Abstract
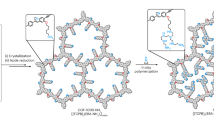
Capture of CO2 from the air offers a promising approach to addressing climate change and achieving carbon neutrality goals1,2. However, the development of a durable material with high capacity, fast kinetics and low regeneration temperature for CO2 capture, especially from the intricate and dynamic atmosphere, is still lacking. Here a porous, crystalline covalent organic framework (COF) with olefin linkages has been synthesized, structurally characterized and post-synthetically modified by the covalent attachment of amine initiators for producing polyamines within the pores. This COF (termed COF-999) can capture CO2 from open air. COF-999 has a capacity of 0.96 mmol g–1 under dry conditions and 2.05 mmol g–1 under 50% relative humidity, both from 400 ppm CO2. This COF was tested for more than 100 adsorption–desorption cycles in the open air of Berkeley, California, and found to fully retain its performance. COF-999 is an exceptional material for the capture of CO2 from open air as evidenced by its cycling stability, facile uptake of CO2 (reaches half capacity in 18.8 min) and low regeneration temperature (60 °C).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All experimental data are available in the main text or Supplementary Information. Computational results are available on Zenodo (https://doi.org/10.5281/zenodo.13382234) (ref. 42). Source data are provided with this paper.
Change history
28 October 2024
In the version of the article initially published, in Fig. 3c, the x axis read “0, 0.2, 0.2, 0.4, 0.8, 1.0” and has now been amended to “0, 0.2, 0.4, 0.6, 0.8, 1.0” in the HTML and PDF versions of the article.
04 December 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41586-024-08464-z
References
Lackner, K., Ziock, H.-J. & Grimes, P. Carbon dioxide extraction from air: is it an option? in 24th Annual Technical Conference on Coal Utilization and Fuel Systems (Clearwater, 1999).
Lackner, K. S. et al. The urgency of the development of CO2 capture from ambient air. Proc. Natl Acad. Sci. USA 109, 13156–13162 (2012).
Sanz-Pérez, E. S., Murdock, C. R., Didas, S. A. & Jones, C. W. Direct capture of CO2 from ambient air. Chem. Rev. 116, 11840–11876 (2016).
Shi, X. et al. Sorbents for the direct capture of CO2 from ambient air. Angew. Chem. Int. Ed. 59, 6984–7006 (2020).
Zhu, X. et al. Recent advances in direct air capture by adsorption. Chem. Soc. Rev. 51, 6574–6651 (2022).
Brethomé, F. M., Williams, N. J., Seipp, C. A., Kidder, M. K. & Custelcean, R. Direct air capture of CO2 via aqueous-phase absorption and crystalline-phase release using concentrated solar power. Nat. Energy 3, 553–559 (2018).
Keith, D. W., Holmes, G., St. Angelo, D. & Heidel, K. A process for capturing CO2 from the atmosphere. Joule 2, 1573–1594 (2018).
Shekhah, O. et al. Made-to-order metal-organic frameworks for trace carbon dioxide removal and air capture. Nat. Commun. 5, 4228 (2014).
McDonald, T. M. et al. Capture of carbon dioxide from air and flue gas in the alkylamine-appended metal-organic framework mmen-Mg2(dobpdc). J. Am. Chem. Soc. 134, 7056–7065 (2012).
Bien, C. E. et al. Bioinspired metal-organic framework for trace CO2 capture. J. Am. Chem. Soc. 140, 12662–12666 (2018).
Chen, O. I.-F. et al. Water-enhanced direct air capture of carbon dioxide in metal-organic frameworks. J. Am. Chem. Soc. 146, 2835–2844 (2024).
Nugent, P. et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 495, 80–84 (2013).
Deutz, S. & Bardow, A. Life-cycle assessment of an industrial direct air capture process based on temperature–vacuum swing adsorption. Nat. Energy 6, 203–213 (2021).
