Abstract
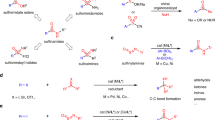
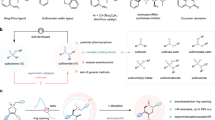
Achiral sulfur functional groups, such as sulfonamide, sulfone, thiol and thioether, are common in drugs and natural products. By contrast, chiral sulfur functional groups are often neglected as pharmacophores1,2,3, although sulfoximine, with its unique physicochemical and pharmacokinetic properties4,5, has been recently incorporated into several clinical candidates. Thus, other sulfur stereogenic centres, such as sulfinate ester, sulfinamide, sulfonimidate ester and sulfonimidamide, have started to attract attention. The diversity and complexity of these sulfur stereogenic centres have the potential to expand the chemical space for drug discovery6,7,8,9,10. However, the installation of these structures enantioselectively into drug molecules is highly challenging. Here we report straightforward access to enantioenriched sulfinate esters via asymmetric condensation of prochiral sulfinates and alcohols using pentanidium as an organocatalyst. We successfully coupled a wide range of sulfinates and bioactive alcohols stereoselectively. The initial sulfinates can be prepared from existing sulfone and sulfonamide drugs, and the resulting sulfinate esters are versatile for transformations to diverse chiral sulfur pharmacophores. Through late-stage diversification11,12 of celecoxib and other drug derivatives, we demonstrate the viability of this unified approach towards sulfur stereogenic centres.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Tilby, M. J. & Willis, M. C. How do we address neglected sulfur pharmacophores in drug discovery? Expert Opin. Drug Discov. 16, 1227–1231 (2021).
Lücking, U. Neglected sulfur(VI) pharmacophores in drug discovery: exploration of novel chemical space by the interplay of drug design and method development. Org. Chem. Front. 6, 1319–1324 (2019).
Kitamura, S. et al. Sulfur(VI) fluoride exchange (SuFEx)-enabled high-throughput medicinal chemistry. J. Am. Chem. Soc. 142, 10899–10904 (2020).
Mader, P. & Kattner, L. Sulfoximines as rising stars in modern drug discovery? Current status and perspective on an emerging functional group in medicinal chemistry. J. Med. Chem. 63, 14243–14275 (2020).
Han, Y. et al. Application of sulfoximines in medicinal chemistry from 2013 to 2020. Eur. J. Med. Chem. 209, 112885 (2021).
Koizumi, M., Hiratake, J., Nakatsu, T., Kato, H. & Oda, J. I. A potent transition-state analogue inhibitor of Escherichia coli asparagine synthetase A. J. Am. Chem. Soc. 121, 5799–5800 (1999).
Altenburg, B. et al. Chiral analogues of PFI-1 as BET inhibitors and their functional role in myeloid malignancies. ACS Med. Chem. Lett. 11, 1928–1934 (2020).
Foote, K. M. et al. Discovery and characterization of AZD6738, a potent inhibitor of ataxia telangiectasia mutated and rad3 related (ATR) kinase with application as an anticancer agent. J. Med. Chem. 61, 9889–9907 (2018).
Lücking, U. et al. The lab oddity prevails: discovery of pan-CDK inhibitor (R)-S-cyclopropyl-S-(4-{[4-{[(1R,2R)-2-hydroxy-1-methylpropyl]oxy}-5-(trifluorome thyl)pyrimidin-2-yl]amino}phenyl)sulfoximide (BAY 1000394) for the treatment of cancer. ChemMedChem 8, 1067–1085 (2013).
Bentley, R. Role of sulfur chirality in the chemical processes of biology. Chem. Soc. Rev. 34, 609–624 (2005).
Börgel, J. & Ritter, T. Late-stage functionalization. Chem 6, 1877–1887 (2020).
Guillemard, L., Kaplaneris, N., Ackermann, L. & Johansson, M. J. Late-stage C–H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem. 5, 522–545 (2021).
Schreiber, S. L. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science 287, 1964–1969 (2000).
Galloway, W. R., Isidro-Llobet, A. & Spring, D. R. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Commun. 1, 80 (2010).
Quasdorf, K. W. & Overman, L. E. Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature 516, 181–191 (2014).
Feng, M., Tang, B., Liang, S. H. & Jiang, X. Sulfur containing scaffolds in drugs: synthesis and application in medicinal chemistry. Curr. Top. Med. Chem. 16, 1200–1216 (2016).
