Abstract
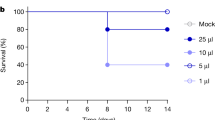
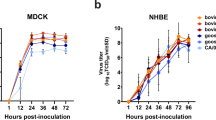
The outbreak of clade 2.3.4.4b highly pathogenic avian influenza viruses of the H5N1 subtype (HPAI H5N1) in dairy cattle in the USA has so far resulted in spillover infections of at least 14 farm workers1,2,3, who presented with mild respiratory symptoms or conjunctivitis, and one individual with no known animal exposure who was hospitalized but recovered3,4. Here we characterized A/Texas/37/2024 (huTX37-H5N1), a virus isolated from the eyes of an infected farm worker who developed conjunctivitis5. huTX37-H5N1 replicated efficiently in primary human alveolar epithelial cells, but less efficiently in corneal epithelial cells. Despite causing mild disease in the infected worker, huTX37-H5N1 proved lethal in mice and ferrets and spread systemically, with high titres in both respiratory and non-respiratory organs. Importantly, in four independent experiments in ferrets, huTX37-H5N1 transmitted by respiratory droplets in 17–33% of transmission pairs, and five of six exposed ferrets that became infected died. PB2-631L (encoded by bovine isolates) promoted influenza polymerase activity in human cells, suggesting a role in mammalian adaptation similar to that of PB2-627K (encoded by huTX37-H5N1). In addition, bovine HPAI H5N1 virus was found to be susceptible to polymerase inhibitors both in vitro and in mice. Thus, HPAI H5N1 virus derived from dairy cattle transmits by respiratory droplets in mammals without previous adaptation and causes lethal disease in animal models.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Deep-sequencing data have been deposited to the Sequence Read Archive (SRA) under bioproject accession no. PRJNA1163435. SRA accession numbers for individual samples are provided in Supplementary Table 4. Source data are provided with this paper.
References
CDC Reports Fourth Human Case of H5 Bird Flu Tied to Dairy Cow Outbreak. CDC www.cdc.gov/media/releases/2024/p-0703-4th-human-case-h5.html (2024).
Cluster of Influenza A(H5) Cases Associated with Poultry Exposure at Two Facilities — Colorado, July 2024. CDC www.cdc.gov/mmwr/volumes/73/wr/mm7334a1.htm (2024).
H5 Bird Flu: Current Situation. CDC www.cdc.gov/bird-flu/situation-summary/index.html (2024).
CDC Confirms Human H5 Bird Flu Case in Missouri. CDC www.cdc.gov/media/releases/2024/s0906-birdflu-case-missouri.html (2024).
Uyeki, T. M. et al. Highly pathogenic avian influenza A(H5N1) virus infection in a dairy farm worker. N. Engl. J. Med. 390, 2028–2029 (2024).
HPAI Confirmed Cases in Livestock. USDA APHIS www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/hpai-confirmed-cases-livestock (accessed 20 September 2024).
Eisfeld, A. J. et al. Pathogenicity and transmissibility of bovine H5N1 influenza virus. Nature 633, 426–432 (2024).
Guan, L. et al. Cow’s milk containing avian Iifluenza A(H5N1) virus – heat inactivation and infectivity in mice. N. Engl. J. Med. 391, 87–90 (2024).
Detections of Highly Pathogenic Avian Influenza in Mammals. USDA APHIS www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/mammals (2024).
Confirmations of Highly Pathogenic Avian Influenza in Commercial and Backyard Flocks. USDA APHIS www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/commercial-backyard-flocks (2024).
Hatta, M., Gao, P., Halfmann, P. & Kawaoka, Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293, 1840–1842 (2001).
Subbarao, E. K., London, W. & Murphy, B. R. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J. Virol. 67, 1761–1764 (1993).
CDC Reports A(H5N1) Ferret Study Results. CDC www.cdc.gov/bird-flu/spotlights/ferret-study-results.html (2024).
Hatta, M. et al. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS Pathog. 3, 1374–1379 (2007).
Maemura, T. et al. Characterization of highly pathogenic clade 2.3.4.4b H5N1 mink influenza viruses. EBioMedicine 97, 104827 (2023).
Imai, M. et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486, 420–428 (2012).
Idoko-Akoh, A. et al. Creating resistance to avian influenza infection through genome editing of the ANP32 gene family. Nat. Commun. 14, 6136 (2023).
