Abstract
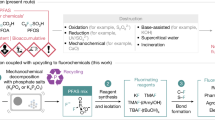
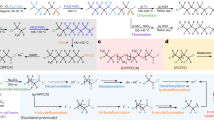
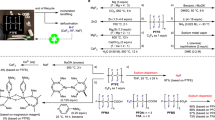
Polyfluoroalkyl and perfluoroalkyl substances (PFASs) are found in many everyday consumer products, often because of their high thermal and chemical stabilities, as well as their hydrophobic and oleophobic properties1. However, the inert carbon–fluorine (C–F) bonds that give PFASs their properties also provide resistance to decomposition through defluorination, leading to long-term persistence in the environment, as well as in the human body, raising substantial safety and health concerns1,2,3,4,5. Despite recent advances in non-incineration approaches for the destruction of functionalized PFASs, processes for the recycling of perfluorocarbons (PFCs) as well as polymeric PFASs such as polytetrafluoroethylene (PTFE) are limited to methods that use either elevated temperatures or strong reducing reagents. Here we report the defluorination of PFASs with a highly twisted carbazole-cored super-photoreductant KQGZ. A series of PFASs could be defluorinated photocatalytically at 40–60 °C. PTFE gave amorphous carbon and fluoride salts as the major products. Oligomeric PFASs such as PFCs, perfluorooctane sulfonic acid (PFOS), polyfluorooctanoic acid (PFOA) and derivatives give carbonate, formate, oxalate and trifluoroacetate as the defluorinated products. This allows for the recycling of fluorine in PFASs as inorganic fluoride salt. The mechanistic investigation reveals the difference in reaction behaviour and product components for PTFE and oligomeric PFASs. This work opens a window for the low-temperature photoreductive defluorination of the ‘forever chemicals’ PFASs, especially for PTFE, as well as the discovery of new super-photoreductants.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the main text or the supplementary materials.
Change history
26 November 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41586-025-09871-6
References
Evich, M. G. et al. Per- and polyfluoroalkyl substances in the environment. Science 375, eabg9065 (2022).
Washington, J. W. et al. Nontargeted mass-spectral detection of chloroperfluoropolyether carboxylates in New Jersey soils. Science 368, 1103–1107 (2020).
Singh, K., Kumar, N., Yadav, A. K., Singh, R. & Kumar, K. Per- and polyfluoroalkyl substances (PFAS) as a health hazard: current state of knowledge and strategies in environmental settings across Asia and future perspectives. Chem. Eng. J. 475, 145065 (2023).
Sunderland, E. M. et al. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 29, 131–147 (2019).
Gaballah, S. et al. Evaluation of developmental toxicity, developmental neurotoxicity, and tissue dose in zebrafish exposed to GenX and other PFAS. Environ. Health Perspect. 128, 047005 (2020).
Bentel, M. J. et al. Defluorination of per- and polyfluoroalkyl substances (PFASs) with hydrated electrons: structural dependence and implications to PFAS remediation and management. Environ. Sci. Technol. 53, 3718–3728 (2019).
Bentel, M. J. et al. Degradation of perfluoroalkyl ether carboxylic acids with hydrated electrons: structure–reactivity relationships and environmental implications. Environ. Sci. Technol. 54, 2489–2499 (2020).
Liu, Z. et al. Accelerated degradation of perfluorosulfonates and perfluorocarboxylates by UV/sulfite + iodide: reaction mechanisms and system efficiencies. Environ. Sci. Technol. 56, 3699–3709 (2022).
Gao, J. et al. Photochemical degradation pathways and near-complete defluorination of chlorinated polyfluoroalkyl substances. Nat. Water 1, 381–390 (2023).
Hao, S. et al. Hydrothermal alkaline treatment for destruction of per- and polyfluoroalkyl substances in aqueous film-forming foam. Environ. Sci. Technol. 55, 3283–3295 (2021).
Yang, N. et al. Solvent-free nonthermal destruction of PFAS chemicals and PFAS in sediment by piezoelectric ball milling. Environ. Sci. Technol. Lett. 10, 198–203 (2023).
Schaefer, C. E. et al. Electrochemical transformations of perfluoroalkyl acid (PFAA) precursors and PFAAs in groundwater impacted with aqueous film forming foams. Environ. Sci. Technol. 52, 10689–10697 (2018).
Singh, R. K. et al. Rapid removal of poly- and perfluorinated compounds from investigation-derived waste (IDW) in a pilot-scale plasma reactor. Environ. Sci. Technol. 53, 11375–11382 (2019).
Baumgartner, R., Stieger, G. K. & McNeill, K. Complete hydrodehalogenation of polyfluorinated and other polyhalogenated benzenes under mild catalytic conditions. Environ. Sci. Technol. 47, 6545–6553 (2013).
