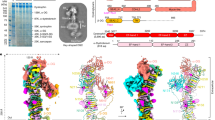
Abstract
The dystrophin glycoprotein complex (DGC) has a crucial role in maintaining cell membrane stability and integrity by connecting the intracellular cytoskeleton with the surrounding extracellular matrix1,2,3. Dysfunction of dystrophin and its associated proteins results in muscular dystrophy, a disorder characterized by progressive muscle weakness and degeneration4,5. Despite the important roles of the DGC in physiology and pathology, its structural details remain largely unknown, hindering a comprehensive understanding of its assembly and function. Here we isolated the native DGC from mouse skeletal muscle and obtained its high-resolution structure. Our findings unveil a markedly divergent structure from the previous model of DGC assembly. Specifically, on the extracellular side, β-, γ- and δ-sarcoglycans co-fold to form a specialized, extracellular tower-like structure, which has a central role in complex assembly by providing binding sites for α-sarcoglycan and dystroglycan. In the transmembrane region, sarcoglycans and sarcospan flank and stabilize the single transmembrane helix of dystroglycan, rather than forming a subcomplex as previously proposed6,7,8. On the intracellular side, sarcoglycans and dystroglycan engage in assembly with the dystrophin–dystrobrevin subcomplex through extensive interaction with the ZZ domain of dystrophin. Collectively, these findings enhance our understanding of the structural linkage across the cell membrane and provide a foundation for the molecular interpretation of many muscular dystrophy-related mutations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The cryo-EM maps of the mouse DGC have been deposited at the Electron Microscopy Data Bank (https://www.ebi.ac.uk/pdbe/emdb/) under the accession codes EMD-39569 and EMD-39568. The corresponding atomic coordinate data has been deposited at the Protein Data Bank (http://www.rcsb.org) under the accession code 8YT8. All data analysed during this study are included in this Article and its Supplementary Information. Any other relevant reagents and materials are available from the corresponding author upon request.
Code availability
No code was used for this study.
References
Campbell, K. P. & Kahl, S. D. Association of dystrophin and an integral membrane glycoprotein. Nature 338, 259–262 (1989).
Ibraghimov-Beskrovnaya, O. et al. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 355, 696–702 (1992).
Ervasti, J. M. & Campbell, K. P. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J. Cell Biol. 122, 809–823 (1993).
McNally, E. M. & Pytel, P. Muscle diseases: the muscular dystrophies. Annu. Rev. Pathol. 2, 87–109 (2007).
Duan, D, S., Goemans, N., Takeda, S., Mercuri, E. & Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Primers 7, 13 (2021).
Durbeej, M. & Campbell, K. P. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr. Opin. Genet. Dev. 12, 349–361 (2002).
Wilson, D. G. S., Tinker, A. & Iskratsch, T. The role of the dystrophin glycoprotein complex in muscle cell mechanotransduction. Commun. Biol. 5, 1022 (2022).
Gao, Q. Q. & McNally, E. M. The dystrophin complex: structure, function, and implications for therapy. Compr. Physiol. 5, 1223–1239 (2015).
Gumerson, J. D. & Michele, D. E. The dystrophin-glycoprotein complex in the prevention of muscle damage. J. Biomed. Biotechnol. 2011, 210797 (2011).
Lapidos, K. A., Kakkar, R. & McNally, E. M. The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ. Res. 94, 1023–1031 (2004).
Belhasan, D. C. & Akaaboune, M. The role of the dystrophin glycoprotein complex on the neuromuscular system. Neurosci. Lett. 722, 134833 (2020).
Pilgram, G. S. K., Potikanond, S., Baines, R. A., Fradkin, L. G. & Noordermeer, J. N. The roles of the dystrophin-associated glycoprotein complex at the synapse. Mol. Neurobiol. 41, 1–21 (2010).
Constantin, B. Dystrophin complex functions as a scaffold for signalling proteins. Boichim. Biophys. Acta 1838, 635–642 (2014).