Good, M. R., Ji, W., Fernandez-Quintero, M. L., Ward, A. B. & Guthmiller, J. A single mutation in dairy cow-associated H5N1 viruses increases receptor binding breadth. Preprint at bioRxiv https://doi.org/10.1101/2024.06.22.600211 (2024).
Chopra, P. et al. Receptor binding specificity of a bovine A(H5N1) influenza virus. Preprint at bioRxiv https://doi.org/10.1101/2024.07.30.605893 (2024).
Santos, J. J. S. et al. Bovine H5N1 influenza virus binds poorly to human-type sialic acid receptors. Preprint at bioRxiv https://doi.org/10.1101/2024.08.01.606177 (2024).
Andreev, K. et al. Antiviral susceptibility of highly pathogenic avian influenza A(H5N1) viruses circulating globally in 2022-2023. J. Infect. Dis. 229, 1830–1835 (2024).
Nguyen, T. Q. et al. Emergence and interstate spread of highly pathogenic avian influenza A(H5N1) in dairy cattle. Preprint at bioRxiv https://doi.org/10.1101/2024.05.01.591751 (2024).
Imai, M. et al. A highly pathogenic avian H7N9 influenza virus isolated from a human is lethal in some ferrets infected via respiratory droplets. Cell Host Microbe 22, 615–626 (2017).
Belser, J. A., Sun, X., Pulit-Penaloza, J. A. & Maines, T. R. Fatal infection in ferrets after ocular inoculation with highly pathogenic avian influenza A(H5N1) virus. Emerg. Infect. Dis. 30, 1484–1487 (2024).
Pulit-Penaloza, J. A. et al. Highly pathogenic avian influenza A(H5N1) virus of clade 2.3.4.4b isolated from a human case in Chile causes fatal disease and transmits between co-housed ferrets. Emerg. Microbes Infect. 13, 2332667 (2024).
Restori, K. H. et al. Risk assessment of a highly pathogenic H5N1 influenza virus from mink. Nat. Commun. 15, 4112 (2024).
Zhang, X. et al. Enhanced pathogenicity and neurotropism of mouse-adapted H10N7 influenza virus are mediated by novel PB2 and NA mutations. J. Gen. Virol. 98, 1185–1195 (2017).
Long, J. S. et al. Species difference in ANP32A underlies influenza A virus polymerase host restriction. Nature 529, 101–104 (2016).
Staller, E. et al. Structures of H5N1 influenza polymerase with ANP32B reveal mechanisms of genome replication and host adaptation. Nat. Commun. 15, 4123 (2024).
Worobey, M. et al. Preliminary report on genomic epidemiology of the 2024 H5N1 influenza A virus outbreak in U.S. cattle (part 1 of 2). Virological https://virological.org/t/preliminary-report-on-genomic-epidemiology-of-the-2024-h5n1-influenza-a-virus-outbreak-in-u-s-cattle-part-1-of-2/970 (2024).
Worobey, M. et al. Preliminary report on genomic epidemiology of the 2024 H5N1 influenza A virus outbreak in U.S. cattle (part 2 of 2). Virological https://virological.org/t/preliminary-report-on-genomic-epidemiology-of-the-2024-h5n1-influenza-a-virus-outbreak-in-u-s-cattle-part-2-of-2/971 (2024).
Halwe, N. J. et al. H5N1 clade 2.3.4.4b dynamics in experimentally infected calves and cows. Nature https://doi.org/10.1038/s41586-024-08063-y (2024).
Massin, P., van der Werf, S. & Naffakh, N. Residue 627 of PB2 is a determinant of cold sensitivity in RNA replication of avian influenza viruses. J. Virol. 75, 5398–5404 (2001).
Crescenzo-Chaigne, B., van der Werf, S. & Naffakh, N. Differential effect of nucleotide substitutions in the 3’ arm of the influenza A virus vRNA promoter on transcription/replication by avian and human polymerase complexes is related to the nature of PB2 amino acid 627. Virology 303, 240–252 (2002).
CDC A(H5N1) Bird Flu Response Update August 9, 2024. CDC www.cdc.gov/bird-flu/spotlights/ah5n1-response-update.html (2024).
Capelastegui, F. et al. Pilot of asymptomatic swabbing of humans following exposures to confirmed avian influenza A(H5) in avian species in England, 2021/2022. Influenza Other Respir. Viruses 17, e13187 (2023).
Toner, E. S. et al. Assessment of serosurveys for H5N1. Clin. Infect. Dis. 56, 1206–1212 (2013).
