Abstract
Neural coding has traditionally been examined through changes in firing rates and latencies in response to different stimuli1,2,3,4,5. However, populations of neurons can also exhibit transient bursts of spiking activity, wherein neurons fire in a specific temporal order or sequence6,7,8. The human brain may utilize these neuronal sequences within population bursts to efficiently represent information9,10,11,12, thereby complementing the well-known neural code based on spike rate or latency. Here we examined this possibility by recording the spiking activity of populations of single units in the human anterior temporal lobe as eight participants performed a visual categorization task. We find that population spiking activity organizes into bursts during the task. The temporal order of spiking across the activated units within each burst varies across stimulus categories, creating unique stereotypical sequences for individual categories as well as for individual exemplars within a category. The information conveyed by the temporal order of spiking activity is separable from and complements the information conveyed by the units’ spike rates or latencies following stimulus onset. Collectively, our data provide evidence that the human brain contains a complementary code based on the neuronal sequence within bursts of population spiking to represent information.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data used in this study are available at https://research.ninds.nih.gov/zaghloul-lab/downloads. Source data are provided with this paper.
Code availability
Except where otherwise noted, computational analyses were performed using custom written Matlab scripts. Custom code used for analysis is available at https://research.ninds.nih.gov/zaghloul-lab/downloads.
References
Perkel, D. H. & Bullock, T. H. Neural coding. Neurosci. Res. Program Bull. 6, 221–348 (1968).
Rieke, F., Warland, D., de Ruyter van Steveninck, R. & Bialek, W. Spikes: Exploring the Neural Code (MIT Press, 1997).
Shadlen, M. N. & Newsome, W. T. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J. Neurosci. 18, 3870–3896 (1998).
Raiguel, S. E., Xiao, D. K., Marcar, V. L. & Orban, G. A. Response latency of macaque area MT/V5 neurons and its relationship to stimulus parameters. J. Neurophysiol. 82, 1944–1956 (1999).
Celebrini, S., Thorpe, S., Trotter, Y. & Imbert, M. Dynamics of orientation coding in area VI of the awake primate. Vis. Neurosci. 10, 811–825 (1993).
Luczak, A., McNaughton, B. L. & Harris, K. D. Packet-based communication in the cortex. Nat. Rev. Neurosci. 16, 745–755 (2015).
Nguyen, N. D. et al. Cortical reactivations predict future sensory responses. Nature 625, 110–118 (2024).
Tanabe, S., Lee, H., Wang, S. & Hudetz, A. G. Spontaneous and visual stimulation evoked firing sequences are distinct under desflurane anesthesia. Neuroscience 528, 54–63 (2023).
Thorpe, S. & Gautrais, J. in Computational Neuroscience: Trends in Research (ed. Bower, J. M.) 113–118 (Springer, 1998).
Van Rullen, R. & Thorpe, S. J. Rate coding versus temporal order coding: What the retinal ganglion cells tell the visual cortex. Neural Comput. 13, 1255–1283 (2001).
Boahen, K. Dendrocentric learning for synthetic intelligence. Nature 612, 43–50 (2022).
Gautrais, J. & Thorpe, S. Rate coding versus temporal order coding: a theoretical approach. BioSystems 48, 57–65 (1998).
Hahn, G., Ponce-Alvarez, A., Deco, G., Aertsen, A. & Kumar, A. Portraits of communication in neuronal networks. Nat. Rev. Neurosci. 20, 117–127 (2019).
Palmigiano, A., Geisel, T., Wolf, F. & Battaglia, D. Flexible information routing by transient synchrony. Nat. Neurosci. 20, 1014–1022 (2017).
Abeles, M. Corticonics: Neural Circuits of the Cerebral Cortex (Cambridge Univ. Press, 1991); https://doi.org/10.1017/CBO9780511574566.
Bermudez-Contreras, E. J. et al. Formation and reverberation of sequential neural activity patterns evoked by sensory stimulation are enhanced during cortical desynchronization. Neuron 79, 555–566 (2013).
Montijn, J. S., Olcese, U. & Pennartz, C. M. A. Visual stimulus detection correlates with the consistency of temporal sequences within stereotyped events of V1 neuronal population activity. J. Neurosci. 36, 8624–8640 (2016).
