Abstract
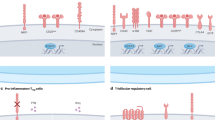
The T cell response to cancer controls disease progression and response to immunotherapy1,2,3. Despite extensive knowledge regarding CD8 T cells, how CD4 T cells contribute to this process is less well understood. Here we identified a population of PD1+TCF1+ CD4 T cells with stem-like properties that are capable of self-renewal and differentiation into canonical CD4 effector cells. Primarily residing in tumour-draining lymph nodes (TDLNs), these tumour-specific CD4 T cells are restricted by T regulatory (Treg) cells to a stem-like fate that predominantly generated induced Treg (iTreg) cells, limiting effector CD8 T cell responses to the tumour. By contrast, upon Treg depletion, stem-like CD4 T cells differentiated into T helper 1 (TH1) cells, and via IFNγ production induced robust effector differentiation from TCF1+ CD8 T cells in TDLNs, a state we defined as ‘active’. Notably, enforcing TBET expression in transferred stem-like CD4 T cells was sufficient to overcome the established restricted T cell state. Despite the presence of Treg cells, endogenous stem-like CD4 T cells actively generated TH1 cells, which were required to restore TDLN effector CD8 T cell differentiation, enhance tumour control and rescue response to immunotherapy. In agreement, TH1 differentiation in patients with kidney cancer predicted successful immunotherapy responses and improved progression-free survival. Together, these findings identify a stem-like CD4 T cell population that through alternative differentiation fates controls the switch between restricted and active T cell states with implications for cancer immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Raw fastq files and associated scRNA sequencing have been uploaded to the NCBI Gene Expression Omnibus (GEO) database under identifier GSE274801. Other relevant data are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Change history
06 November 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41586-024-08303-1
References
Galon, J. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964 (2006).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Borst, J. et al. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 18, 635–647 (2018).
Speiser, D. E. et al. CD4+ T cells in cancer. Nat. Cancer 4, 317–329 (2023).
Cohen, M. et al. The interaction of CD4+ helper T cells with dendritic cells shapes the tumor microenvironment and immune checkpoint blockade response. Nat. Cancer 3, 303–317 (2022).
Magen, A. et al. Intratumoral dendritic cell–CD4+ T helper cell niches enable CD8+ T cell differentiation following PD-1 blockade in hepatocellular carcinoma. Nat. Med. 29, 1389–1399 (2023).
Cui, C. et al. Neoantigen-driven B cell and CD4 T follicular helper cell collaboration promotes anti-tumor CD8 T cell responses. Cell 184, 6101–6118.e13 (2021).
Sharma, P. et al. Immune checkpoint therapy—current perspectives and future directions. Cell 186, 1652–1669 (2023).
Binnewies, M. et al. Unleashing type-2 dendritic cells to drive protective antitumor CD4+ T cell immunity. Cell 177, 556–571.e16 (2019).
Kim, J. M., Rasmussen, J. P. & Rudensky, A. Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8, 191–197 (2007).
Lahl, K. et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 204, 57–63 (2007).
Spitzer, M. H. et al. Systemic immunity is required for effective cancer immunotherapy. Cell 168, 487–502.e15 (2017).
Wei, S. C. et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell 170, 1120–1133.e17 (2017).
Alonso, R. et al. Induction of anergic or regulatory tumor-specific CD4+ T cells in the tumor-draining lymph node. Nat. Commun. 9, 2113 (2018).
Nishikawa, H. & Sakaguchi, S. Regulatory T cells in tumor immunity. Int. J. Cancer 127, 759–767 (2010).
Jansen, C. S. et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 576, 465–470 (2019).
Zhou, L., Chong, M. M. & Littman, D. R. Plasticity of CD4+ T cell lineage differentiation. Immunity 30, 646–655 (2009).
Zhu, J., Yamane, H. & Paul, W. E. Differentiation of erffector CD4 T cell populations. Annu. Rev. Immunol. 28, 445–489 (2010).
Brummelman, J. et al. High-dimensional single cell analysis identifies stem-like cytotoxic CD8+ T cells infiltrating human tumors. J. Exp. Med. 215, 2520–2535 (2018).
Hong, S.-W. et al. Immune tolerance of food is mediated by layers of CD4+ T cell dysfunction. Nature 607, 762–768 (2022).
Kalekar, L. A. et al. CD4+ T cell anergy prevents autoimmunity and generates regulatory T cell precursors. Nat. Immunol. 17, 304–314 (2016).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Im, S. J. et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016).
Siddiqui, I. et al. Intratumoral Tcf1+ PD-1+ CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50, 195–211.e10 (2019).
Xia, Y. et al. BCL6-dependent TCF-1+ progenitor cells maintain effector and helper CD4+ T cell responses to persistent antigen. Immunity 55, 1200–1215.e6 (2022).
