Abstract
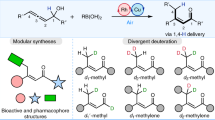
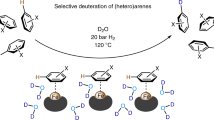
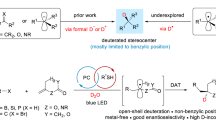
The incorporation of deuterium in organic molecules has widespread applications in medicinal chemistry and materials science1,2. For example, the deuterated drugs austedo3, donafenib4 and sotyktu5 have been recently approved. There are various methods for the synthesis of deuterated compounds with high deuterium incorporation6. However, the reductive deuteration of aromatic hydrocarbons—ubiquitous chemical feedstocks—to saturated cyclic compounds has rarely been achieved. Here we describe a scalable and general electrocatalytic method for the reductive deuteration and deuterodefluorination of (hetero)arenes using a prepared nitrogen-doped electrode and deuterium oxide (D2O), giving perdeuterated and saturated deuterocarbon products. This protocol has been successfully applied to the synthesis of 13 highly deuterated drug molecules. Mechanistic investigations suggest that the ruthenium–deuterium species, generated by electrolysis of D2O in the presence of a nitrogen-doped ruthenium electrode, are key intermediates that directly reduce aromatic compounds. This quick and cost-effective methodology for the preparation of highly deuterium-labelled saturated (hetero)cyclic compounds could be applied in drug development and metabolism studies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available in the main text or in Supplementary Information.
References
Pirali, T., Serafini, M., Cargnin, S. & Genazzani, A. A. Applications of deuterium in medicinal chemistry. J. Med. Chem. 62, 5276–5297 (2019).
Atzrodt, J., Derdau, V., Kerr, W. J. & Reid, M. Deuterium- and tritium-labelled compounds: applications in the life sciences. Angew. Chem. Int. Ed. 57, 1758–1784 (2018).
Schmidt, C. First deuterated drug approved. Nat. Biotechnol. 35, 493–494 (2017).
Keam, S. J. & Duggan, S. Donafenib: first approval. Drugs 81, 1915–1920 (2021).
Mullard, A. First de novo deuterated drug poised for approval. Nat. Rev. Drug Discov. 21, 623–625 (2022).
Kopf, S. et al. Recent developments for the deuterium and tritium labeling of organic molecules. Chem. Rev. 122, 6634–6718 (2022).
Simmons, E. M. & Hartwig, J. F. On the interpretation of deuterium kinetic isotope effects in C–H bond functionalizations by transition-metal complexes. Angew. Chem. Int. Ed. 51, 3066–3072 (2012).
Tullo, A. H. Your next TV could contain uncommon isotopes. CEN Glob. Enterp. 100, 21–22 (2022).
Jansen-van Vuuren, R. D., Jedlovcnik, L., Kosmrlj, J., Massey, T. E. & Derdau, V. Deuterated drugs and biomarkers in the COVID-19 pandemic. ACS Omega 7, 41840–41858 (2022).
Shi, L. et al. Optical imaging of metabolic dynamics in animals. Nat. Commun. 9, 2995 (2018).
De Feyter, H. M. et al. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci. Adv. 4, eaat7314 (2018).
Gant, T. G. Using deuterium in drug discovery: leaving the label in the drug. J. Med. Chem. 57, 3595–3611 (2014).
Zhang, M., Yuan, X. A., Zhu, C. & Xie, J. Deoxygenative deuteration of carboxylic acids with D2O. Angew. Chem. Int. Ed. 58, 312–316 (2019).
Farizyan, M., Mondal, A., Mal, S., Deufel, F. & van Gemmeren, M. Palladium-catalyzed nondirected late-stage C–H deuteration of arenes. J. Am. Chem. Soc. 143, 16370–16376 (2021).
Pieters, G. et al. Regioselective and stereospecific deuteration of bioactive aza compounds by the use of ruthenium nanoparticles. Angew. Chem. Int. Ed. 53, 230–234 (2014).
Atzrodt, J., Derdau, V., Fey, T. & Zimmermann, J. The renaissance of H/D exchange. Angew. Chem. Int. Ed. 46, 7744–7765 (2007).
Yu, R. P., Hesk, D., Rivera, N., Pelczer, I. & Chirik, P. J. Iron-catalysed tritiation of pharmaceuticals. Nature 529, 195–199 (2016).
Loh, Y. Y. et al. Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds. Science 358, 1182–1187 (2017).
Li, W. et al. Scalable and selective deuteration of (hetero)arenes. Nat. Chem. 14, 334–341 (2022).
He, T., Klare, H. F. T. & Oestreich, M. Perdeuteration of deactivated aryl halides by H/D exchange under superelectrophile catalysis. J. Am. Chem. Soc. 144, 4734–4738 (2022).