Skaggs, W. E., McNaughton, B. L., Wilson, M. A. & Barnes, C. A. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6, 149–172 (1996).
Shahbaba, B. et al. Hippocampal ensembles represent sequential relationships among an extended sequence of nonspatial events. Nat. Commun. 13, 787 (2022).
Hahnloser, R. H. R., Kozhevnikov, A. A. & Fee, M. S. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature 797, 796–797 (2002).
Yiling, Y. et al. Robust encoding of natural stimuli by neuronal response sequences in monkey visual cortex. Nat. Commun. 14, 3021 (2023).
Harnad, S. in Handbook of Categorization in Cognitive Science, vol. 1908 (eds Cohen, H. & Lefebvre, C.) 19–43 (Elsevier, 2005).
Jang, A. I., Wittig, J. H., Inati, S. K. & Zaghloul, K. A. Human cortical neurons in the anterior temporal lobe reinstate spiking activity during verbal memory retrieval. Curr. Biol. 27, 1700–1705 (2017).
Wittig, J. H., Jang, A. I., Cocjin, J. B., Inati, S. K. & Zaghloul, K. A. Attention improves memory by suppressing spiking-neuron activity in the human anterior temporal lobe. Nat. Neurosci. 21, 808–810 (2018).
Vaz, A. P., Wittig, J. H., Inati, S. K. & Zaghloul, K. A. Replay of cortical spiking sequences during human memory retrieval. Science 367, 1131–1134 (2020).
Tong, A. P. S., Vaz, A. P., Wittig, J. H., Inati, S. K. & Zaghloul, K. A. Ripples reflect a spectrum of synchronous spiking activity in human anterior temporal lobe. eLife 10, e68401 (2021).
Liu, K., Sibille, J. & Dragoi, G. Preconfigured patterns are the primary driver of offline multi-neuronal sequence replay. Hippocampus 29, 275–283 (2019).
Ji, D. & Wilson, M. A. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 10, 100–107 (2007).
Xie, W. et al. The medial temporal lobe supports the quality of visual short-term memory representation. Nat. Hum. Behav. 7, 627–641 (2023).
Nádasdy, Z., Hirase, H., Czurkó, A., Csicsvari, J. & Buzsáki, G. Replay and time compression of recurring spike sequences in the hippocampus. J. Neurosci. 19, 9497–9507 (1999).
Reber, T. P. et al. Representation of abstract semantic knowledge in populations of human single neurons in the medial temporal lobe. PLoS Biol. 17, 1–17 (2019).
Ohayon, S., Freiwald, W. A. & Tsao, D. Y. What makes a cell face selective? The importance of contrast. Neuron 74, 567–581 (2012).
Resulaj, A., Ruediger, S., Olsen, S. R. & Scanziani, M. First spikes in visual cortex enable perceptual discrimination. eLife 7, e34044 (2018).
McGill, W. J. Multivariate information transmission. Psychometrika 19, 97–116 (1954).
Timme, N., Alford, W., Flecker, B. & Beggs, J. M. Synergy, redundancy, and multivariate information measures: an experimentalist’s perspective. J. Comput. Neurosci. 36, 119–140 (2014).
Kubkowski, M. & Mielniczuk, J. Asymptotic distributions of empirical interaction information. Methodol. Comput. Appl. Probab. 23, 291–315 (2021).
Luczak, A., McNaughton, B. L. & Kubo, Y. Neurons learn by predicting future activity. Nat. Mach. Intell. 4, 62–72 (2022).
Sotomayor-Gómez, B., Battaglia, F. P. & Vinck, M. Differential population coding of natural movies through spike counts and temporal sequences. Preprint at bioRxiv https://doi.org/10.1101/2023.06.27.546669 (2023).
Gonzalo Cogno, S. et al. Minute-scale oscillatory sequences in medial entorhinal cortex. Nature 625, 338–344 (2024).
Buzsáki, G. & Tingley, D. Space and time: the hippocampus as a sequence generator. Trends Cogn. Sci. 22, 853–869 (2018).
Norman, Y. et al. Hippocampal sharp-wave ripples linked to visual episodic recollection in humans. Science 365, eaax1030 (2019).
Vaz, A. P., Inati, S. K., Brunel, N. & Zaghloul, K. A. Coupled ripple oscillations between the medial temporal lobe and neocortex retrieve human memory. Science 363, 975–978 (2019).