Hoffman, E. P., Brown, R. H. & Kunkel, L. M. Dystrophin—the protein product of the Duchenne muscular-dystrophy locus. Cell 51, 919–928 (1987).
Koenig, M., Monaco, A. P. & Kunkel, L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell 53, 219–228 (1988).
Rybakova, I. N., Patel, J. R. & Ervasti, J. M. The dystrophin complex forms a mechanically strong link between the sarcolemma and costameric actin. J. Cell Biol. 150, 1209–1214 (2000).
Bhat, H. F. et al. ABC of multifaceted dystrophin glycoprotein complex (DGC). J. Cell. Physiol. 233, 5142–5159 (2018).
Deyst, K. A., Bowe, M. A., Leszyk, J. D. & Fallon, J. R. The α-dystroglycan-β-dystroglycan complex. Membrane organization and relationship to an agrin receptor. J. Biol. Chem. 270, 25956–25959 (1995).
Holt, K. H., Crosbie, R. H., Venzke, D. P. & Campbell, K. P. Biosynthesis of dystroglycan: processing of a precursor propeptide. FEBS Lett. 468, 79–83 (2000).
Martin, P. T. Dystroglycan glycosylation and its role in matrix binding in skeletal muscle. Glycobiology 13, 55R–66R (2003).
Sciandra, F. et al. Identification of the β-dystroglycan binding epitope within the C-terminal region of α-dystroglycan. Eur. J. Biochem. 268, 4590–4597 (2001).
Crosbie, R. H., Heighway, J., Venzke, D. P., Lee, J. C. & Campbell, K. P. Sarcospan, the 25-kDa transmembrane component of the dystrophin-glycoprotein complex. J. Biol. Chem. 272, 31221–31224 (1997).
Wein, N., Alfano, L. & Flanigan, K. M. Genetics and emerging treatments for Duchenne and Becker muscular dystrophy. Pediatr. Clin. North. Am. 62, 723–742 (2015).
Mah, J. K. et al. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscular Disord 24, 482–491 (2014).
Nigro, V. & Savarese, M. Genetic basis of limb-girdle muscular dystrophies: the 2014 update. Acta Myol 33, 1–12 (2014).
Brown, S. C. et al. Abnormalities in alpha-dystroglycan expression in MDC1C and LGMD2I muscular dystrophies. Am. J. Pathol. 164, 727–737 (2004).
Michele, D. E. et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature 418, 417–422 (2002).
Barresi, R. & Campbell, K. P. Dystroglycan: from biosynthesis to pathogenesis of human disease. J. Cell Sci. 119, 199–207 (2006).
Waite, A., Brown, S. C. & Blake, D. J. The dystrophin-glycoprotein complex in brain development and disease. Trends Neurosci. 35, 487–496 (2012).
Tsubata, S. et al. Mutations in the human δ-sarcoglycan gene in familial and sporadic dilated cardiomyopathy. J. Clin. Invest. 106, 655–662 (2000).
Guiraud, S. et al. The pathogenesis and therapy of muscular dystrophies. Annu. Rev. Genomics Hum. Genet. 16, 281–308 (2015).
Norwood, F. L. M., Sutherland-Smith, A. J., Keep, N. H. & Kendrick-Jones, J. The structure of the N-terminal actin-binding domain of human dystrophin and how mutations in this domain may cause Duchenne or Becker muscular dystrophy. Structure 8, 481–491 (2000).
Muthu, M., Richardson, K. A. & Sutherland-Smith, A. J. The crystal structures of dystrophin and utrophin spectrin repeats: implications for domain boundaries. PLoS ONE 7, e40066 (2012).
Huang, X. et al. Structure of a WW domain containing fragment of dystrophin in complex with β-dystroglycan. Nat. Struct. Biol. 7, 634–638 (2000).