Du, X. et al. A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy. Cell Res. 28, 416–432 (2018).
Simpson, T. R. et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti–CTLA-4 therapy against melanoma. J. Exp. Med. 210, 1695–1710 (2013).
Prokhnevska, N. et al. CD8+ T cell activation in cancer comprises an initial activation phase in lymph nodes followed by effector differentiation within the tumor. Immunity 56, 107–124.e5 (2023).
Connolly, K. A. et al. A reservoir of stem-like CD8+ T cells in the tumor-draining lymph node preserves the ongoing antitumor immune response. Sci. Immunol. 6, eabg7836 (2021).
Li, Z. et al. In vivo labeling reveals continuous trafficking of TCF-1+ T cells between tumor and lymphoid tissue. J. Exp. Med. 219, e20210749 (2022).
Alfei, F. et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 571, 265–269 (2019).
Gearty, S. V. et al. An autoimmune stem-like CD8 T cell population drives type 1 diabetes. Nature 602, 156–161 (2022).
Eberhardt, C. S. et al. Functional HPV-specific PD-1+ stem-like CD8 T cells in head and neck cancer. Nature 597, 279–284 (2021).
Moguche, A. O. et al. ICOS and Bcl6-dependent pathways maintain a CD4 T cell population with memory-like properties during tuberculosis. J. Exp. Med. 212, 715–728 (2015).
Shin, B. et al. Effector CD4 T cells with progenitor potential mediate chronic intestinal inflammation. J. Exp. Med. 215, 1803–1812 (2018).
Zou, D. et al. CD4+ T cell immunity is dependent on an intrinsic stem-like program. Nat. Immunol. 25, 66–76 (2024).
Kratchmarov, R. et al. TCF1–LEF1 co-expression identifies a multipotent progenitor cell (TH2-MPP) across human allergic diseases. Nat. Immunol. 25, 902–915 (2024).
Matloubian, M., Concepcion, R. J. & Ahmed, R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68, 8056–8063 (1994).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021).
Jones, M. G., Rosen, Y. & Yosef, N. Interactive, integrated analysis of single-cell transcriptomic and phylogenetic data with PhyloVision. Cell Rep. Methods 2, 100200 (2022).
Bolotin, D. A. et al. MiXCR: software for comprehensive adaptive immunity profiling. Nat. Methods 12, 380–381 (2015).
Browaeys, R., Saelens, W. & Saeys, Y. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat. Methods 17, 159–162 (2020).
Wilkinson, A. C. et al. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature 571, 117–121 (2019).
Labun, K. et al. CHOPCHOP v3: expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res. 47, W171–W174 (2019).
Acknowledgements
This work was supported by the James M. Cox Foundation, and J. C. Kennedy, funding from the Prostate Cancer Foundation and pilot funding from the Winship Cancer Institute supported by the Dunwoody Country Club Senior Men’s Association; H.T.K. was supported by the Cancer Research Institute Lloyd J. Old STAR program. NCI grants 1R01CA280069 (to H.T.K.) and U01-CA113913 (to M.G.S.); DOD grants W81XWH-20-1-0525 (to H.T.K. and V.A.M.) and HT9425-23-1-0318 (H.T.K.). H.T.K. was supported by ARPA-H funding (1AY1AX000001) C.B.M. was supported by the Howard Hughes Medical Institute Hanna H. Gray Fellowship (GT16001), the Burroughs Wellcome Fund – PDEP Fellowship (1022362) and the Cancer Research Institute Bristol Myers Squibb Fellowship (CRI4061). The authors acknowledge the Yerkes NHP Genomics Core (P51 OD011132, NIH S10 OD026799) and the Emory Flow Cytometry Core (UL1TR002378). All Tetramers were acquired from the NIH tetramer core, supported by contract 75N93020D0000.
Author information
Authors and Affiliations
Contributions
M.A.C. and H.T.K. designed the study, analysed data and composed the manuscript. M.A.C. conducted experiments with support from N.P., E.S., P.G. and R.M.V. R.G., L.D. and P.G. collected human tissue samples and organized clinical data. M.A.B., S.J., V.N., V.A.M. and M.G.S. provided clinical samples. N.P., E.S., C.B.M. and R.M.V, provided critical expertise and assisted in result interpretation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Phenotypic characterization of PD1 + CD45RA- CD4 T cells in kidney, bladder and prostate cancer patients.
