Abstract
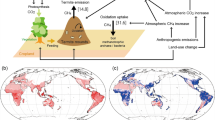
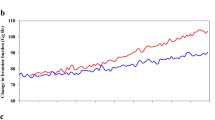
Atmospheric methane (CH4) is a potent greenhouse gas, and its mole fraction has more than doubled since the preindustrial era1. Fossil fuel extraction and use are among the largest anthropogenic sources of CH4 emissions, but the precise magnitude of these contributions is a subject of debate2,3. Carbon-14 in CH4 (14CH4) can be used to distinguish between fossil (14C-free) CH4 emissions and contemporaneous biogenic sources; however, poorly constrained direct 14CH4 emissions from nuclear reactors have complicated this approach since the middle of the 20th century4,5. Moreover, the partitioning of total fossil CH4 emissions (presently 172 to 195 teragrams CH4 per year)2,3 between anthropogenic and natural geological sources (such as seeps and mud volcanoes) is under debate; emission inventories suggest that the latter account for about 40 to 60 teragrams CH4 per year6,7. Geological emissions were less than 15.4 teragrams CH4 per year at the end of the Pleistocene, about 11,600 years ago8, but that period is an imperfect analogue for present-day emissions owing to the large terrestrial ice sheet cover, lower sea level and extensive permafrost. Here we use preindustrial-era ice core 14CH4 measurements to show that natural geological CH4 emissions to the atmosphere were about 1.6 teragrams CH4 per year, with a maximum of 5.4 teragrams CH4 per year (95 per cent confidence limit)—an order of magnitude lower than the currently used estimates. This result indicates that anthropogenic fossil CH4 emissions are underestimated by about 38 to 58 teragrams CH4 per year, or about 25 to 40 per cent of recent estimates. Our record highlights the human impact on the atmosphere and climate, provides a firm target for inventories of the global CH4 budget, and will help to inform strategies for targeted emission reductions9,10.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Code availability
The code for the firn air inverse model and atmospheric box model (MATLAB) is available from the corresponding author upon request.
References
Meinshausen, M. et al. Historical greenhouse gas concentrations for climate modelling (CMIP6). Geosci. Model Dev. 10, 2057–2116 (2017).
Saunois, M. et al. The global methane budget 2000–2012. Earth Syst. Sci. Data 8, 697–751 (2016).
Schwietzke, S. et al. Upward revision of global fossil fuel methane emissions based on isotope database. Nature 538, 88–91 (2016); corrigendum 543, 452 (2017).
Lassey, K. R., Etheridge, D. M., Lowe, D. C., Smith, A. M. & Ferretti, D. F. Centennial evolution of the atmospheric methane budget: what do the carbon isotopes tell us? Atmos. Chem. Phys. 7, 2119–2139 (2007).
Zazzeri, G., Acuña Yeomans, E. & Graven, H. D. Global and regional emissions of radiocarbon from nuclear power plants from 1972 to 2016. Radiocarbon 60, 1067–1081 (2018).
Etiope, G. Natural Gas Seepage: The Earth's Hydrocarbon Degassing Vol. 1 (Springer International Publishing, 2015).
Etiope, G., Ciotoli, G., Schwietzke, S. & Schoell, M. Gridded maps of geological methane emissions and their isotopic signature. Earth Syst. Sci. Data 11, 1–22 (2019).
Petrenko, V. V. et al. Minimal geological methane emissions during the Younger Dryas–Preboreal abrupt warming event. Nature 548, 443–446 (2017).
Höglund-Isaksson, L. Global anthropogenic methane emissions 2005–2030: technical mitigation potentials and costs. Atmos. Chem. Phys. 12, 9079–9096 (2012).
Howarth, R. W. Methane emissions and climatic warming risk from hydraulic fracturing and shale gas development: implications for policy. Eng. Emis. Con. Tech. 3, 45–54 (2015).
Nicewonger, M. R., Aydin, M., Prather, M. J. & Saltzman, E. S. Large changes in biomass burning over the last millennium inferred from paleoatmospheric ethane in polar ice cores. Proc. Natl Acad. Sci. USA 115, 12413–12418 (2018).
Bock, M. et al. Glacial/interglacial wetland, biomass burning, and geologic methane emissions constrained by dual stable isotopic CH4 ice core records. Proc. Natl Acad. Sci. USA 114, E5778–E5786 (2017).
Lassey, K. R., Lowe, D. C. & Smith, A. M. The atmospheric cycling of radiomethane and the “fossil fraction” of the methane source. Atmos. Chem. Phys. 7, 2141–2149 (2007).
Etiope, G., Milkov, A. V. & Derbyshire, E. Did geologic emissions of methane play any role in Quaternary climate change? Global Planet. Change 61, 79–88 (2008).
Petrenko, V. V. et al. Measurements of 14C in ancient ice from Taylor Glacier, Antarctica constrain in situ cosmogenic 14CH4 and 14CO production rates. Geochim. Cosmochim. Ac. 177, 62–77 (2016).