Finn, P., Charlton, M., Edmund, G., Jirgensons, A., & Loza, E. 2-Amino-N-(arylsulfinyl)-acetamide compounds as inhibitors of bacterial aminoacyl-trna synthetase. patent WO2018065611A1 (2018).
Chinthakindi, P. K. et al. Sulfonimidamides in medicinal and agricultural chemistry. Angew. Chem. Int. Ed. 56, 4100–4109 (2017).
Yu, H., Li, Z. & Bolm, C. Copper-catalyzed transsulfinamidation of sulfinamides as a key step in the preparation of sulfonamides and sulfonimidamides. Angew. Chem. Int. Edn 57, 15602–15605 (2018).
Chatterjee, S., Makai, S. & Morandi, B. Hydroxylamine-derived reagent as a dual oxidant and amino group donor for the iron-catalyzed preparation of unprotected sulfinamides from thiols. Angew. Chem. Int. Edn 60, 758–765 (2021).
Davies, T. Q. et al. Harnessing sulfinyl nitrenes: a unified one-pot synthesis of sulfoximines and sulfonimidamides. J. Am. Chem. Soc. 142, 15445–15453 (2020).
Wojaczynska, E. & Wojaczynski, J. Modern stereoselective synthesis of chiral sulfinyl compounds. Chem. Rev. 120, 4578–4611 (2020).
Aota, Y., Kano, T. & Maruoka, K. Asymmetric synthesis of chiral sulfoximines via the S-arylation of sulfinamides. J. Am. Chem. Soc. 141, 19263–19268 (2019).
Mendonca Matos, P., Lewis, W., Argent, S. P., Moore, J. C. & Stockman, R. A. General method for the asymmetric synthesis of N–H sulfoximines via C–S bond formation. Org. Lett. 22, 2776–2780 (2020).
Greed, S. et al. Synthesis of highly enantioenriched sulfonimidoyl fluorides and sulfonimidamides by stereospecific sulfur–fluorine exchange (SuFEx) reaction. Chem. Eur. J. 26, 12533–12538 (2020).
Dong, S. et al. Organocatalytic kinetic resolution of sulfoximines. J. Am. Chem. Soc. 138, 2166–2169 (2016).
Brauns, M. & Cramer, N. Efficient kinetic resolution of sulfur-stereogenic sulfoximines by exploiting CpXRhIII-catalyzed C–H functionalization. Angew. Chem. Int. Edn 58, 8902–8906 (2019).
Shen, B., Wan, B. & Li, X. Enantiodivergent desymmetrization in the rhodium(III)-catalyzed annulation of sulfoximines with diazo compounds. Angew. Chem. Int. Edn 57, 15534–15538 (2018).
Sun, Y. & Cramer, N. Enantioselective synthesis of chiral-at-sulfur 1,2-benzothiazines by CpX RhIII-catalyzed C–H functionalization of sulfoximines. Angew. Chem. Int. Edn 57, 15539–15543 (2018).
Zhou, T. et al. Efficient synthesis of sulfur-stereogenic sulfoximines via Ru(II)-catalyzed enantioselective C–H functionalization enabled by chiral carboxylic acid. J. Am. Chem. Soc. 143, 6810–6816 (2021).
Tang, Y. & Miller, S. J. Catalytic enantioselective synthesis of pyridyl sulfoximines. J. Am. Chem. Soc. 143, 9230–9235 (2021).
Tilby, M. J., Dewez, D. F., Hall, A., Martinez Lamenca, C. & Willis, M. C. Exploiting configurational lability in aza-sulfur compounds for the organocatalytic enantioselective synthesis of sulfonimidamides. Angew. Chem. Int. Edn 60, 25680–25687 (2021).
Kagan, H. B. & Rebiere, F. Some routes to chiral sulfoxides with very high enantiomeric excesses. Synlett 1990, 643–650 (1990).
Fernandez, I., Khiar, N., Llera, J. M. & Alcudia, F. Asymmetric synthesis of alkane- and arenesulfinates of diacetone-D-glucose (DAG): an improved and general route to both enantiomerically pure sulfoxides. J. Org. Chem. 57, 6789–6796 (1992).
Lu, B. Z. et al. New general sulfinylating process for asymmetric synthesis of enantiopure sulfinates and sulfoxides. Org. Lett. 7, 1465–1468 (2005).
Evans, J. W., Fierman, M. B., Miller, S. J. & Ellman, J. A. Catalytic enantioselective synthesis of sulfinate esters through the dynamic resolution of tert-butanesulfinyl chloride. J. Am. Chem. Soc. 126, 8134–8135 (2004).