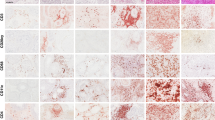
a) ScRNAseq analysis of PD1 + CD45RA- CD4 T cells in kidney cancer patients (n = 2). Bar plot showing cluster distribution between patients. b) UMAP projections of selected genes across all clusters. c) VISION GSEA using defined human CD4 T cell lineage (Treg, Th1, Tfh) and proliferation (cell cycle) signatures. UMAP projections show the top 10% scoring cells and the enrichment score for the signature is represented as violin plots for each cluster. Means shown for every violin plot and were analyzed by one-way ANOVA with Tukey’s multiple-comparison tests. d-e) Total quantification (d) and frequency of PD1+ (e) CD4 T cell infiltration in kidney (K n = 125), prostate (P n = 6) and bladder (B n = 17) tumors. f) Frequency of activated CD4 T cell populations based on transcription factor expression in human bladder (n = 17) and prostate (n = 6) tumors. g) Representative TCF1 and KI67 expression in activated CD4 T cells infiltrating human kidney tumors (n = 21). Mean ± 95% CI are represented and were analyzed by two-sided unpaired Mann Whitney U test. h) Representative plots of various phenotypic markers expressed in activated CD4 T cells in human kidney tumors. Mean ± s.d. are represented in each summary plot and were analyzed by Kruskal-Wallis test with Dunn’s multiple-comparison tests (n = 14–125 kidney cancer patients for each marker). i) Quantification of PD1 + CD4 T cell infiltration in kidney tumor draining lymph nodes, shown as the percent of total CD4 T cells (n = 12). j-k) Representative lineage transcription factor and PD1 expression in PD1 + CD45RA- CD4 T cell populations in kidney tumor draining lymph nodes (n = 9–12). Mean ± s.d. are represented in each summary plot and were analyzed by Kruskal-Wallis test with Dunn’s multiple-comparison tests. l) Phenotype of activated CD4 T cells in kidney tumor draining lymph nodes (n = 5–12 patients per marker). Mean ± s.d. are represented in each summary plot and were analyzed by Kruskal-Wallis test with Dunn’s multiple-comparison tests. Each point represents an individual patient. The activated populations were defined based on the following gates: Tregs: CD28 + FOXP3 + TBET- EOMES-, EOMES: CD28 + FOXP3- TBET-EOMES +, TFH: CD28 + FOXP3-TBET-EOMES-TCF1 + BCL6 +, Th1: CD28- FOXP3-TBET +, TCF1+lin-: CD28 + FOXP3-TBET-EOMES-TCF1 + BCL6-.
Extended Data Fig. 2 Tumor PD1 + TCF1+lin- CD4 T cells retain proliferation and differentiation capacity.
Representative CTV staining and expression of selected differentiation markers after five days of culturing TCF1+ lin- and CD39 + CD4 T cells in unstimulated (U) or a) Th0 (green, TCF1+ n = 11, CD39+ n = 8) d) Eomes (red, TCF1+ n = 9, CD39+ n = 6) and e) TFH (pink, TCF1+ n = 6, CD39+ n = 6) stimulating conditions. Summary plots show the frequency of cells within each population positive for the indicated marker on day 5. Each point represents an individual patient. Multiple conditions were performed with the same patient depending on cell sorting numbers. Median ± 95% CI are represented and analyzed by two-sided unpaired Mann–Whitney U test. U vs. S within the same population were compared across conditions. b) Frequency of original plated cells undergoing division for TCF1+ or CD39 + CD4 T cells in Th0 conditions. Median is represented and analyzed by two-sided unpaired Mann-Whitney U test. c) Frequency of TCF1+ or CD39 + CD4 T cells expressing the indicated marker on day 5 under Th1 (TCF1+ n = 13, CD39+ n = 2) or Treg stimulation (TCF1+ n = 10, CD39+ n = 5). Median ± 95% CI are represented and analyzed by two-sided unpaired Mann–Whitney U test. f) Summary plots represent the frequency of cells within each sorted population (TCF1+ lin- or CD39+) expressing the indicated transcription factor after 5 days of culture in each of the respective conditions tested. Cytokine stimulation conditions were the following: Th0 (IL-2), Th1 (IL-2 and IL-12), Treg (IL-2, TGF-beta, and anti-IFN gamma), Tfh (Activin A, IL-12), or Eomes (IL2, IL-12, IL-4, aIFNg). g-h) Representative plots of various markers on sorted TCF1+ lin- or CD39 + CD4 T cells after 5-days of 1:1 co-culture with patient matched dendritic cells (n = 7 patients for TCF1+ lin- and n = 4 patients for CD39+). Medians are shown and analyzed by two-sided unpaired Mann–Whitney U test. i) Frequency of original plated cells undergoing division for TCF1+ or CD39 + CD4 T cells after DC co-cultures. Medians are shown and analyzed by two-sided unpaired Mann–Whitney U test. j) Representative plot of TBET expression on TCF1+ lin- CD4 T cells in DC co-cultures with exogenous IL-12 (no IL12 n = 7, IL12 n = 4). Mean ± s.d. are shown and analyzed by two-sided unpaired Mann–Whitney U test between each division. k) Experimental design to test the capacity of PD1 + CD39 + CD4 T cells to proliferate with 4000U/ml of exogenous IL-2. l) Proliferation and FOXP3 expression on sorted CD39 + CD4 T cells after 5-days of CD3/CD2/CD28 bead stimulation with 10U/ml (low, n = 3) or 4000U/ml (high, n = 4) of exogenous IL-2. Medians are shown and analyzed by two-sided unpaired Mann–Whitney U test. m) UMAP projections of the distribution of TCR clonotypes corresponding to cells in the Treg (blue) or Eomes (red) clusters. Summary bar graph shows the cluster distribution of the 3 most dominant TCR clonotypes across all clusters for one patient. The number of cells sharing the respective TCR clonotype is indicated below. n) Correlation matrix showing the number of clonotypes shared by each population in the tumor for both patients.