Petrenko, V. V. et al. 14CH4 measurements in Greenland Ice: investigating last glacial termination CH4 sources. Science 324, 506–508 (2009).
Severinghaus, J. P. et al. Deep air convection in the firn at a zero-accumulation site, central Antarctica. Earth Planet. Sci. Lett. 293, 359–367 (2010).
Buizert, C. et al. Gas transport in firn: multiple-tracer characterisation and model intercomparison for NEEM, Northern Greenland. Atmos. Chem. Phys. 12, 4259–4277 (2012).
Rommelaere, V., Arnaud, L. & Barnola, J.-M. Reconstructing recent atmospheric trace gas concentrations from polar firn and bubbly ice data by inverse methods. J. Geophys. Res. Atmos. 102, 30069–30083 (1997).
Trudinger, C. et al. Reconstructing atmospheric histories from measurements of air composition in firn. J. Geophys. Res. Atmos. 107, (2002).
Smil, V. Energy Transitions: Global and National Perspectives (ABC-CLIO, 2016).
Hua, Q., Barbetti, M. & Rakowski, A. Z. Atmospheric radiocarbon for the period 1950–2010. Radiocarbon 55, 2059–2072 (2013).
Etiope, G. & Klusman, R. W. Microseepage in drylands: flux and implications in the global atmospheric source/sink budget of methane. Global Planet. Change 72, 265–274 (2010).
McGinnis, D. F., Greinert, J., Artemov, Y., Beaubien, S. & Wüest, A. Fate of rising methane bubbles in stratified waters: how much methane reaches the atmosphere? J. Geophys. Res. Oceans 111, C09007 (2006).
Leonte, M. et al. Rapid rates of aerobic methane oxidation at the feather edge of gas hydrate stability in the waters of Hudson Canyon, US Atlantic Margin. Geochim. Cosmochim. Acta 204, 375–387 (2017).
Sparrow, K. J. et al. Limited contribution of ancient methane to surface waters of the U.S. Beaufort Sea shelf. Sci. Adv. 4, eaao4842 (2018).
Nicewonger, M. R., Verhulst, K. R., Aydin, M. & Saltzman, E. S. Preindustrial atmospheric ethane levels inferred from polar ice cores: a constraint on the geologic sources of atmospheric ethane and methane. Geophys. Res. Lett. 43, 214–221 (2016).
Alvarez, R. A. et al. Assessment of methane emissions from the U.S. oil and gas supply chain. Science 361, 186–188 (2018).
Acknowledgements
This work was supported by US NSF awards OPP-1203779 (V.V.P.) OPP-1203686, OPP-0230452, ANT-0839031 (J.P.S.) ARC-1204084, ARC-1702920 (C.B.), a Packard Fellowship for Science and Engineering (V.V.P.), the National Institute of Water and Atmospheric Research through the Greenhouse Gases, Emissions and Carbon Cycle Science Programme (T.B.) and the Australian Government for the Centre for Accelerator Science at ANSTO through the National Collaborative Research Infrastructure Strategy (A.M.S.). We thank J. McConnell and P. Vallelonga for the interpretation of the ice core CFA data; P. Neff and E. Steig for sharing the ice-thinning model code; L. Davidge, J. Edwards, M. Pacicco and A. Adolph for assistance with firn air and ice core sampling; M. Jayred, L. Albershardt, T. Kuhl, D. Kirkpatrick and the US Ice Drilling programme for ice-drilling support; K. Gorham, J. Jenkins, D. Einerson, Polar Field Services and the 109th New York Air National Guard for logistical support; the Australian Antarctic Science Program for supporting the Law Dome drilling and firn air sampling and CSIRO GASLAB, in particular R. Langenfelds, for analysis of the firn air sample trace gas concentrations.
Author information
Authors and Affiliations
Contributions
B.H. and V.V.P. designed the study and conducted field logistical and scientific preparations; B.H., V.V.P., M.N.D., C.B., P.F.P., R.B., J.S. and X.F. collected samples at Summit; B.H. measured [CO] and extracted CH4 and CO from firn air and ice core samples; C.B. developed the firn modelling code; B.H. and M.N.D. developed the box-model calculations; Q.H. and B.Y. graphitized the 14C samples; A.M.S. measured 14C; P.F.P. and I.V. measured δ13CO; S.E.M. measured δ13CH4; C.H. measured [CH4] and halogenated trace gases under the supervision of R.F.W.; E.D. supervised the firn air trace gas measurements; J.P.S. measured δXe/Kr, δKr/N2, δXe/N2 and δNe/N2 and collected Megadunes firn air samples; R.B. measured the δ15N of N2, the δ18O of O2, δO2/N2 and δAr/N2; D.E. collected and supervised the analyses of the Law Dome firn air samples; T.B. extracted CH4 from Megadunes and Law Dome samples; B.H. and V.V.P. analysed the data and B.H. drafted the manuscript with contribution from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains details regarding ice core and firn air sample collection and analyses, procedural and in situ cosmogenic corrections for 14CH4, forward and inverse modeling of firn air and ice core data, atmospheric box modeling of 14CH4 and 13CH4. It also contains 5 Supplementary Figures and 8 Supplementary Tables.