Kerr, W. J. et al. Site-selective deuteration of N-heterocycles via iridium-catalyzed hydrogen isotope exchange. ACS Catal. 7, 7182–7186 (2017).
Nilsson, G. N. & Kerr, W. J. The development and use of novel iridium complexes as catalysts for ortho-directed hydrogen isotope exchange reactions. J. Label. Compd. Radiopharm. 53, 662–667 (2010).
Czeskis, B. et al. Deuterated active pharmaceutical ingredients: a science-based proposal for synthesis, analysis, and control. Part 1: framing the problem. J. Label. Compd. Radiopharm. 62, 690–694 (2019).
Qiu, X. et al. Cleaving arene rings for acyclic alkenylnitrile synthesis. Nature 597, 64–69 (2021).
Roche, S. P. & Porco, J. A. Jr Dearomatization strategies in the synthesis of complex natural products. Angew. Chem. Int. Ed. 50, 4068–4093 (2011).
Zhuo, C. X., Zhang, W. & You, S. L. Catalytic asymmetric dearomatization reactions. Angew. Chem. Int. Ed. 51, 12662–12686 (2012).
Nairoukh, Z., Wollenburg, M., Schlepphorst, C., Bergander, K. & Glorius, F. The formation of all-cis-(multi)fluorinated piperidines by a dearomatization-hydrogenation process. Nat. Chem. 11, 264–270 (2019).
The Njarðarson Group Top 200 Small Molecule Pharmaceuticals by Retail Sales in 2021 (Univ. Arizona, 2021); https://bpb-us-e2.wpmucdn.com/sites.arizona.edu/dist/9/130/files/2024/08/Top-200-Small-Molecules-2021V3.pdf.
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Joannou, M. V., Bezdek, M. J. & Chirik, P. J. Pyridine(diimine) molybdenum-catalyzed hydrogenation of arenes and hindered olefins: insights into precatalyst activation and deactivation pathways. ACS Catal. 8, 5276–5285 (2018).
Smith, J. A. et al. Preparation of cyclohexene isotopologues and stereoisotopomers from benzene. Nature 581, 288–293 (2020).
Novaes, L. F. T. et al. Electrocatalysis as an enabling technology for organic synthesis. Chem. Soc. Rev. 50, 7941–8002 (2021).
Horn, E. J. et al. Scalable and sustainable electrochemical allylic C–H oxidation. Nature 533, 77–81 (2016).
Badalyan, A. & Stahl, S. S. Cooperative electrocatalytic alcohol oxidation with electron-proton-transfer mediators. Nature 535, 406–410 (2016).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Zeng, L. et al. Asymmetric-waveform alternating current-promoted silver catalysis for C–H phosphorylation. Nat. Synth. 2, 172–181 (2023).
Francke, R. & Little, R. D. Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem. Soc. Rev. 43, 2492–2521 (2014).
Li, R. et al. One-pot H/D exchange and low-coordinated iron electrocatalyzed deuteration of nitriles in D2O to alpha,beta-deuterio aryl ethylamines. Nat. Commun. 13, 5951 (2022).
Kurimoto, A., Sherbo, R. S., Cao, Y., Loo, N. W. X. & Berlinguette, C. P. Electrolytic deuteration of unsaturated bonds without using D2. Nat. Catal. 3, 719–726 (2020).
Zhu, J., Hu, L., Zhao, P., Lee, L. Y. S. & Wong, K.-Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 120, 851–918 (2020).
Yoshida, J., Kataoka, K., Horcajada, R. & Nagaki, A. Modern strategies in electroorganic synthesis. Chem. Rev. 108, 2265–2299 (2008).
Sajiki, H., Sawama, Y. & Monguchi, Y. Efficient H–D exchange reactions using heterogeneous platinum-group metal on carbon-H2–D2O system. Synlett 23, 959–972 (2012).
Wiesenfeldt, M. P., Nairoukh, Z., Li, W. & Glorius, F. Hydrogenation of fluoroarenes: direct access to all-cis-(multi)fluorinated cycloalkanes. Science 357, 908–912 (2017).
Nishi, T. et al. Studies on 2-oxoquinoline derivatives as blood platelet aggregation inhibitors. II. 6-[3-(1-Cyclohexyl-5-tetrazolyl)propoxy]-1,2-dihydro-2-oxoquinoline and related compounds. Chem. Pharm. Bull. 31, 1151–1157 (1983).