Extended Data Fig. 3 Tumor specific CD4 T cells activate in TDLNs and rapidly acquire a TCF1+lin phenotype.
a) Representative I-AbGP66 tetramer staining and phenotype of GP66 + CD4 T cells in TDLNs one-week after TRAMPC1-GP inoculation. b-c) Phenotypic characterization of GP66 + CD4 T cells in TDLNs (b) or tumors (c) of 5-week TRAMPC1-GP bearing mice. Data are representative of 3–5 independent experiments (n > 5 mice for each marker). d) Representative CD44 and PD1 staining and phenotype of bulk PD1 + CD4 T cells in TDLNs of 5-week TRAMPC1-GP bearing mice. Summary plot shows the total numbers of activated (CD44 + PD1 +) CD4 T cell populations in each tissue. Kinetics plot shows the total number of activated CD4 T cells in each phenotype within TDLNs (n > = 5 mice for each timepoint for each individual experiment). e) Frequency of PD1 + CD4 T cells expressing FOXP3 in TDLNs 1-week after tumor inoculation or secondary lymphoid tissues 8-days after LCMV Armstrong infection. Data are representative of 2 independent experiments (n = 5–7 mice per group for each timepoint). f-g) Phenotype of GP66+ (f) or bulk PD1+ (g) CD4 T cells 12-days after B16-GP inoculation in TDLNs. Data are representative of 2 independent experiments (n = 10). h) Representative CD44 and PD1 staining, and phenotype of bulk PD1 + CD4 T cells in TDLNs of day 14 MC38 bearing mice. Data are representative of 2 independent experiments (n = 11). i) Representative TCF1 and BCL6 staining in GP66 + CD4 T cells on D8 LCMV Armstrong infected mice in the spleen. Summary plot shows the frequency of virus specific CD4 T cells in each phenotype. Data are representative of 2 independent experiments (n = 10). j) Phenotype of endogenous CD44 + PD1 + CD4 T cells in subcutaneous D21–28 RENCA-HA TDLNs and tumor (n = 8–12 mice). k) Phenotype of PD1 + CD4 T cells in orthotopic RENCA-HA-Luciferase in tumors 15-days after orthotopic implant (n = 9 mice).
Extended Data Fig. 4 TCF1+lin- CD4 T cells are stem-like cells that are actively restrained in the tumor response.
a) Experimental design to characterize SMARTA differentiation kinetics. b-e) Total numbers and representative phenotype of recovered SMARTAs in TDLNs and tumor 1- to 5-weeks after transfer in TRAMPC1-GP mice. Summary graphs show median or mean ± s.d. Data are representative of 1-2 independent experiments for each timepoint (n = 4–6 mice per group for each timepoint). f) Experimental design and total number of recovered SMARTAs in TDLNs and tumor 4-weeks post transfer. g) Representative histogram of PD-1 expression on recovered SMARTAs in TDLNs and tumor as compared to naïve CD4 T cells. h-i) Phenotypic analysis of SMARTAs in TDLNs and tumor 4-weeks post transfer (n = 9). Medians are shown for each summary plot. j) Representative phenotype of endogenous GP66 + CD4 T cells (top) or transferred SMARTAs (bottom) in the indicated tissue 8 days after LCMV Armstrong infection. Summary plots show median of the total number of GP66+ or SMARTAs in each tissue. k) Representative histograms for Th1 (TBET + TCF1-) and Tfh (TBET- TCF1 + CXCR5 +) populations for the endogenous GP66+ or SMARTAs in the spleen 8 days after LCMV Armstrong infection. Naïve (CD44- PD1-) CD4 T cells are plotted as a reference for each marker. Data are representative of 2 independent experiments (n = 8 recipient mice). l) Experimental design to test how PDL1 therapy affects stem-like CD4 differentiation in TRAMPC1-GP refractory tumor model. m) Tumor kinetics as shown by tumor diameter shown as mean ± s.d. for Untx and aPDL-1 treated mice and analyzed by two-sided unpaired Mann–Whitney U test (n = 5–7 mice per group). n) Phenotype of GP66 + CD4 T cells in TDLNs (top) and tumor (bottom) in Untx or aPDL-1 treated mice 14-days after treatment in TRAMPC1-GP bearing mice. Mean ± s.d. represented and analyzed by two-sided unpaired Mann–Whitney U test (n = 5–7 mice per group). o) Tumor kinetics shown as mean ± s.e.m. and phenotype of PD1 + CD4 T cells for Untx and PDL-1 treated mice in MC38 responsive tumor model. Median shown for phenotype summaries. Statistical comparisons were analyzed by two-sided unpaired Mann–Whitney U test (n = 7 mice per group). p) Phenotype of bulk activated CD4 T cells for untreated and PDL-1 treated mice in RENCA-HA responsive tumor model. Mean ± s.d. are represented and analyzed by two-sided unpaired Mann–Whitney U test (n = 4 mice per group).