Shibata, N. et al. Cinchona alkaloid/sulfinyl chloride combinations: enantioselective sulfinylating agents of alcohols. J. Am. Chem. Soc. 127, 1374–1375 (2005).
Peltier, H. M., Evans, J. W. & Ellman, J. A. Catalytic enantioselective sulfinyl transfer using cinchona alkaloid catalysts. Org. Lett. 7, 1733–1736 (2005).
Zong, L. & Tan, C. H. Phase-transfer and ion-pairing catalysis of pentanidiums and bisguanidiniums. Acc. Chem. Res. 50, 842–856 (2017).
Zhang, X. et al. An enantioconvergent halogenophilic nucleophilic substitution (SN2X) reaction. Science 363, 400–404 (2019).
Fujiwara, Y. et al. Practical and innate carbon-hydrogen functionalization of heterocycles. Nature 492, 95–99 (2012).
Smith, J. M., Dixon, J. A., deGruyter, J. N. & Baran, P. S. Alkyl sulfinates: radical precursors enabling drug discovery. J. Med. Chem. 62, 2256–2264 (2019).
Goh, J., Maraswami, M. & Loh, T. P. Synthesis of vinylic sulfones in aqueous media. Org. Lett. 23, 1060–1065 (2021).
Fier, P. S. & Maloney, K. M. NHC-catalyzed deamination of primary sulfonamides: a platform for late-stage functionalization. J. Am. Chem. Soc. 141, 1441–1445 (2019).
Gauthier, D. R. Jr & Yoshikawa, N. A general, one-pot method for the synthesis of sulfinic acids from methyl sulfones. Org. Lett. 18, 5994–5997 (2016).
DiRocco, D. A. et al. A multifunctional catalyst that stereoselectively assembles prodrugs. Science 356, 426–430 (2017).
Wang, Y. et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395, 1569–1578 (2020).
Slusarczyk, M. et al. Application of ProTide technology to gemcitabine: a successful approach to overcome the key cancer resistance mechanisms leads to a new agent (NUC-1031) in clinical development. J. Med. Chem. 57, 1531–1542 (2014).
Zenzola, M., Doran, R., Degennaro, L., Luisi, R. & Bull, J. A. Transfer of electrophilic NH using convenient sources of ammonia: direct synthesis of NH sulfoximines from sulfoxides. Angew. Chem. Int. Edn 55, 7203–7207 (2016).
Izzo, F., Schafer, M., Stockman, R. & Lücking, U. A new, practical one-pot synthesis of unprotected sulfonimidamides by transfer of electrophilic NH to sulfinamides. Chem. Eur. J. 23, 15189–15193 (2017).
Acknowledgements
We acknowledge support from Nanyang Technological University for Tier 1 grant (RG2/20). This research was supported by the Ministry of Education, Singapore, under its Academic Research Fund Tier 2 (MOE2019-T2-1-091). This work was supported by the A*STAR Computational Resource Centre through the use of its high-performance computing facilities. The computational work for this article was partially performed on resources of the National Supercomputing Centre, Singapore (https://www.nscc.sg).
Author information
Authors and Affiliations
Contributions
C.-H.T. and X.Z. conceived the research; X.Z. is responsible for experimental design and data analysis; X.Z., E.C.X.A., and Z.Y. performed the experiments and compounds testing; C.W.K. contributed to mechanistic discussion; C.-H.T. and X.Z. prepared the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Edward Anderson, Danielle Schultz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Sections 1–11 and references.
Rights and permissions
About this article
Cite this article
Zhang, X., Ang, E.C.X., Yang, Z. et al. Synthesis of chiral sulfinate esters by asymmetric condensation. Nature 604, 298–303 (2022). https://doi.org/10.1038/s41586-022-04524-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-022-04524-4
This article is cited by
-
Copper-catalysed asymmetric cross-coupling reactions tolerant of highly reactive radicals
Nature Chemistry (2026)
-
Chiral anionic ProPhenol ligand enabled nickel catalyzed enantioselective synthesis of sulfinamides
Science China Chemistry (2026)
-
Enantioselective organocatalytic construction of axially chiral sulfoxides
Nature Synthesis (2025)
-
Reductive sulfinylation by nucleophilic chain isomerization of sulfonylpyridinium
Nature Communications (2025)
-
Asymmetric S=N-embedded polyaromatic construction via enantioselective Pd-catalyzed C–H activation
Nature Communications (2025)