Norman, Y., Raccah, O., Liu, S., Parvizi, J. & Malach, R. Hippocampal ripples and their coordinated dialogue with the default mode network during recent and remote recollection. Neuron 109, 2767–2780.e5 (2021).
Verzhbinsky, I. A. et al. Co-occurring ripple oscillations facilitate neuronal interactions between cortical locations in humans. Proc. Natl Acad. Sci. USA 121, e2312204121 (2024).
Luczak, A., Barthó, P., Marguet, S. L., Buzsáki, G. & Harris, K. D. Sequential structure of neocortical spontaneous activity in vivo. Proc. Natl Acad. Sci. USA 104, 347–352 (2007).
Hemberger, M., Shein-Idelson, M., Pammer, L. & Laurent, G. Reliable sequential activation of neural assemblies by single pyramidal cells in a three-layered cortex. Neuron 104, 353–369.e5 (2019).
Branco, T., Clark, B. A. & Häusser, M. Dendritic discrimination of temporal input sequences in cortical neurons. Science 329, 1671–1675 (2010).
Pouget, A., Dayan, P. & Zemel, R. Information processing with population codes. Nat. Rev. Neurosci. 1, 125–132 (2000).
Ma, W. J., Beck, J. M., Latham, P. E. & Pouget, A. Bayesian inference with probabilistic population codes. Nat. Neurosci. 9, 1432–1438 (2006).
Beck, J. M. et al. Probabilistic Population Codes for Bayesian Decision Making. Neuron 60, 1142–1152 (2008).
Maimon, G. & Assad, J. A. Beyond Poisson: increased spike-time regularity across primate parietal cortex. Neuron 62, 426–440 (2009).
Averbeck, B. B. Poisson or not Poisson: differences in spike train statistics between parietal cortical areas. Neuron 62, 310–311 (2009).
Christopher Decharms, R. & Merzenich, M. M. Primary cortical representation of sounds by the coordination of action-potential timing. Nature 381, 610–613 (1996).
Gawne, T. J., Kjaer, T. W. & Richmond, B. J. Latency: another potential code for feature binding in striate cortex. J. Neurophysiol. 76, 1356–1360 (1996).
Steinmetz, P. N. et al. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature 404, 187–190 (2000).
Willenbockel, V. et al. Controlling low-level image properties: the SHINE toolbox. Behav. Res. Methods 42, 671–684 (2010).
Joshua, M., Elias, S., Levine, O. & Bergman, H. Quantifying the isolation quality of extracellularly recorded action potentials. J. Neurosci. Methods 163, 267–282 (2007).
Thorpe, S., Delorme, A. & Van Rullen, R. Spike-based strategies for rapid processing. Neural Netw. 14, 715–725 (2001).
Farooq, U., Sibille, J., Liu, K. & Dragoi, G. Strengthened temporal coordination within pre-existing sequential cell assemblies supports trajectory replay. Neuron 103, 719–733.e7 (2019).
Chandrashekar, G. & Sahin, F. A survey on feature selection methods. Comput. Electr. Eng. 40, 16–28 (2014).
Cohen, M. X. Analyzing Neural Time Series Data: Theory and Practice (MIT Press, 2014).
Prechelt, L. in Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics) vol. 7700 LECTU 55–69 (1998).
Estefan, D. P. et al. Volitional learning promotes theta phase coding in the human hippocampus. Proc. Natl Acad. Sci. USA 118, e2021238118 (2021).
Bakdash, J. Z. & Marusich, L. R. Repeated measures correlation. Front. Psychol. 8, 456 (2017).
Xie, W. & Zhang, W. Effortfulness of visual working memory: gauged by physical exertion. J. Exp. Psychol. Gen. 152, 2074–2093 (2023).
Yu, Z. et al. Beyond t test and ANOVA: applications of mixed-effects models for more rigorous statistical analysis in neuroscience research. Neuron 110, 21–35 (2022).
Rosenthal, R. & Rubin, D. B. r equivalent: a simple effect size indicator. Psychol. Methods 8, 492–496 (2003).
Xie, W., Bainbridge, W. A., Inati, S. K., Baker, C. I. & Zaghloul, K. A. Memorability of words in arbitrary verbal associations modulates memory retrieval in the anterior temporal lobe. Nat. Hum. Behav. 4, 937–948 (2020).
