et al (2019) https://doi.org/10.1038/s41...
Jeansok J Kim1 and Joseph E. LeDoux2
1Department of Psychology, University of Washington, Seattle, WA 98195
2Center for Neural Science, New York University, New York, NY 10003
Animal research on threat processing in the brain can potentially contribute to the development of more effective, and non-invasive, treatments for psychopathologies such as phobias, posttraumatic stress disorder (PTSD), and other maladies related to fear. In a recent article “Neural circuits underlying a psychotherapeutic regimen for fear disorders” (21 Feb., p. 339), Hee-Sup Shin and his colleagues [1] reported that alternating bilateral sensory stimulation (ABS) significantly abolished what is often called "fear memory" in mice. The study was premised on Eye Movement Desensitization and Reprocessing (EMDR) psychotherapy, founded by psychologist Francine Shapiro [2]. In 1987, while walking in nature, she inadvertently made a self-discovery that her distressing thoughts dissipated as her eyes shifted back and forth, scanning her surroundings [3]. EMDR has, over the years, risen in popularity as a treatment for a host of fear-related illnesses, but also many other conditions, including schizophrenia, tinnitus, and eating disorders.
Since mice cannot be instructed to move their eyes back and forth while imagining dangerous events, Baek and colleagues devised a clever proxy of the human EMDR procedure. Mice were first trained with a 3 kHz tone and aversive footshock pairings (i.e., so-called Pavlovian fear conditioning). During extinction of the conditioned memory, repeated tone presentations were each accompanied by ABS involving bi-directional flashing lights. The results aligned with the therapeutic expectations of EMDR. Specifically, mice exposed to extinction-ABS pairings exhibited a significant decrease in auditory fear memory that did not return with a passage of time (spontaneous recovery), or with a change in environment (renewal). In contrast, animals that experienced standard extinction or unpaired extinction/ABS showed spontaneous recovery and renewal, effects which also occur after cognitive behavior therapy, which is, in part, based on extinction. Next, Shin and colleagues used state of the art methods (single unit recordings, optogenetics, genetically modified mouse) to demonstrate that a specific increase in tonic (but not bursting) neural activity in a specific circuit involving the superior colliculus-mediodorsal thalamus was both necessary and sufficient to produce lasting effects of ABS on the conditioned responses.
These findings, though impressive, need to be cautiously interpreted in relation to EMDR. For one thing, what is called "fear" in preclinical studies of animals typically conflates the experience of fear, which is a central part of human suffering in fear disorders, with behavioral responses to threats [4,5]. Ignoring such differences perpetuates anthropomorphic presuppositions about animal minds, and trivializes the complexity of human fear, which in no small measure depends on cognitive, social and cultural factors. Another important issue, not unrelated to the first, is that the effectiveness of EMDR relative to other treatments is still the subject of considerable debate [6-9], in part because the underlying mechanisms of psychotherapies in general, and EMDR in particular, has been elusive. The suggestion that a specific mechanism underlying EMDR has been discovered in mice might tip the balance in the debate, but for the wrong reason. While the subcortical circuits implicated in the mouse study are common between rodents and
humans, the experience of fear itself may well involve cortical circuits that
are lacking in mice. One important outcome of the mouse study would be a movement in the EMDR community to attempt to determine the degree to which positive effects of EMDR are due to a direct reduction of the experience of fear, as opposed to changes in behavioral and physiological effects (such as a reduction in pathological avoidance and/or hyperarousal), which might secondarily affect the experience of fear.
References
1. Baek J, Lee S, Cho T, Kim S-W, Kim M, Yoon Y, Kim KK, Byun J, Kim SJ, Jeong J, Shin H-S (2019) Neural circuits underlying a psychotherapeutic regimen for fear disorders. Nature 566:339-343.
2. Shapiro F (2018) Eye Movement Desensitization and Reprocessing (EMDR) Therapy (third edition). The Guilford Press: New York.