Uyeki, T. M. et al. Seroprevalence of antibodies to avian influenza A (H5) and A (H9) viruses among market poultry workers, Hanoi, Vietnam, 2001. PLoS ONE 7, e43948 (2012).
CDC A(H5N1) Bird Flu Response Update June 28, 2024. CDC www.cdc.gov/bird-flu/spotlights/h5n1-response-06282024.html (2024).
Belser, J. A., Lash, R. R., Garg, S., Tumpey, T. M. & Maines, T. R. The eyes have it: influenza virus infection beyond the respiratory tract. Lancet Infect. Dis. 18, E220–E227 (2018).
Belser, J. A., Rota, P. A. & Tumpey, T. M. Ocular tropism of respiratory viruses. Microbiol. Mol. Biol. Rev. 77, 144–156 (2013).
Herfst, S. et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336, 1534–1541 (2012).
Tumpey, T. M. et al. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science 315, 655–659 (2007).
Fukao, K. et al. Combination treatment with the cap-dependent endonuclease inhibitor baloxavir marboxil and a neuraminidase inhibitor in a mouse model of influenza A virus infection. J. Antimicrob. Chemother. 74, 654–662 (2019).
Mentre, F. et al. Dose regimen of favipiravir for Ebola virus disease. Lancet Infect. Dis. 15, 150–151 (2015).
Ryan, D. M., Ticehurst, J., Dempsey, M. H. & Penn, C. R. Inhibition of influenza virus replication in mice by GG167 (4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid) is consistent with extracellular activity of viral neuraminidase (sialidase). Antimicrob. Agents Chemother. 38, 2270–2275 (1994).
Baranovich, T. et al. The neuraminidase inhibitor oseltamivir is effective against A/Anhui/1/2013 (H7N9) influenza virus in a mouse model of acute respiratory distress syndrome. J. Infect. Dis. 209, 1343–1353 (2014).
Sidwell, R. W. et al. Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice. Antimicrob. Agents Chemother. 51, 845–851 (2007).
Taniguchi, K. et al. Inhibition of avian-origin influenza A(H7N9) virus by the novel cap-dependent endonuclease inhibitor baloxavir marboxil. Sci. Rep. 9, 3466 (2019).
Neumann, G. et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl Acad. Sci. USA 96, 9345–9350 (1999).
Eisfeld, A. J., Neumann, G. & Kawaoka, Y. Influenza A virus isolation, culture and identification. Nat. Protoc. 9, 2663–2681 (2014).
Shepard, S. S. et al. Viral deep sequencing needs an adaptive approach: IRMA, the iterative refinement meta-assembler. BMC Genomics 17, 708 (2016).
Pattinson, D. IRI-UW-Bioinformatics/flu-ngs. GitHub https://github.com/IRI-UW-Bioinformatics/flu-ngs/releases/tag/v1.0.1 (2024).
Grubaugh, N. D. et al. An amplicon-based sequencing framework for accurately measuring intrahost virus diversity using PrimalSeq and iVar. Genome Biol. 20, 8 (2019).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. Preprint at https://arxiv.org/abs/1303.3997 (2013).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Murakami, S. et al. Establishment of canine RNA polymerase I-driven reverse genetics for influenza A virus: its application for H5N1 vaccine production. J. Virol. 82, 1605–1609 (2008).
Ozawa, M. et al. Contributions of two nuclear localization signals of influenza A virus nucleoprotein to viral replication. J. Virol. 81, 30–41 (2007).
Meetings of the WHO working group on surveillance of influenza antiviral susceptibility—Geneva, November 2011 and June 2012. WHO https://iris.who.int/handle/10665/241965 (2011).
Acknowledgements
This work was supported by the National Institute of Allergy and Infectious Diseases Centers of Excellence for Influenza Research and Response (contract no. 75N93021C00014), non-sponsored discretionary funding and by grants from the Japan Agency for Medical Research and Development (nos. JP24wm0125002, JP243fa627001 and JP24fk0108626 to Y.K.). We thank H. D. Nguyen and P. Jester for technical assistance, and S. Watson for editing the manuscript. The illustrations used in Fig. 8 were created with BioRender (https://biorender.com)7.