Douvris, C. & Ozerov, O. V. Hydrodefluorination of perfluoroalkyl groups using silylium-carborane catalysts. Science 321, 1188–1190 (2008).
Trang, B. et al. Low-temperature mineralization of perfluorocarboxylic acids. Science 377, 839–845 (2022).
Puts, G. J., Crouse, P. & Ameduri, B. M. Polytetrafluoroethylene: synthesis and characterization of the original extreme polymer. Chem. Rev. 119, 1763–1805 (2019).
Améduri, B. & Hori, H. Recycling and the end of life assessment of fluoropolymers: recent developments, challenges and future trends. Chem. Soc. Rev. 52, 4208–4247 (2023).
Yang, X. et al. A chemical route from PTFE to amorphous carbon nanospheres in supercritical water. Chem. Commun. 342–343 (2004).
Simon, C. M. & Kaminsky, W. Chemical recycling of polytetrafluoroethylene by pyrolysis. Polym. Degrad. Stab. 62, 1–7 (1998).
Ellis, D. A., Mabury, S. A., Martin, J. W. & Muir, D. C. G. Thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment. Nature 412, 321–324 (2001).
Koch, E.-C. Metal‐Fluorocarbon Based Energetic Materials (Wiley, 2011).
Nelson, E., Kilduff, T. J. & Benderly, A. A. Bonding of Teflon. Ind. Eng. Chem. 50, 329–330 (1958).
Yoshino, K. et al. Conducting polymer prepared from teflon. Jpn. J. Appl. Phys. 21, L301–L302 (1982).
Chakrabarti, N. & Jacobus, J. The chemical reduction of poly(tetrafluoroethylene). Macromolecules 21, 3011–3014 (1988).
Costello, C. A. & McCarthy, T. J. Surface modification of poly(tetrafluoroethylene) with benzoin dianion. Macromolecules 17, 2940–2942 (1984).
Costello, C. A. & McCarthy, T. J. Surface-selective introduction of specific functionalities onto poly(tetrafluoroethylene). Macromolecules 20, 2819–2828 (1987).
Kavan, L., Dousek, F. P., Janda, P. & Weber, J. Carbonization of highly oriented poly(tetrafluoroethylene). Chem. Mater. 11, 329–335 (1999).
Sheldon, D. J., Parr, J. M. & Crimmin, M. R. Room temperature defluorination of poly(tetrafluoroethylene) by a magnesium reagent. J. Am. Chem. Soc. 145, 10486–10490 (2023).
Wu, Y., Kim, D. & Teets, T. S. Photophysical properties and redox potentials of photosensitizers for organic photoredox transformations. Synlett 33, 1154–1179 (2022).
Liang, K. et al. Intermolecular oxyarylation of olefins with aryl halides and TEMPOH catalyzed by the phenolate anion under visible light. Chem. Sci. 11, 6996–7002 (2020).
Kim, H., Kim, H., Lambert, T. H. & Lin, S. Reductive electrophotocatalysis: merging electricity and light to achieve extreme reduction potential. J. Am. Chem. Soc. 142, 2087–2092 (2020).
Wu, S., Schiel, F. & Melchiorre, P. A general light-driven organocatalytic platform for the activation of inert substrates. Angew. Chem. Int. Ed. 62, e202306364 (2023).
Halder, S., Mandal, S., Kundu, A., Mandal, B. & Adhikari, D. Super-reducing behavior of benzo[b]phenothiazine anion under visible-light photoredox condition. J. Am. Chem. Soc. 145, 22403–22412 (2023).
MacKenzie, I. A. et al. Discovery and characterization of an acridine radical photoreductant. Nature 580, 76–81 (2020).
Cole, J. P. et al. Organocatalyzed birch reduction driven by visible light. J. Am. Chem. Soc. 142, 13573–13581 (2020).
Xiao, Z. F. et al. Iridium-catalyzed cyclization of isoxazolines and alkenes: divergent access to pyrrolidines, pyrroles, and carbazoles. Org. Lett. 18, 5672–5675 (2016).
Luan, Z. H., Qu, J. P. & Kang, Y. B. Discovery of oxygen α-nucleophilic addition to α,β-unsaturated amides catalyzed by redox-neutral organic photoreductant. J. Am. Chem. Soc. 142, 20942–20947 (2020).
Wang, S. D., Yang, B., Zhang, H., Qu, J. P. & Kang, Y. B. Reductive cleavage of C–X or N–S bonds catalyzed by super organoreductant CBZ6. Org. Lett. 25, 816–820 (2023).
Yabuta, T., Hayashi, M. & Matsubara, R. Photocatalytic reductive C–O bond cleavage of alkyl aryl ethers by using carbazole catalysts with cesium carbonate. J. Org. Chem. 86, 2545–2555 (2021).