3. Lilienfeld SO, Arkowitz H (2007) Can moving your eyes back and forth help to ease anxiety? Scientific American 17:10-11.
4. LeDoux JE (2014) Coming to terms with fear. Proceedings of the National Academy of Sciences of the United States of America 111:2871-2878.
5. LeDoux JE, Pine DS (2016) Using neuroscience to help understand fear and anxiety: a two-system framework. American Journal of Psychiatry 173:1083-1093.
6. Herbert JD, Lilienfeld SO, Lohr JM, Montgomery RW, O’Donohue WT, Rosen GM, Tolin DF (2000) Science and pseudoscience in the development of eye movement desensitization of eye movement desensitization and
reprocessing: implications for clinical psychology. Clinical Psychology Review 20:945-971.
7. Davidson PR, Parker KC (2002) Eye movement desensitization and reprocessing (EMDR): a meta-analysis. Journal of Consulting and Clinical Psychology 69:305-316.
8. Chen L, Zhang G, Hu M, Liang X (2015) Eye movement desensitization and reprocessing versus cognitive-behavioral therapy for adult posttraumatic stress disorder: systematic review and meta-analysis. The Journal of Nervous and Mental Disease 203:443-451.
9. van den Berg DPG, de Bont PAJM, van der Vleugel BM, de Roos C, de Jongh A, Van Minnen A, van der Gaag M (2015) Prolonged exposure vs eye movement desensitization and reprocessing vs waiting list for posttraumatic stress disorder in patients with a psychotic disorder: a randomized clinical trial. JAMA Psychiatry 72:259-267.
J.J.K. and J.E.L. discussed the content and wrote the comments.
Lori Zoellner
Lost in Translation—Rodents Watching Flashing LED Chips to Treat Their PTSD: A Clinical Comment on Baek et al. (2019)
Lori A. Zoellner1, David F. Tolin2, Jonathan S. Abramowitz3, & Norah C. Feeny4
1Department of Psychology, University of Washington, Seattle, WA 98195
2Anxiety Disorders Center, The Institute of Living/Hartford Hospital, Hartford, CT 06106
3Department of Psychology and Neuroscience, University of North Carolina, Chapel Hill, Chapel Hill, NC 27599
4Department of Psychological Science, Case Western Reserve University, Cleveland, OH 44106
As experts in the treatment of fear-based disorders, we excitedly look to our colleagues in behavioral neuroscience to explore unique mechanisms of change, helping isolate neural processes and make our interventions more effective and durable. The recent article by Jinhee Baek and colleagues “Neural Circuits Underlying a Psychotherapeutic Regimen for Fear Disorders” (21 Feb., p. 339), premised on Eye Movement Desensitization and Reprocessing (EMDR) psychotherapy, argues that alternating bilateral sensory stimulation (ABS) abolished "fear memory" in mice [1]. This is a strong claim. Its premises and extrapolation to psychotherapy leave us simply “lost in the translation” between basic science and applied clinical science and make us question the broader relevance of their findings.
Though the Baek et al. study was based on the premise of EMDR as a treatment for PTSD, it is noted that other treatments—specifically, cognitive processing therapy (CPT) and prolonged exposure (PE)—have better empirical support as first line interventions for PTSD [2-4]. The vast majority of those who receive these therapies (91.6%) make reliable improvements in symptoms [5] which are retained for five to ten years after treatment, and only a minority retain their PTSD diagnosis at this long-term follow-up (22.2% of CPT, 17.5% of PE) [6]. Moreover, optimistic estimates of evidence quality for CPT and PE suggest that the efficacy of both substantially and meaningfully outstrip the efficacy of EMDR [7]. Thus, the extant literature does not substantiate the argument that EMDR confers superior or more durable outcomes than other trauma-focused psychotherapies, as implied by Baek et al.