Author information
Authors and Affiliations
Contributions
Author contributions are provided according to Contributor Roles Taxonomy (CRediT). Conceptualization: A.J.E., P.J.H., G.N. and Y.K. Data curation: C.G., T.M., L.G., A.J.E., A.B., M.K. and R.U. Formal analysis: A.J.E., M.K., R.U., L.B. and S.Y. Funding acquisition: Y.K. Investigation: C.G., T.M., L.G., A.J.E., A.B., M.K., R.U., M.I., T.W., L.B., R.P., R.D. and S.Y. Methodology: C.G., T.M., L.G., A.J.E., A.B., M.K., R.U., M.I., L.B., R.D., P.J.H., S.Y., G.N. and Y.K. Project administration: A.J.E., P.J.H., S.T. and G.N. Resources: Y.S. and Y.K. Software: L.B. and R.P. Supervision: Y.K. Validation: C.G., T.M., L.G., A.J.E., A.B., M.K., R.U., T.W., L.B. and R.D. Visualization: A.J.E., M.K. and R.U. Writing—original draft: A.J.E., G.N. and Y.K. Writing—review and editing: C.G., T.M., L.G., A.J.E., A.B., M.K., R.U., M.I., S.T., T.W., L.B., R.P., R.D., Y.S., P.J.H., S.Y., G.N. and Y.K. Author contributions to specific experiments: ALI epithelial cell infection experiments were performed by T.M., L.G., A.J.E., A.B. and T.W. Mouse pathogenicity experiments were performed by C.G., L.G., A.J.E., A.B. and T.W. Ferret pathogenicity and transmission experiments were performed by C.G., T.M., L.G., A.J.E., A.B. and T.W. Minireplicon assays were performed by T.M. Receptor-binding studies were performed by T.M. Antiviral susceptibility testing experiments were performed by M.K., R.U., M.I. and S.Y. Sequence and deep-sequencing analysis was performed by L.B., R.P., R.D. and G.N.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Clinical data and virus titres associated with ferrets used to assess respiratory droplet transmission.
For infected ferrets (donors) shown in Fig. 4 (N = 6 biologically independent animals per dose), daily body weights (A), body temperatures (B), and survival (C) are given. In panels (A) and (B), data points represent mean values and error bars represent the standard deviation. Survival curves in panel (C) were compared by using a log-rank Mantel-Cox test and the p-value is reported in the figure panel. Panel (D) shows virus titres in tissues of the same animals collected at the time of euthanasia or within 14 h of death. Each dot represents the titre of an individual ferret. °C, degrees Celsius; d, days; PFU, plaque forming units; PFU/g, plaque forming units per gram of tissue; PFU/ml, plaque forming units per millilitre.
Extended Data Fig. 2 huTX37-H5N1 receptor binding activity.
Four-fold serial dilutions of α2,3 and α2,6 sialylglycopolymers adhered to microtitre plates were incubated with 32 hemagglutination (HA) units of the indicated viruses or PBS (negative control). After washing, virus binding was detected by an anti-HA human monoclonal antibody (CR9114) and an HRP-conjugated secondary antibody. The absorbance values for each condition with each virus or PBS are shown. Each dot represents a single biologically independent replicate value. Three independent replicate experiments are shown.
Supplementary information
Supplementary Table 1
Non-synonymous mutations detected in inocula used to infect ferrets and the nasal swabs of infected and exposed ferrets in respiratory droplet transmission experiments. Shown are mutations that resulted in amino acid changes in at least 3% of sequence reads.
Supplementary Table 2
Amino acid consensus sequences of virus inoculum, and of viruses isolated from nasal swabs of infected and exposed animals.
Supplementary Table 3
Forward and reverse primers used in deep sequencing for each genome segment.
Supplementary Table 4
SRA accession numbers for deep-sequencing data associated with inocula used to infect ferrets and ferret nasal swab samples.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gu, C., Maemura, T., Guan, L. et al. A human isolate of bovine H5N1 is transmissible and lethal in animal models. Nature 636, 711–718 (2024). https://doi.org/10.1038/s41586-024-08254-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-08254-7
This article is cited by
-
Steps to prevent and respond to an H5N1 epidemic in the USA
Nature Medicine (2025)
-
Superior replication, pathogenicity, and immune evasion of a Texas dairy cattle H5N1 virus compared to a historical avian isolate
Scientific Reports (2025)
-
Efficacy of baloxavir marboxil against bovine H5N1 virus in mice
Nature Communications (2025)
-
Broad neuraminidase antibodies confer protection against seasonal and avian influenza viruses
Nature Communications (2025)
-
Bovine H5N1 binds poorly to human-type sialic acid receptors
Nature (2025)