Extended Data Fig. 5 Phenotypic and transcriptional profiling of antigen specific CD4 T cells generated after Treg depletion in cancer.
a-c) Phenotypic analysis of GP66+ and bulk PD1 + CD4 T cells in TDLNs, blood and tumor 5-days after Treg depletion. Median or mean ± s.e.m are shown and analyzed by two-sided unpaired Mann Whitney U or Kruskal-Wallis test with Dunn’s multiple-comparison tests when appropriate. Each individual timepoint represents an independent experiment (n = 2–17 mice per group for each time point). d) Schematic of experimental designs for B16F10-GP or MC38 tumors. e-f) Representative TBET by FOXP3 staining of bulk activated CD4 T cells in B16F10-GP (e) or MC38 (f) TDLNs 5-days after Treg depletion. Data represents 3-4 independent experiments (n = 4–5 mice per group for each experiment). Medians are shown and were analyzed by two sided unpaired Mann-Whitney U test or Kruskal-Wallis test with Dunn’s multiple-comparison tests when appropriate. g) Schematic of experimental design and phenotypic analysis of transferred SMARTAs in B16F10-GP TDLNs 5-days after Treg depletion. Data represents 2 independent experiments (n = 5 mice per group). Medians are represented in each summary plot and were analyzed by two-sided unpaired Mann-Whitney U test. h) ScRNAseq analysis of bulk activated (CD44 + PD1 +) CD4 T cells sorted from TDLNs of Untx or Treg depleted TRAMPC1-GP mice 5-days after depletion (n = 4-12 pooled mice per group). Naïve (CD44- PD1-) CD4 T cells were included as a control. UMAP projection and cluster distribution of activated CD4 T cells in TDLNs for both groups. i-j) Normalized expression of transcription factors and selected genes defining each cluster. k) VISION GSEA analysis using a signature from precursor TCF1+ BCL6low CD4 T cells from chronic LCMV infection for Untx and Treg depleted mice. Mean is represented in each violin and were analyzed by one-way ANOVA with Tukey’s multiple comparison tests. l) Transcriptional comparison of stem-like CD4 T cell cluster between groups. Volcano plots show fold change versus -log(p-value) for each gene. m) UMAP projection of the distribution of all the TCR clonotypes in the TCF1+ stem-like cluster in untreated mice (top). Cluster distribution of the 10 most dominant TCR clonotypes belonging to the Treg cluster in Untx mice. The number of cells sharing the respective TCR clonotype is indicated below (bottom).
Extended Data Fig. 6 Stem-like CD4 T cells are the source of Th1 cells in response to Treg depletion and aCTLA4 therapy.
a-b) Phenotypic analysis of recovered stem and Treg SMARTAs in TDLNs (a) and tumors (b) 7 days after transfer in WT or Treg depleted (DTR) recipients for each condition. Medians are represented in each summary graph and analyzed by Kruskal-Wallis test with Dunn’s multiple-comparison tests. Data are representative of 2 independent experiments (n = 13 for stem-like and n = 5 for Tregs). c) Representative FOXP3 and CTLA4 staining with accompanying CTLA4 geometric MFI in TDLNs and tumors in TRAMPC1-GP bearing mice. Medians are shown and analyzed by two sided unpaired Mann-Whitney U test (n = 10 mice). d) Experimental design to test how Tregs suppress stem-like differentiation through CTLA4 in TRAMPC1-GP bearing mice. e) Tumor kinetics shown as mean tumor diameter ± s.d. in untreated (n = 9), aCTLA4 4F10 (n = 9), or aCTLA4 9H10 (n = 9) mice and analyzed by Kruskal-Wallis test with Dunn’s multiple-comparison tests. f-g) Representative f) GP66 staining and g) phenotype of tumor specific CD4 T cells in TDLNs in each treatment group. h) Phenotype of bulk PD1 + CD4 T cells for each treatment group in tumors. Medians are represented in each summary graph and were analyzed by Kruskal-Wallis test with Dunn’s multiple-comparisons tests (n = 9 for each group). i) Experimental design to test the effects of aCTLA4 therapy in orthotopic RENCA-HA-Luciferase mice. j) Phenotype analysis of bulk PD1 + CD4 T cells in the tumor for untreated or 9H10 CTLA4 treated orthotopic RENCA-HA-Luc mice. Medians are shown and were analyzed by two sided unpaired Mann-Whitney U test (n = 9 for Untx and n = 5 for 9H10 aCTLA4 group).