Miao, Y., He, Z., Zhu, X., Izikowitz, D. & Li, J. Operating temperatures affect direct air capture of CO2 in polyamine-loaded mesoporous silica. Chem. Eng. J. 426, 131875 (2021).
Rim, G., Feric, T. G., Moore, T. & Park, A. H. A. Solvent impregnated polymers loaded with liquid-like nanoparticle organic hybrid materials for enhanced kinetics of direct air capture and point source CO2 capture. Adv. Funct. Mater. 31, 2010047 (2021).
Choe, J. H. et al. Boc protection for diamine-appended MOF adsorbents to enhance CO2 recyclability under realistic humid conditions. J. Am. Chem. Soc. 146, 646–659 (2024).
Barsoum, M. L. et al. Probing structural transformations and degradation mechanisms by direct observation in SIFSIX-3-Ni for direct air capture. J. Am. Chem. Soc. 146, 6557–6565 (2024).
Carneiro, J. S. A. et al. Insights into the oxidative degradation mechanism of solid amine sorbents for CO2 capture from air: roles of atmospheric water. Angew. Chem. Int. Ed. 62, e2023028 (2023).
Yaghi, O. M., Kalmutzki, M. J. & Diercks, C. S. Introduction to Reticular Chemistry: Metal‐Organic Frameworks and Covalent Organic Frameworks (Wiley, 2019).
Diercks, C. S. & Yaghi, O. M. The atom, the molecule, and the covalent organic framework. Science 355, eaal158 (2017).
Li, H., Dilipkumar, A., Abubakar, S. & Zhao, D. Covalent organic frameworks for CO2 capture: from laboratory curiosity to industry implementation. Chem. Soc. Rev. 52, 6294–6329 (2023).
Lyu, H., Li, H., Hanikel, N., Wang, K. & Yaghi, O. M. Covalent organic frameworks for carbon dioxide capture from air. J. Am. Chem. Soc. 144, 12989–12995 (2022).
Lin, J.-B. et al. A scalable metal-organic framework as a durable physisorbent for carbon dioxide capture. Science 374, 1464–1469 (2021).
Quang, D. V. et al. Effect of moisture on the heat capacity and the regeneration heat required for CO2 capture process using PEI impregnated mesoporous precipitated silica. Greenhouse Gases Sci. Technol. 5, 91–101 (2015).
Jin, E. et al. Two-dimensional sp2 carbon–conjugated covalent organic frameworks. Science 357, 673–676 (2017).
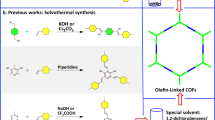
Lyu, H., Diercks, C. S., Zhu, C. & Yaghi, O. M. Porous crystalline olefin-linked covalent organic frameworks. J. Am. Chem. Soc. 141, 6848–6852 (2019).
Pawley, G. S. Unit-cell refinement from powder diffraction scans. J. Appl. Crystallogr. 14, 357–361 (1981).
Brunauer, S., Emmett, P. H. & Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938).
Ji, W. et al. Removal of GenX and perfluorinated alkyl substances from water by amine-functionalized covalent organic frameworks. J. Am. Chem. Soc. 140, 12677–12681 (2018).
Mao, H. et al. A scalable solid-state nanoporous network with atomic-level interaction design for carbon dioxide capture. Sci. Adv. 8, eabo6849 (2022).
McCabe, W. L., Smith, J. C. & Harriott P. Unit Operations of Chemical Engineering 7th edn (McGraw Hill, 2004).
Panda, D., Kulkarni, V. & Singh, S. K. Evaluation of amine-based solid adsorbents for direct air capture: a critical review. React. Chem. Eng. 8, 10–40 (2023).
Kolle, J. M., Fayaz, M. & Sayari, A. Understanding the effect of water on CO2 adsorption. Chem. Rev. 121, 7280–7345 (2021).
Ilkaeva, M. et al. Assessing CO2 capture in porous sorbents via solid-state NMR-assisted adsorption techniques. J. Am. Chem. Soc. 145, 8764–8769 (2023).