Sap, J. B. I. et al. Organophotoredox hydrodefluorination of trifluoromethylarenes with translational applicability to drug discovery. J. Am. Chem. Soc. 142, 9181–9187 (2020).
Chen, K., Berg, N., Gschwind, R. & König, B. Selective single C(sp3)–F bond cleavage in trifluoromethylarenes: merging visible-light catalysis with Lewis acid activation. J. Am. Chem. Soc. 139, 18444–18447 (2017).
Wang, H. & Jui, N. T. Catalytic defluoroalkylation of trifluoromethylaromatics with unactivated alkenes. J. Am. Chem. Soc. 140, 163–166 (2018).
Picheau, E., Amar, S., Derré, A., Pénicaud, A. & Hof, F. An introduction to the combustion of carbon materials. Chem. Eur. J. 28, e202200117 (2022).
Patrick, J. S., Pradeep, T., Luo, H., Ma, S. & Cooks, R. G. Gas-phase C-F bond cleavage in perfluorohexane using W-, Si-, P-, Br-, and I-containing ions: comparisons with reactions at fluorocarbon surfaces. J. Am. Soc. Mass. Spectrom. 9, 1158–1167 (1998).
Vogt, D. B., Seath, C. P., Wang, H. & Jui, N. T. Selective C–F functionalization of unactivated trifluoromethylarenes. J. Am. Chem. Soc. 141, 13203–13211 (2019).
Campbell, M. W. et al. Photochemical C–F activation enables defluorinative alkylation of trifluoroacetates and -acetamides. J. Am. Chem. Soc. 143, 19648–19654 (2021).
Ye, J. H., Bellotti, P., Heusel, C. & Glorius, F. Photoredox-catalyzed defluorinative functionalizations of polyfluorinated aliphatic amides and esters. Angew. Chem. Int. Ed. 61, e202115456 (2022).
Nagai, Y., Smith, R. L.Jr., Inomata, H. & Arai, K. Direct observation of polyvinylchloride degradation in water at temperatures up to 500°C and at pressures up to 700 MPa. J. Appl. Polym. Sci. 106, 1075–1086 (2007).
Campbell, S. F., Stephens, R. & Tatlow, J. C. Perfluorocycloalkenyl-lithium compounds. Chem. Commun. 151–152 (1967).
Acknowledgements
This paper is dedicated to Yong Tang at Shanghai institute of Organic Chemistry on the occasion of his 60th birthday. We appreciate the financial support from the National Key R&D Program of China (no. 2021YFA1500100) and the National Natural Science Foundation of China (22271268). We thank Q. Li at the University of Science and Technology of China for assistance with the X-ray photoelectron spectroscopy measurements and characterizations. We thank K. Gong at the University of Science and Technology of China for helpful discussions about solid-state magic-angle spinning nuclear magnetic resonance characterizations.
Author information
Authors and Affiliations
Contributions
H.Z. performed all experiments and analysed the data in the main text and supplementary information, with guidance from Y.-B.K. and J.-P.Q., unless otherwise stated. J.-X.C. assisted H.Z., synthesized the photocatalyst shown in Fig. 1 and performed the reactions in Fig. 4a,b and the analysis. Y.-B.K. and J.-P.Q. conceived the research, designed the experiments, supervised experiments and analyses, interpreted the data, generated figures and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A patent application has been filed by the University of Science and Technology of China on photocatalytic defluorination of polyfluoroalkyl and perfluoroalkyl substances.
Peer review
Peer review information
Nature thanks Jinyong Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Sections 1–16, including Supplementary Figs. 1–105, Supplementary Data and Supplementary References.
Supplementary Video 1
Combustion experiment of the defluorinated amorphous carbon product. The defluorinated amorphous carbon product sustains smouldering and stays red-hot without any flame when it is heated by an alcohol burner. The red-hot ember becomes a black solid when the alcohol burner is removed.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Chen, JX., Qu, JP. et al. Photocatalytic low-temperature defluorination of PFASs. Nature 635, 610–617 (2024). https://doi.org/10.1038/s41586-024-08179-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-08179-1
This article is cited by
-
Rethinking sustainable pathways for PFAS
Nature Chemistry (2026)
-
Room-temperature defluorination of PTFE and PFAS via sodium dispersion
Nature Communications (2025)
-
Upcycling of PTFE and PVDF to fluorochemicals through mechanochemical process
Nature Communications (2025)
-
How to get rid of toxic ‘forever chemical’ pollution
Nature (2025)
-
Solar-driven defluorination via hydroxyl radical spillover for complete mineralization of organofluorine pollutants without fluoride byproducts
Communications Chemistry (2025)