Extended Data Fig. 7 CD4 T cell help is required for effector CD8 differentiation in TDLNs.
a-c) Th1 differentiation and tumor growth kinetics shown as mean ± s.d. for a) TRAMPC1-GP (Untx n = 8, aCD8 n = 6), b) B16F10-GP (n = 3 mice per group) and c) MC38 tumors (n = 4 mice per group) for Untx mice, Treg depleted mice, total CD4-depleted mice (GK1.5 clone), and combination of Treg and CD8 T cell (Lyt3.2 clone) depleted mice. Medians shown in summary plots. Statistical comparisons were performed using two-sided unpaired Mann-Whitney U test or Kruskal-Wallis test with Dunn’s multiple-comparison tests when appropriate. d-e) Phenotypic analysis of d) GP33+ and e) SPAS1 + CD8 T cells in TRAMPC1-GP TDLNs 5-days after Treg or total CD4 T cell depletions. f) Phenotype of GP33 + CD8 T cells in tumor 5-days after Treg or total CD4 T cell depletion. Every time point represents an independent experiment (n = 5–17 mice per group). Medians or mean ± s.e.m are represented in each summary plot and were analyzed by Kruskal-Wallis test with Dunn’s multiple-comparison tests. g) Schematic of experimental designs. h-i) Phenotypic analysis of bulk activated CD8 T cells in h) B16F10-GP i) or MC38 TDLNs after Treg or total CD4 depletion. Summary plots show total number or frequency of activated CD8 T cells expressing the indicated marker at the respective time points (n = 3–7 mice per group per timepoint for each independent experiment). Medians are represented in each summary plot and were analyzed by Kruskal-Wallis test with Dunn’s multiple-comparison tests. j) Experimental design. ScRNAseq of sorted naïve and activated (CD44 + PD1 +) CD8 T cells from TDLNs of Untx, Treg depleted, and total CD4 depleted TRAMPC1-GP bearing mice 5-days after depletion (n = 4–12 pooled mice per group). k-m) Cluster distributions and UMAP projections of normalized expression of genes defining the naïve, LN-stem, and effector CD8 clusters in all conditions. n) Transcriptional comparisons of LN-stem and effector CD8 T cells between groups. Volcano plots show fold change versus -log(p-value) for each gene within each respective comparison. o) Transcriptional comparison of selected genes across LN-stem (green) and effector (red) clusters between Untx, Treg depleted, and CD4 depleted groups. The color and size of the circles represent the normalized expression and proportion of cells expressing that gene, respectively. p) VISION GSEA using an IFN signaling signature. Signature enrichment score is represented as violin plots for LN-stem (green) and effector (red) clusters in the respective groups. Mean is represented in each violin and were analyzed by one-way ANOVA with Tukey’s multiple comparison tests (n = 4–12 pooled mice per group).
Extended Data Fig. 8 Localized Th1 response is sufficient to induce endogenous stem-like to Th1 differentiation and improve response to immunotherapy in the presence of Tregs.
a) Phenotype and cytokine production after GP61-77 stimulation of transferred SMARTAs across all groups in TDLNs. Summary plots show the frequency of SMARTAs expressing TBET and producing IFNg. b) Phenotype and cytokine production after GP61-77 stimulation of endogenous GP66 + CD4 T cells in TDLNs for each group. Summary plots show the total number of GP66 + CD4 T cells expressing TBET and producing IFNg. c-d) Representative GP66 staining in TDLNs for each group and summary of the phenotype of endogenous bulk and GP66 + CD4 T cell expressing Tbet in TDLNs and tumors. e-h) Phenotype of endogenous bulk activated (CD44 + PD1 +) or GP33 + CD8 T cells in e) TDLNs, f) blood and g) tumor for each group. Data represents 3 independent experiments. a-g) Median are represented in each summary plot and were analyzed by Kruskal Wallis test with Dunn’s multiple-comparison tests (n = 4–6 mice per group for each experiment). h) Individual tumor growth kinetics of TRAMPC1-GP mice after SMARTA transfer and tumor weights at endpoint for each group. Summary tumor weights represented as grams for each group. Medians are shown and were analyzed by Kruskal Wallis test with Dunn’s multiple-comparison tests (n = 4–6 mice per group for each independent experiment). i) Experimental design. j) Phenotype and cytokine production after GP61-77 stimulation of transferred WT or TetON TBET SMARTAs in TDLNs. k) Phenotype of endogenous PD1 + CD4 T cells in the tumor. Summaries show the frequency and number of Th1 and Tregs infiltrating the tumor. Data represents two independent experiments and were analyzed by two-sided unpaired Mann Whitney U test (n = 5–7 mice per group for each experiment).