Bozic, D., Sciandra, F., Lamba, D. & Brancaccio, A. The structure of the N-terminal region of murine skeletal muscle α-dystroglycan discloses a modular architecture. J. Biol. Chem. 279, 44812–44816 (2004).
Briggs, D. C. et al. Structural basis of laminin binding to the LARGE glycans on dystroglycan. Nat. Chem. Biol. 12, 810–814 (2016).
Ramaswamy, K. S. et al. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J. Physiol. 589, 1195–1208 (2011).
Singh, J. et al. Proteolytic enzymes and altered glycosylation modulate dystroglycan function in carcinoma cells. Cancer Res. 64, 6152–6159 (2004).
Jung, D., Yang, B., Meyer, J., Chamberlain, J. S. & Campbell, K. P. Identification and characterization of the dystrophin anchoring site on beta-dystroglycan. J. Biol. Chem. 270, 27305–27310 (1995).
Chan, Y. M. & Kunkel, L. M. In vitro expressed dystrophin fragments do not associate with each other. FEBS Lett. 410, 153–159 (1997).
SadouletPuccio, H. M., Rajala, M. & Kunkel, L. M. Dystrobrevin and dystrophin: An interaction through coiled-coil motifs. Proc. Natl Acad. Sci. USA 94, 12413–12418 (1997).
Swiderski, K. et al. Phosphorylation within the cysteine-rich region of dystrophin enhances its association with β-dystroglycan and identifies a potential novel therapeutic target for skeletal muscle wasting. Hum. Mol. Genet. 23, 6697–6711 (2014).
Ilsley, J. L., Sudol, M. & Winder, S. J. The interaction of dystrophin with β-dystroglycan is regulated by tyrosine phosphorylation. Cell Signal 13, 625–632 (2001).
Ge, X. & Wang, J. W. Structural mechanism of bacteriophage lambda tail’s interaction with the bacterial receptor. Nat. Commun. 15, 4185 (2024).
Hynes, R. O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002).
Luo, B. H., Carman, C. V. & Springer, T. A. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25, 619–647 (2007).
Guo, C. et al. Absence of α7 integrin in dystrophin-deficient mice causes a myopathy similar to Duchenne muscular dystrophy. Hum. Mol. Genet. 15, 989–998 (2006).
Rooney, J. E. et al. Severe muscular dystrophy in mice that lack dystrophin and α7 integrin. J. Cell Sci. 119, 2185–2195 (2006).
Hodges, B. L. et al. Altered expression of the α7β1 integrin in human and murine muscular dystrophies. J. Cell Sci. 110, 2873–2881 (1997).
Marshall, J. L. & Crosbie-Watson, R. H. Sarcospan: a small protein with large potential for Duchenne muscular dystrophy. Skelet. Muscle 3, 1 (2013).
Yan, Z. et al. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature 517, 50–55 (2015).
Diniz, G. et al. Sarcolemmal alpha and gamma sarcoglycan protein deficiencies in Turkish siblings with a novel missense mutation in the alpha sarcoglycan gene. Pediatr. Neurol. 50, 640–647 (2014).
Duggan, D. J. et al. Mutations in the sarcoglycan genes in patients with myopathy. New Engl. J. Med. 336, 618–624 (1997).
Piccolo, F. et al. Primary adhalinopathy—a common-cause of autosomal recessive muscular-dystrophy of variable severity. Nat. Genet. 10, 243–245 (1995).
Carrie, A. et al. Mutational diversity and hot spots in the alpha-sarcoglycan gene in autosomal recessive muscular dystrophy (LGMD2D). J. Med. Genet. 34, 470–475 (1997).
Saha, M. et al. Impact of PYROXD1 deficiency on cellular respiration and correlations with genetic analyses of limb-girdle muscular dystrophy in Saudi Arabia and Sudan. Physiol. Genomics 50, 929–939 (2018).
Kawai, H. et al. Adhalin gene mutations in patients with autosomal recessive childhood onset muscular dystrophy with adhalin deficiency. J. Clin. Invest. 96, 1202–1207 (1995).