Acknowledgements
The authors thank R. Rosenthal and K. Boahen for providing insightful comments, M. Trotta and A. Jang for task development, and A. Vaz for code and suggestions. This work was made possible by the Intramural Research Programs of the National Institute of Neurological Disorders and Stroke (ZIA-NS003144, K.A.Z.) and the NIH Pathway to Independence Award (K99NS126492, W.X.). We are indebted to all patients who have selflessly volunteered their time to participate in this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: K.A.Z., W.X. and J.H.W. Methodology: J.H.W., W.X., M.E.-K., S.N.J., S.K.I. and K.A.Z. Software: J.W.H. and W.X. Validation: W.X. Formal analysis: J.H.W., W.X. and J.I.C. Investigation: J.H.W., W.X., J.I.C., S.K.I. and K.A.Z. Resources: K.A.Z. Data curation: J.H.W., W.X. and S.N.J. Writing, original draft: W.X. Writing, review and editing: K.A.Z., W.X., J.H.W. and J.I.C. Visualization: W.X., J.H.W. and J.I.C. Supervision: K.A.Z.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Shuo Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Behavioral task details, data structure in the current study, and unit quality metrics.
a, An example trial using images from the four predefined taxonomic categories (top panel) along with trial counts for each participant in each experimental session (bottom panel). b An example trial using images of four selected U.S. presidents (as PERSON exemplars) along with trial counts for each participant in each experimental session. c, An example trial using images representing four arrow directions along with trial counts for each participant in each experimental session. d, Average trial counts, accuracy, and response times for each trial type. e, Across 8 participants with recordings providing meaningful unit data, we identified 18 unique recordings from 13 experimental sessions across 12 physical arrays. As units recorded from different arrays or from different experimental sessions on subsequent days can exhibit variations, we treat each recording as a separate sample. In total, we identified 2110 putative single units across the 18 recordings. To account for the multi-level data structure in our statistical analysis, we employ a mixed-effects modelling approach to assess the effects of interest, allowing for generalization across different recordings while accounting for variances at the participant, session, and array levels. f, We quantified the quality of each identified unit by calculating signal-to-noise ratio (SNR) and a normalized isolation score (from 0 to 1) to capture the consistency of a unit’s waveform across spikes and how well a unit’s waveform can be separated from the waveforms of other units and noise snippets. Across participants, the mean SNR for all identified units is 1.98 ± 0.06 (median = 1.99) and the mean isolation score for all identified units is 0.94 ± 0.01 (median = 0.95). The average spike rate is around 1 Hz (log spike rate around 0).
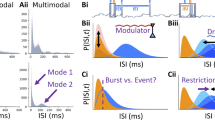
Extended Data Fig. 2 Burst detection.
a, Spike data extracted from an example recording, along with the detected bursts, based on individual smoothing and thresholding parameters identified to ensure a false positive (FP) rate smaller than 0.1 as compared with surrogate data. The inter-burst intervals appear to be non-uniformly distributed. b, Surrogate spike data generated by a Poisson process to maintain the same average spike rate per unit over time, along with the detected bursts using the same smoothing and thresholding parameters. The inter-burst intervals in this surrogate data appear to be uniformly distributed. c, Systematic variation of the smoothing and thresholding parameters for each individual recording to identify the parameter set that results in a low FP rate, quantified as the ratio of detected bursts between the surrogate and the original data. d, The best parameter sets across recordings fall within a narrow range, indicating some homogeneity in bursting behavior across recordings. Color values represent the number of recordings. e, Population spiking bursts coincide with an increase in 80–120 Hz ripple rate recorded from the same micro-electrodes, observable at the level of individual bursts (left) and across recordings (right). Error areas represent the s.e.m. f, The number of detected bursts tends to covary with the number of total available units (Spearman ρ = 0.75, p = 0.00030). However, it does not depend on the average spike rate (Spearman ρ = 0.25, p = 0.31) or the number of included trials (Spearman ρ = 0.02, p = 0.94). N = 18 recordings. Two-tailed uncorrected p-values were calculated based on Spearman rank-order correlation. g, Increasing the FP rate leads to more detected bursts. We maintained a FP rate of 0.1 in the current study. Each data point and connected line represent the results from an individual recording.