Extended Data Fig. 9 Th1-derived IFNg is required for effective anti-tumor immunity.
a) Experimental design for systemic IFNg block in TRAMPC1-GP mice. b) TRAMPC1-GP tumor kinetics shown as tumor diameter. Data are representative of 2 independent experiments. Mean ± s.e.m. shown and were analyzed by Kruskal Wallis test with Dunn’s multiple comparison tests between groups (Untx n = 11, Treg dep. n = 13, Treg dep + aIFNg n = 8). c) Total numbers and phenotypic analysis of tumor specific GP66 + CD4 T cells in TDLNs 5-days after Treg depletion and IFNg block. d) Phenotypic analysis of GP33 + CD8 T cells in TDLNs in TRAMPC1-GP bearing mice after 5-days of Treg depletion and IFNg block. Medians are represented in each summary plot and were analyzed by One-way ANOVA test with Tukey’s multiple comparison tests between groups (n = 6-13 mice per group). e) Experimental design for systemic IFNg and IL-12 block in B16-F10-GP mice. f-g) Phenotypic analysis of CD44 + PD1 + CD4 or GP33 + CD8 T cells in TDLNs of B16F10-GP bearing mice 5-days after each depletion or cytokine blocks. Medians are represented in each summary plot and were analyzed by One-way ANOVA test with Tukey’s multiple comparison tests between groups (n = 4–8 mice per group). h) Cytokine production after GP61-77 stimulation of transferred TetON or IFNg KO TetON SMARTAs in TDLNs of TRAMPC1-GP mice. i) Individual tumor growth kinetics of TRAMPC1-GP mice after SMARTA transfer for each group. j) Phenotype and cytokine production (GP61-77 peptide stimulation) of endogenous GP66+ or bulk PD1 + CD4 T cells in TDLNs of mice receiving WT or IFNg KO TetON TBET SMARTAS. k-l) Phenotypic analysis of GP33 + CD8 T cells in TDLNs (k) and tumor (l) of mice receiving WT or IFNg KO TetON TBET SMARTAS. Median are represented in each summary plot and were analyzed by two-sided unpaired Mann Whitney U test between groups. (n = 6–8 mice per group).
Extended Data Fig. 10 IFNg is intrinsically required for tumor specific CD8 LN-stem to effector differentiation.
a) Schematic to make IFNgR1 KO P14s using an HSC BM chimera lentiviral system. b-d) Phenotypic analysis of transferred VEX+ P14s in WT mice with and without doxycycline administration and Treg depleted (DTR) mice without doxycycline treatment in TDLNs and tumor. Medians are represented in each summary plot (n = 4–5 mice per group). e-f) Representative flow cytometry stain of IFNgR1 and PD1 expression in WT and KO P14s after transfer into FOXP3-DTR TRAMPC1-GP mice. Endogenous GP33 + CD8 T cells in each respective mouse are included as a comparison. Medians are represented in each summary plot from one representative experiment and analyzed by two-sided unpaired Mann Whitney U test between WT and KO (n = 7 mice). g-h) Phenotype of transferred WT and IFNgR1 KO P14s in TDLNs 5-days after Treg depletion. i) Total number and phenotypic analysis of endogenous GP33 + CD8 T cell numbers in TDLNs in mice with transferred WT and KO P14s. j) P14 number and phenotype in the tumor 5-days after Treg depletion. Data are representative of 2 independent experiments (n = 5–6 mice per group for each experiment). Median are represented in each summary plot and were analyzed by two-sided unpaired Mann-Whitney U test. k) Representative phenotype of activated (PD1 + CD45RA-) CD4 T cells in primary tumors upon surgical resection in a cohort of 47 kidney cancer patients that received immunotherapy. Patients where stratified based on the % of total TBET + PD1 + CD4 T cells in the resected primary tumors prior to therapy. l) Representative phenotype of activated (PD1 + CD45RA-) CD8 T cells in Th1 low and Th1 high patients. Medians are represented and analyzed by two-sided unpaired Mann-Whitney U test (n = 47 kidney cancer patients). m) Spearman correlation between TBET + CD4 T cells and effector GZMB + CD8 T cell populations as a percent of total cells in non-metastatic draining lymph nodes from kidney cancer patients (n = 12). n) Proposed models of T cell differentiation states in TDLNs. Restricted TDLN state. Tregs actively suppress stem-like CD4 T cells, preventing their differentiation into Th1 cells and promoting iTreg differentiation. Minimal CD4 T cell help is provided in this state and CD8 T cells are maintained in an activated stem-like state in TDLNs. Anti-tumor response is not optimal, and tumor outcompetes the T cell response. Active TDLN state. In the absence of Treg suppression stem-like CD4 T cells undergo Th1 differentiation. Th1 CD4s secrete IFNg which promotes stem-like to effector CD8 T cell differentiation. Anti-tumor effector response is optimal, and tumor is controlled. Stem-like CD4 T cell differentiation can be targeted and stimulated to generate Th1 cells in the presence of Tregs. Stem to Th1 CD4 differentiation is then sufficient to switch between active and restricted states and rescue response to immunotherapy.