We also note that Baek et al. focus on the role of eye movements in the efficacy of EMDR therapy. EMDR is a multicomponent intervention primarily involving (a) repeatedly focusing on traumatic memories, and (b) visually tracking the therapist's back-and-forth finger movements. Thus, an important scientific question is “What is the active ingredient in EMDR: exposure to the traumatic memory or the lateral eye movements?” Notably, in humans, the evidence suggests that the lateral eye movements do not make a contribution over and above the effects of exposure on key outcomes such as PTSD severity [8,9]. Mechanistically, eye movements likely reflect nothing substantial or simply a distraction-like task [10].
We propose that rather than focusing on second-tier treatments [11] with dubious mechanisms of action, translational science would be best served by focusing on neurobiological mechanisms of well-established PTSD treatments such as CPT and PE and by developing more ecologically valid fear conditioning and extinction paradigms. Helpful research would include studies of optimal ways of engaging frontal and hippocampal regions, which modulate amygdala responses to conditioned stimuli [12]. Principles of inhibitory learning [13,14] suggest potential modifications to exposure therapy that maximize prefrontal cortex (PFC) engagement by developing new, non-threat associations and by enhancing retrieval of these newly-learned associations. In addition, continued research into the processes of memory consolidation/reconsolidation and neuronal plasticity would be helpful. Finally, compounds that promote brain activity implicated in extinction consolidation and recall such as yohimbine, an 2-adrenergic receptor antagonist [15], methylene blue, an autoxidizing agent [16], and d-cycloserine (DCS) a partial NMDA receptor agonist [17], serve as proof of concept that the biological mechanisms of cognitive behavioral therapies can be potentiated [18].
The animal model of PTSD used by Baek et al. may not be appropriate for modeling this complex human disorder. PTSD is characterized by persistent fear that is resistant to extinction, often following an evolutionary-primed conditioning event (e.g., sexual assault) with potent visual, auditory, olfactory, and somatic stimuli. Multiple footshocks in rodents simply do not capture this complexity of “fear memory” in humans. Further, individual differences are seldom modeled in rodent paradigms. The vast majority of individuals after powerful conditioning events do not develop chronic psychopathology; rather, the majority experience natural recovery [19,20]. Indeed, PTSD is often considered a failure of natural extinction [21,22]; yet resistance to initial extinction is simply not modeled. Finally, in humans, the majority of conditioned stimuli (CS) and contexts have some evolutionary-primed, negative valence prior to conditioning (e.g., dark alley behind a bar). “CSs” in humans are not typically arbitrary, neutral stimuli (e.g., auditory tone), rather they are often ambiguous and prone to overgeneralization. A “fear memory” in a rodent as implied by the present study simply does not capture the clinical complexity of fear memories seen in PTSD.
Baek et al. go well beyond their data when they conclude that (a) ABS abolished the “fear memory” in rodents and (b) their study provides a neurobiological basis substantiating durable gains of bilateral eye movements in EMDR. Moreover, EMDR does not have as strong of empirical support as other interventions, and its purported mechanisms are not well supported. Thus, suggesting these results support an “…animal model for psychotherapy…” is akin to rodents talking to a psychotherapist—the translation simply fails.
References
1. Baek J, Lee S, Cho T, Kim S-W, Kim M, Yoon Y, Kim KK, Byun J, Kim SJ, Jeong J, Shin H-S (2019) Neural circuits underlying a psychotherapeutic regimen for fear disorders. Nature 566:339-343.
2. Cusack K, Jonas DE, Forneris CA, Wines C, Sonis J., Middleton JC, Feltner C, Brownley KA, Olmsted KR, Greenblatt A, Weil A, Gaynes BN (2016) Psychological treatments for adults with posttraumatic stress disorder: a systematic review and meta-analysis. Clin Psychol Rev 43:128-141.
3. Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, Foa EB (2010) A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin Psychol Rev 30(6):635-641.
4. Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, & Friedman MJ (2013) Meta analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry 74(6):e551-e557. doi:10.4088/JCP.12r08225
5. Jayawickreme N, Cahill SP, Riggs DS, Rauch SA, Resick PA, Rothbaum BO, Foa, EB (2014) Primum non nocere (first do no harm): symptom worsening and improvement in female assault victims after prolonged exposure for PTSD. Depress Anxiety 31(5):412-419.
6. Resick PA, Williams LF, Suvak MK, Monson CM, Gradus JL (2012) Long-term outcomes of cognitive–behavioral treatments for posttraumatic stress disorder among female rape survivors. J Consult Clin Psychol 80(2):201–210. doi:10.1037/a0026602
7. Sakaluk JK, Williams A, Kilshaw R, Rhyner KT (2019) Evaluating the evidential value of empirically supported psychological treatments (ESTs): a meta-scientific review. J Abnorm Psychol.
8. Lee CW, Cuijpers P (2013) A meta-analysis of the contribution of eye movements in processing emotional memories. J Behav Ther Exp Psychiatry 44(2):231-239.
9. Devilly GJ, Ono M, Lohr JM (2014) The use of meta-analytic software to derive hypotheses for EMDR. J Behav Ther Exp Psychiatry 45:223–225.
10. Engelhard IM, McNally RJ, van Schie K (2019) Retrieving and modifying traumatic memories: Recent research relevant to three controversies. Curr Dir Psychol Sci 28(1):91–96.
11. American Psychological Association (2017) Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. https://www.apa.org/ptsd-gu...
12. Delgado MR, Nearing KI, Ledoux JE, Phelps EA (2008) Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron 59(5):829-838. doi:S08966273(08)00579-5 [pii] 10.1016/j.neuron.2008.06.029
13. Bouton ME (1993) Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psycholol Bull 114(1):80-99.
14. Craske MG, Treanor M, Conway CC, Zbozinek T, & Vervliet B (2014) Maximizing exposure therapy: an inhibitory learning approach. Behav Res Ther 58:10-23. doi:10.1016/j.brat.2014.04.006
15. Smits JA, Rosenfield D, Davis ML, Julian K, Handelsman PR, Otto MW, Tuerk P, Shiekh M, Rosenfield B, Hofmann SG, Powers MB (2014) Yohimbine enhancement of exposure therapy for social anxiety disorder: a randomized controlled trial. Biol Psychiatry 75:840-846.
16. Zoellner LA, Telch M, Foa EB, Farach FJ, McLean CP, Gallop R, Bluett EJ, Cobb A, Gonzalez-Lima F (2017) Enhancing extinction learning in posttraumatic stress disorder with brief daily imaginal exposure and methylene blue: a randomized controlled trial. J Clin Psychiatry 78(7):e782-e789.
17. Otto MW, Pollack MH, Dowd SM, Hofmann SG, Pearlson G, Szuhany KL, Gueorguieva R, Krystal JH, Simon NM, Tolin DF (2016) Randomized trial of d‐cycloserine enhancement of cognitive‐behavioral therapy for panic disorder. Depress Anxiety 33(8):737-745.
18. Mataix-Cols D, Fernandez de la Cruz L, Monzani B, Rosenfield D, Andersson E, Perez-Vigil A, et al. (2017) D-cycloserine augmentation of exposure-based cognitive behavior therapy for anxiety, obsessive-compulsive, and posttraumatic stress disorders: A systematic review and meta-analysis of individual participant data. JAMA Psychiatry, 74(5):501-510. doi:10.1001/jamapsychiatry.2016.3955
19. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995) Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52(12):1048-60
20. Rothbaum BO, Foa EB, Riggs DS, Murdock T, Walsh W (1992) A prospective examination of post‐traumatic stress disorder in rape victims. J Trauma Stress 5(3):455-475.
21. Mahan AL, Ressler KJ (2012) Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci 35(1):24-35.
22. Maren S, Holmes A. Stress and fear extinction (2016) Neuropsychopharmacology 41(1):58.
L. A. Z., D. F. T., J. S. A., and N.C.F. discussed the content and wrote the comments.