Extended Data Fig. 3 Unit selection and evaluation of the sequence-based classifier.
a, To identify units that exhibit reliable ranks within bursts, we calculated the mean and standard deviation (σ) of a unit’s rank across all bursts. Conceptually, units involved in sequence-based coding should demonstrate a reliable mean rank (μrank) across bursts relative to shuffled data. Units involved in sequence-based coding but with a rank consistently in the middle of a sequence may not be distinguished from the null distribution but would exhibit small variance in their rank, σrank. Hence, we considered units showing either a reliable μrank (pboostrap < .05 in either direction, two-tailed) or a small σrank (pboostrap < .05 in the predicted direction, one-tailed) in at least one stimulus category as sequence-related units. b, The number of sequence-related units in each recording. Overall, sequence-related units account for 52.6% of all units. c, Across independent data folds, we assessed within-category sequence similarity relative to between-category sequences based on sequence-related units. Within-category sequence similarity is significantly greater than between-category similarity across participants. d, Sequence-based decoding accuracy depends on unit selection. Including units with less reliable ranks decreases classification accuracy across recordings (true vs. shuffled trial labels in mixed-effects modelling: t(34) = 1.84, p = 0.075). e, Using spiking data from a subset of participants who completed the arrow trials, we find that neither sequence-based nor rate-based classification could reliably differentiate arrow directions. f, Sequence-based classification accuracy is unaffected by the timing of the held-out burst relative to a trial’s response time or to the stimulus presentation. Data are shown as the mean ± s.e.m., with results from individual recordings shown as dots and/or lines color-coded by participant. N = 18 recordings. Two-tailed uncorrected p-values were calculated using a linear mixed-effects model, accounting for participant-, session-, and array-level variances.
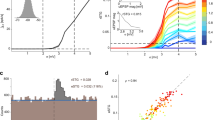
Extended Data Fig. 4 Building a rate-based classifier and its performance over time.
a, For each trial type (taxonomic, presidents, or arrow), we built and tested a rate-based classifier using non-overlapping data as training and testing datasets. In each iteration, we used the training data to build the classifier using a one-vs-all logistic regression with early stopping. We then applied the training weights to the independent testing data to generate a prediction of the test stimulus label. If the predicted label is consistent with the test stimulus label, then the classification is considered accurate. We performed this analysis both using the data at each individual 200-ms time window of the task and using the aggregate spike rates data across all units within time windows from 100 to 1400 ms following stimulus onset. In the former case, the features used in the classifier are the population unit activity at a single time window with the feature length equivalent to the number of units. In the latter case, the features used are the population unit activity across time windows with the feature length equivalent to the number of units multiplied by the number of time windows. b, Across N = 18 recordings, a rate-based classifier can significantly decode category-specific information following stimulus onset for taxonomic categories with the classification accuracy peaking around the same time across stimulus categories. c, Overall classification accuracy for taxonomic categories is significantly higher than chance from 300 to 1120 ms after stimulus onset to (first cluster mean ± s.e.m.: 32.2% ± 2.0%; pcorrected < .05). The two recordings in which decoding accuracy exceeds 50% are from participant NIH086 in two separate sessions, capturing 167 and 212 units, respectively. Thin lines indicate individual recordings and thick line group average. Cluster-based two-tailed p values are corrected at the 0.05 level.
Extended Data Fig. 5 Associations among spike timing measures.
a, A unit’s spike rank within a burst is strongly correlated with spike latency from burst onset (τb, mean Spearman ρ = 0.76, range: 0.60 to 0.87), compared with spike latency from stimulus onset (τo, mean Spearman ρ = 0.06, range: 0.02 to 0.11). The former is over 10 times that for the latter, suggesting that rank-based measures better capture spike timing within bursts than from stimulus onset. b, Spike rates during both task and baseline periods are correlated with τo (e.g., mean Spearman ρ between task-period spike rate and τo = −0.43, range: −0.51 to −0.33). This relation is weaker between task-period spike rate and a unit’s spike timing within bursts (rate & rank, mean Spearman ρ = −0.09, range: −0.16 to −0.02; rate & τb, mean Spearman ρ = −0.24, range: −0.35 to −0.11). Single-unit measures are shown as dots following z-score normalization within each burst. c-d, Changes in spike rate from baseline correlate significantly with τo, but not with spike rank within bursts or τb, indicating that information conveyed by rank or τb differs from that by spike rate changes. e, In an example recording, mean τo across units varies by stimulus category, while the relative order of τo remains consistent across categories. In contrast, τb values are not consistent across categories, suggesting potential for information coding. f, Mean τo across units provides more stimulus information than the relative order of τo. Conversely, the relative order of τb provides more information than mean τb. Solid blue and dashed bars indicate significant and non-significant (n.s.) classification accuracy relative to chance with Bonferroni correction (pcorrected < .05), respectively. Data are shown as the mean ± s.e.m., with individual data color-coded by participant. Two-tailed uncorrected p-values were calculated using a linear mixed-effects model.