Rights and permissions
About this article
Cite this article
Hmiel, B., Petrenko, V.V., Dyonisius, M.N. et al. Preindustrial 14CH4 indicates greater anthropogenic fossil CH4 emissions. Nature 578, 409–412 (2020). https://doi.org/10.1038/s41586-020-1991-8
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-020-1991-8
This article is cited by
-
Improved estimation of carbon dioxide and methane using machine learning with satellite observations over the Arabian Peninsula
Scientific Reports (2025)
-
Methane-rich gas emissions from natural geologic seeps can be chemically distinguished from anthropogenic leaks
Communications Earth & Environment (2025)
-
Radiocarbon monoxide indicates increasing atmospheric oxidizing capacity
Nature Communications (2025)
-
Methane emissions decreased in fossil fuel exploitation and sustainably increased in microbial source sectors during 1990–2020
Communications Earth & Environment (2024)
-
Widespread natural methane and oil leakage from sub-marine Arctic reservoirs
Nature Communications (2023)


Deguello
Methane is methane whether it comes from the ground or tundra, a rotting tree/organism, a cow or termites, and the gas is not a threat to the climate. Just because a molecule contains carbon and is capable of capturing heat energy in a laboratory does not mean it captures heat energy in the atmosphere. There also has to be energy present in the absorbable radiation bands upon which the molecule works for energy capture to be in the equation. That is where and why ambient methane fails to collect energy. It is competing with a vastly more prevalent gas in its limited sphere of influence.
Before you permit yourself to get all scare-defied over more methane being released into the atmosphere, and even if you buy into recent (since WWII) surface temperature rise being as a result of increased greenhouse gasses, do your research and find that methane is an irrelevant gas in the theoretical causes because of the limited bands of energy from which it can possibly absorb and from those two bands upon which it can act, it must share that potential with one more prevalent, which has already done the job almost completely in those bands leaving nothing much for methane to work upon. Those who promote gloom & doom from impending release of stores of methane wrongly assume the gas would have unlimited stores of energy upon which it could draw to heat the planet should that release occur. Therein lies the failure of this sub-theory even assuming such release is possible and imminent. There is no such pool of energy.
The energy beamed by the sun comes to Earth in the form of short waves, is absorbed by the planet, and some is transmitted back to space in the form of long waves in various bands of energy. Warmists' Anthropogenic Global Warming Theory holds that greenhouse gasses intercept by absorption and transmit back to Earth a percentage of the long wave radiation energy in natural balance until humans destroy the balance by over supplying unnatural amounts of greenhouse gasses by which such process and added heat causes more of the principle greenhouse gas, water vapor, to be produced accelerating the process in an ever heightening loop of heating Gaia. Methane is a "greenhouse gas." The misnamed process acts nothing like a greenhouse, BTW, and empirical measurements, the acid test of science, do not reflect water vapor increasing as required in proportion to CO2 increases or even out of proportion. No increase of water vapor at all in fact has been measured among the several failures of the theory to be sustained by empirical measurement.
Methane (CH4) by its physical properties has only two narrow absorption bands at 3.3 microns and 7.5 microns in the overall broad electro-magnetic spectrum from which it can absorb energy. Theoretically, CH4 is 20 times more effective an absorber than CO2 – in those bands in a laboratory. However, CH4 is only 0.00017% (1.7 parts per million) of the atmosphere. Moreover, both of its bands occur at wavelengths where H2O is already absorbing virtually all energy. Because water vapor is much more plentiful in the atmosphere than methane (or any other GHG), H2O absorbs vastly more energy and is by far the most important greenhouse gas. On any given day, H2O is a percent or two of the atmosphere (1.0-2.0% or 5,882 to 11,764 times as prevalent as methane in the atmosphere, or 5882÷20=294.1 [or 588.4] multiple the absorber as methane); we call that humidity. Hence, any radiation that CH4 might absorb has already been absorbed by H2O in the only radiation bands methane absorbs energy. Once the energy in a band of the spectrum has been sucked dry, no additional absorptive gas can absorb more. Painting a black window another coat will not keep out more light. In other words, the ratio of the percentages of water to methane is such that the effects of CH4 are completely masked by H2O because the absorption of infrared energy in the bands of the spectrum affected by methane has already been saturated by H2O absorption. The amount of CH4 would have to increase at least 300-fold to make it comparable to H2O and even then it would no longer matter because water vapor has beat it to the punch.
There is not much ambient energy in those two little short, stray bands of the radiation spectrum to start with and most of that has already been worked over by H2O from time immemorial leaving only the scraps to poor CH4, which can never effect climate to any appreciable or worrisome amount. Because it absorbs energy in a laboratory does not mean it works that way in a chaotic atmosphere with other agents and processes present.