Supplementary information
Supplementary Data 1
Sorting layouts and lentiviral constructs. a) Sorting layout for stem-like (PD1+CD28+CD26+CD127+CD39−) and effector (PD1+CD28+CD26−CD127−CD39+) CD4 T cells from primary human tumours for functional in vitro assays. Dendritic cells were sorted as CD3−HLA−DR+CD11c+ from matched patient tumours for co-culture assays. b) Sorting layout for mouse TCF1+lin− SMARTAs from TDLNs of TRAMPC1-GP bearing mice 5-weeks after transfer. c) Experimental scheme to make bone marrow chimeras. Donor LSK+ cells were sorted on day 0 and expanded for 14 days in fibronectin coated plates. On day 14, expanded HSCs were spinfected with the lentivirus containing the gene of interest. HSCs were washed the following day and new medium was replaced. On day 16, 5,000–20,000 VEX+ HSCs were sorted to reconstitute congenically mismatched lethally irradiated mice. Chimerism was assessed 8 to 10 weeks after transfer. d) Sorting layout for mouse TCF1+lin− or Treg SMARTAs from TDLNs of TRAMPC1-GP bearing mice for re-transfer experiments into matched tumour mice. e) Lentiviral construct for TBET overexpression experiments. Tbet mRNA (513 amino acids) T2A joined to the VEX reporter was controlled by a tetracycline response element promoter. rtTA mice in the presence of doxycycline can bind and express TBET.
Supplementary Table 1
Antibody Table, lentiviral constructs and guide RNAs. List of human and mouse antibodies with respective fluorochrome, catalogue number, company and dilution used for flow cytometry. Guide RNAs used for generating knockouts using lentiviral constructs or through electroporation. Sequence of construct for TBET overexpression in SMARTAs.
Supplementary Table 2
Differential gene expression between CD4 and CD8 T cell populations in TDLNs between groups in TRAMPC1-GP mice. Comparisons were performed using the FindMarkers() function in Seurat based on the MAST test. Significance was determined as genes with a fold change greater than 0.2 and a P value < 10−10 between groups.
Supplementary Table 3
NicheNet ligand–receptor analysis was performed between TDLN CD4 and CD8 T cell populations in wild-type and Treg-depleted mice. Scoring of the predicted ligand–receptor pairs based on the NicheNet vignette Pearson correlation analysis and we selected the top 10 based on prioritization score for each CD4 helper population.
Supplementary Table 4
List of genes for human and mouse signatures used in GSEA VISION analysis. Last four columns include signatures from CD4 populations from our own scRNA-seq based on significant differential gene expression analyses.
Supplementary Table 5
Descriptive table of cohort of patients with kidney cancer used for survival analysis. Demographics, survival outcomes, and flow cytometry data collected for each patient is listed.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cardenas, M.A., Prokhnevska, N., Sobierajska, E. et al. Differentiation fate of a stem-like CD4 T cell controls immunity to cancer. Nature 636, 224–232 (2024). https://doi.org/10.1038/s41586-024-08076-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-08076-7
This article is cited by
-
Beyond T-cell subsets: stemness and adaptation redefining immunity and immunotherapy
Cellular & Molecular Immunology (2025)
-
SIRT7 regulates T-cell antitumor immunity through modulation BCAA and fatty acid metabolism
Cell Death & Differentiation (2025)
-
Mature tertiary lymphoid structures evoke intra-tumoral T and B cell responses via progenitor exhausted CD4+ T cells in head and neck cancer
Nature Communications (2025)
-
Mapping T helper cell targets with deep learning
Nature Machine Intelligence (2025)
-
Unraveling the gut–immune–kidney axis in kidney stone disease: a two-step Mendelian randomization investigation
Urolithiasis (2025)