Extended Data Fig. 6 Additional analyses to distinguish rate- and sequence-based information.
a, To determine if rate-based information persists across bursting and non-bursting periods, logistic regression classifiers were trained and tested based on population spike rates aggregated separately for these periods. Bursting periods, where a group of units spiked closely in time, were identified by adjusting the smoothing and thresholding parameters of population spike rate calculation for each recording, controlling the false discovery rate (see Extended Data Fig. 2). Bursting and non-bursting spike raster plots were extracted by retaining spike data within and outside bursts, respectively. Instantaneous spike rates of the population of units in these raster plots were calculated (200 ms sliding window, 90% overlap). For burst-only and burst-removed raster plots, all units and critical time windows within 100–1400 ms following stimulus onset in each trial were aggregated to decode taxonomic categories, following the same approach as detailed in the Methods. b, Across recordings, population spike rate significantly distinguishes taxonomic categories better than chance using data either within or outside bursting periods, regardless of whether all units were included (mixed-effects model for burst-only: t(34) = 4.50, p = 0.000076; mixed-effects model for non-burst: t(34) = 2.93, p = 0.0060) or only sequence-related units (mixed-effects model for burst-only: t(34) = 3.84, p = 0.00051; mixed-effects model for non-burst: t(34) = 2.71, p = 0.011). Data are shown as the mean ± s.e.m., with individual recordings shown as dots and lines color-coded by participant. N = 18 recordings. Two-tailed uncorrected p-values were calculated using a linear mixed-effects model, accounting for participant-, session-, and array-level variances. c. Example raster plots for the top five sequence-related units that spiked in bursts across all stimulus categories. These units do not significantly distinguish categories by overall spike rate, but relative rank still retains significant stimulus information (Fig. 5a).
Extended Data Fig. 7 Quantifying stimulus information associated with spike count and rank in burst sequences.
a, In an example burst sequence, a single unit’s spiking activity can be characterized by its spike count (e.g., no spike, spiking once, or multiple times) and its relative rank within the sequence (e.g., early 1/3, middle 1/3, or late 1/3). b, Across burst sequences elicited by images from the same stimulus category, the unit’s counts and ranks can be summarized in a frequency table (left panel), which can be converted into empirical probabilities relative to all observed bursts (right panel). c, Similar frequency tables can be generated for stimuli from all categories for the example unit. Using these frequency and probability tables, the empirical mutual information (I) between spike activity and the presented stimuli can be quantified, either based on spike count or relative rank information. d-e, In the example, the spike count for the current unit contains more information about ANIMAL and PERSON categories. In contrast, spike rank contains more information about the OBJECT category relative to other stimulus categories. This pattern suggests that spike count and rank of the same unit may contain complementary, non-redundant information, effectively capturing different stimulus categories. f, Formal information theory analysis confirms this prediction, showing that the combination of spike count and rank contains more stimulus information than the sum of the information provided by spike count and rank alone (highlighted in red). These findings suggest a synergistic relationship between spike count and rank in representing stimulus information.