Fung, B. M., Khitrin, A. K. & Ermolaev, K. An improved broadband decoupling sequence for liquid crystals and solids. J. Magn. Reson. 142, 97–101 (2000).
Johnson, R. L. & Schmidt-Rohr, K. Quantitative solid-state 13C NMR with signal enhancement by multiple cross polarization. J. Magn. Reson. 239, 44–49 (2014).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Zhou, Z. et al. Computational results for the publication “Carbon dioxide capture from open air using covalent organic frameworks”. Zenodo https://doi.org/10.5281/zenodo.13382234 (2024).
Acknowledgements
Z.Z. thanks H. Lyu, O. I.-F. Chen, Z. Rong and W. Xu (Yaghi Research Group, UC Berkeley) for their discussions. We thank H. Celik and the Core NMR Facility of UC Berkeley Pines Magnetic Resonance Center for spectroscopic assistance. We also thank the UC Berkeley Electron Microscope Laboratory for access and assistance in electron microscopy data collection. This research was supported by the King Abdulaziz City for Science and Technology (Center of Excellence for Nanomaterials and Clean Energy Applications), ATOCO and the Bakar Institute of Digital Materials for the Planet. The NMR instruments used in this work were supported by the National Science Foundation under grant no. 2018784 and by the National Institutes of Health under grant S10OD024998. Z.Z. and O.M.Y. acknowledge the interest and support of Fifth Generation (Love, Tito’s). S.E. thanks the Free State of Saxony and the European Union (Low Surface and Pore Sorption LSPS) for financial support. J.S. is also a distinguished visiting scholar at UC Berkeley.
Author information
Authors and Affiliations
Contributions
Z.Z. and O.M.Y. conceived the idea and led the experimental efforts. Z.Z. designed the COFs and developed synthetic methodologies. Z.Z., T.M., H.Z. and C.L. conducted the synthesis of linkers and COF-999-N3. T.M. and Z.Z. collected and analysed the SEM, PXRD, thermogravimetric analysis and FT-IR data. Z.Z., R.G. and K.W. conducted the NMR experiments. Z.Z., K.W., T.M., S.E. and A.H.A. collected the gas sorption data. Z.Z., H.L. and S.E. performed the breakthrough experiments. S.C. and J.S. led the computational analysis. S.C. conducted the DFT calculations. L.G. advised on the computational setup. M.M.A. and M.O.A. provided valuable suggestions throughout this study. Z.Z., T.M. and O.M.Y. prepared the initial draft and finalized it. All authors contributed to revising the paper.
Corresponding authors
Ethics declarations
Competing interests
COF-999 and its related materials have been filed as US Provisional Patent Application no. 63/587,185 by UC Berkeley. O.M.Y. and Z.Z. are the inventors of this patent. O.M.Y. is a co-founder of ATOCO, aiming at commercializing related technologies. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 CO2 adsorption structures in COF-999.
a, Formation of carbamic acid under dry conditions. b, Formation of carbamic acid/carbamate under humid conditions. c, Formation of bicarbonate under humid conditions. All numbers represent atom distances in pm. C, gray; N, blue; O, red; H, white. Additional structures are shown in Supplementary Information section 14.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Z., Ma, T., Zhang, H. et al. Carbon dioxide capture from open air using covalent organic frameworks. Nature 635, 96–101 (2024). https://doi.org/10.1038/s41586-024-08080-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-08080-x
This article is cited by
-
Towards single-crystalline two-dimensional poly(arylene vinylene) covalent organic frameworks
Nature Chemistry (2026)
-
Fast and selective CO2 capture from outdoor air by covalent organic frameworks
Nature Sustainability (2026)
-
Thiazole-conjugated covalent organic framework membranes enable ultraselective molecular desalination under strongly acidic conditions
Nature Communications (2026)
-
Direct air capture of CO2 for solar fuel production in flow
Nature Energy (2025)
-
Diffusion-driven selective crystallization of high-purity salt through simple and sustainable one-step evaporation
Nature Water (2025)