Acknowledgements
We thank the Center for Electron Microscopy at Wuhan University for their support of the transmission electron microscope work. We acknowledge the support from the National Key R&D Program of China (number 2021YFA1500100, A.L.), the National Natural Science Foundation of China (22031008, A.L.; 212200007, W.L.) and the Science Foundation of Wuhan (2020010601012192, A.L.). We thank S. Chen (WHU), Z. Wei (WHU), G. Wang (WHU) and Y. Xi (NUS) for discussions; X. Zhou from the Core Research Facilities of CCMS (WHU) for assistance with ssNMR analysis; and beamline BL11B and BL14W1 of Shanghai Synchrotron Radiation Facility (SSRF) for providing the beam time.
Author information
Authors and Affiliations
Contributions
A.L., W.L., F.B. and Y.D. conceived and designed the project. F.B., Y.D. and J.X. developed and prepared the catalysts, performed all catalytic experiments and analysed the data. F.B. and Y.L. were responsible for the TEM experiments. F.B. was responsible for the X-ray diffraction experiments. F.B. and Y.L. were responsible for the X-ray photoelectron spectroscopy experiments. D.Y. was responsible for the XAFS experiments. F.B., W.L. and A.L. co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Possible mechanisms.
Pathway 1 supported by our experimental data and reported data, pathway 2 involving D2 as an intermediate.
Extended Data Fig. 2 Reductive deuteration of arenes.
Standard conditions: Undivided cell, Al (+), Ru-N/CF (−) (Ru 3 mol%), a 0.4 mmol, nBu4NBr 0.4 mmol, tBuOD/D2O = 2.5/2.5 mL, constant current, 10 mA, room temperature, argon atmosphere, 8 h to 48 h, isolated yield. a0.2 mmol a was added. bReaction conditions: divided cell, anode cell, CF (+), NaF 0.8 mmol, D2O 10.0 mL; cathode cell, Ru-N/CF (−), a 0.4 mmol, nBu4NBr 0.8 mmol, tBuOD/D2O/HFIP = 5.0/5.0/0.5 mL, constant current, I = 10 mA, room temperature, argon atmosphere, 8 h to 16 h. cRu-N/CF-2 (Ru 5 mol%) was used as cathode.
Extended Data Fig. 3 Reductive deuteration of D-labelled arenes.
Standard conditions: Undivided cell, Al (+), Ru-N/CF (−) (Ru 3 mol%), c 0.4 mmol, nBu4NBr 0.4 mmol, tBuOD/D2O = 2.5/2.5 mL, constant current, 10 mA, room temperature, argon atmosphere, 8 h to 20 h, isolated yield. aReaction conditions: Divided cell, anode cell, CF (+), nBu4NCl 0.8 mmol, D2O 10.0 mL; cathode cell, Ru-N/CF (−), c 0.4 mmol, nBu4NCl 0.8 mmol, tBuOD/D2O = 5.0/5.0 mL, constant current, I = 10 mA, 14 h, room temperature, argon atmosphere.
Extended Data Fig. 4 Deuterodehalogenation.
Standard conditions: Undivided cell, Al (+), Ru-N/CF (−) (Ru 3 mol%), e 0.4 mmol, nBu4NBr 0.8 mmol, tBuOD/D2O = 5.0/5.0 mL, constant current, 10 mA, room temperature, argon atmosphere, 20 h to 72 h, isolated yield. anBu4NBr 0.4 mmol, tBuOD/D2O = 2.5/2.5 mL, t = 8 h to 20 h. bThe position of halogen in the substrates with the number less than five. cRu-N/CF-2 (Ru 5 mol%) was used. dD-incorporation (and yield) was determined by product benzoylation. ePentafluorophenylacetonitrile was used as substrate.
Extended Data Fig. 5 Synthetic applications.
Synthesis of piperazine-d8-containing pharmaceuticals from piperazine-d8 hydrochloride.
Supplementary information
Supplementary Information
This file contains Supplementary Sections 1–16 including Supplementary Figs. 1–28, Data and References—see Contents for details.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bu, F., Deng, Y., Xu, J. et al. Electrocatalytic reductive deuteration of arenes and heteroarenes. Nature 634, 592–599 (2024). https://doi.org/10.1038/s41586-024-07989-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-07989-7
This article is cited by
-
Electrochemical cobalt-catalyzed semi-deuteration of alkynes to access deuterated Z-alkenes
Nature Communications (2025)
-
Synthesis of deuterated acids and bases using bipolar membranes
Nature (2025)
-
Iron(III)-catalyzed activation of C(sp2)-H and C(sp3)-H bonds for H/D exchange of arenes
Nature Communications (2025)
-
Nickel-catalysed deuteration of benzylic C(sp3)–H bonds using D2O
Nature Synthesis (2025)
-
Boosted high-throughput D⁺ transfer from D₂O to unsaturated bonds via Pdδ+ cathode for solvent-free deuteration
Nature Communications (2025)