Extended Data Fig. 8 Stimulus information associated with spike count and/or relative rank within bursts for each recorded unit.
a, Sequence-related units show significantly greater stimulus information disclosed by a unit’s rank within a burst sequence, namely I(r; s), as compared with non-sequence-related units (seq. vs. non-seq. in mixed-effects modelling of I(r; s): t(34) = 2.93, p = 0.0060). b, In contrast, stimulus information disclosed by spike count within a burst sequence, namely I(c; s), is not significantly different between sequence-related and non-sequence-related units (seq. vs. non-seq. units in mixed-effects modelling of I(c; s): t(34) = 1.99, p = 0.055). c, We calculated the interaction information (II) to examine if knowing both the count and rank about a unit’s spiking within a sequence provides more information than knowing only the count or rank, namely I(c, r; s) − I(c; s) − I(r; s). If so, spike count and rank provide synergistic information about the stimulus (i.e., II > 0). Across all 2110 units in 18 recordings, the majority of units (>95%) show a synergistic relation between spike count and rank within burst sequences. Furthermore, this synergistic relationship is significantly greater for sequence-related units as compared with non-sequence-related units (seq. vs. non-seq. units in mixed-effects modelling: t(34) = 3.84, p = 0.00051). Collectively, these data suggest that sequence-related and non-sequence-related units may be functionally different from one another. n.s. = not statistically significant. In the left panel of a-c, each red and blue dot represent results from sequence- and non-sequence-related units, respectively. In the right panel of a-c, data are shown as the mean ± s.e.m., with individual recordings shown as dots and lines color-coded by participant. N = 18 recordings. Two-tailed uncorrected p-values were calculated using a linear mixed-effects model, accounting for participant-, session-, and array-level variances.
Extended Data Fig. 9 Linking a unit’s contribution to population rate code and its rank within neuronal sequences.
a, We assessed each unit’s spike rate sensitivity to different categories by calculating the relative change in the decoding performance of a rate-based classifier when the unit is excluded. A decrease in performance upon exclusion indicates the unit’s importance to the population rate code for that category. This allows us to estimate each unit’s relative contribution to population rate codes across taxonomic categories. For units within a burst, we categorized these contributions into three rank bins based on the order within category-specific template sequences. We then averaged these contributions across units and categories to explore the relationship between a unit’s spiking timing and its sensitivity to visual categorical information. b, Across recordings and categories, units spiking earlier in a sequence contributed more to the population rate code, while later-spiking units contributed less. This relationship was confirmed by a significant repeated-measures correlation (rwithin = −0.43, t(52) = − 3.45, p = 0.0011) between a unit’s rank in category-specific sequences and its contribution to the population rate code, controlling for participant-, session-, or array-level variances. Non-sequence-related units, which lack reliable ranks in stimulus categories, did not show a significant relationship (rwithin = −0.049, t(52) = − 0.35, p = 0.72). These findings suggest that sequence-related units with firing rate responses sensitive to stimulus information tend to activate earlier within a neuronal sequence. P values are reported as two-tailed without correction.
Extended Data Fig. 10 Individual traces of classification accuracy for taxonomic categories as a function of included sequence-related units, separately for rate-based and sequence-based decoding.
a, The number of recorded neuronal units affects classification effect sizes. As the number of units increases, classification accuracies based on task-period spike rates and neuronal sequences during population bursts both improve. Although classification accuracy based on task-period spike rates is generally higher, it appears to plateau as the number of units increases. In contrast, such a plateau is less clear for sequence-based decoding, indicating that with more recorded units, sequence-based information might continue to increase. This finding aligns with recent theories11 and data38 predicting that sequence-based coding could enhance coding efficiency. b, When data is averaged across recordings, normalizing the number of units as a percentage of total recorded units reveals similar trends. Error bars represent s.e.m., and the average number of units within each percentage bin is shown on the right y-axis. Since rate- and sequence-based classifications differ slightly in their analytical procedures, direct comparisons between them can be influenced by factors such as the number of iterations and optimization of decoding parameters. In this case, the differing slopes, rather than absolute magnitudes, of these classification outcomes suggest a potential variation in how these neural codes evolve as more neuronal units are included in the analysis.
Supplementary information
Supplementary Information
The Supplementary Information file includes the Supplementary Discussion, additional references, Supplementary Notes and Supplementary Table 1.
Rights and permissions
About this article
Cite this article
Xie, W., Wittig, J.H., Chapeton, J.I. et al. Neuronal sequences in population bursts encode information in human cortex. Nature 635, 935–942 (2024). https://doi.org/10.1038/s41586-024-08075-8
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-08075-8
This article is cited by
-
Ripple contributions to human memory: making the spiking content count
Nature Reviews Neuroscience (2025)
-
In silico discovery of representational relationships across visual cortex
Nature Human Behaviour (2025)
-
Temporal coding carries more stable cortical visual representations than firing rate over time
Nature Communications (2025)