Duclos, F. et al. β-sarcoglycan: genomic analysis and identification of a novel missense mutation in the LGMD2E Amish isolate. Neuromusc. Disord. 8, 30–38 (1998).
dos Santos, M. R., Jorge, P., Ribeiro, E. M., Pires, M. M. & Guimaraes, A. Noval mutation (Y184C) in exon 4 of the beta-sarcoglycan gene identified in a Portuguese patient. Mutations in brief no. 177. Hum. Mutat. 12, 214–215 (1998).
Bonnemann, C. G. et al. Genomic screening for beta-sarcoglycan gene mutations: Missense mutations may cause severe limb-girdle muscular dystrophy type 2E (LGMD 2E). Hum. Mol. Genet. 5, 1953–1961 (1996).
Bönnemann, C. G. et al. LGMD 2E in Tunisia is caused by a homozygous missense mutation in β-sarcoglycan exon 3. Neuromusc. Disord. 8, 193–197 (1998).
Vermeer, S. et al. Novel mutations in three patients with LGMD2C with phenotypic differences. Pediatr. Neurol. 30, 291–294 (2004).
Nowak, K. J. et al. Severe γ-sarcoglycanopathy caused by a novel missense mutation and a large deletion. Neuromusc. Disord. 10, 100–107 (2000).
Crosbie, R. H. et al. Molecular and genetic characterization of sarcospan:: insights into sarcoglycan–sarcospan interactions. Hum. Mol. Genet. 9, 2019–2027 (2000).
Piccolo, F. et al. A founder mutation in the γ-sarcoglycan gene of Gypsies possibly predating their migration out of India. Hum. Mol. Genet. 5, 2019–2022 (1996).
Duggan, D. J. et al. Mutations in the δ-sarcoglycan gene are a rare cause of autosomal recessive limb-girdle muscular dystrophy (LGMD2). Neurogenetics 1, 49–58 (1997).
Nigro, V. et al. Identification of a novel sarcoglycan gene at 5q33 encoding a sarcolemmal 35 kDa glycoprotein. Hum. Mol. Genet. 5, 1179–1186 (1996).
Moreira, E. S. et al. A first missense mutation in the δ sarcoglycan gene associated with a severe phenotype and frequency of limb-girdle muscular dystrophy type 2 F (LGMD2F) in Brazilian sarcoglycanopathies. J. Med. Genet. 35, 951–953 (1998).
Geis, T. et al. Homozygous dystroglycan mutation associated with a novel muscle-eye-brain disease-like phenotype with multicystic leucodystrophy. Neurogenetics 14, 205–213 (2013).
Dai, Y. et al. Whole exome sequencing identified a novel DAG1 mutation in a patient with rare, mild and late age of onset muscular dystrophy-dystroglycanopathy. J. Cell. Mol. Med. 23, 811–818 (2019).
Feng, J., Yan, J., Buzin, C. H., Towbin, J. A. & Sommer, S. S. Mutations in the dystrophin gene are associated with sporadic dilated cardiomyopathy. Mol. Genet. Metab. 77, 119–126 (2002).
Flanigan, K. M. et al. Rapid direct sequence analysis of the dystrophin gene. Am. J. Hum. Genet. 72, 931–939 (2003).
Vulin, A. et al. The ZZ domain of dystrophin in DMD: making sense of missense mutations. Hum. Mutat. 35, 257–264 (2014).
Goldberg, L. R. et al. A dystrophin missense mutation showing persistence of dystrophin and dystrophin-associated proteins yet a severe phenotype. Ann. Neurol. 44, 971–976 (1998).
Lenk, U. et al. A cysteine 3340 substitution in the dystroglycan-binding domain of dystrophin associated with Duchenne muscular dystrophy, mental retardation and absence of the ERG b-wave. Hum. Mol. Genet. 5, 973–975 (1996).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Pettersen, E. F. et al. UCSF chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D 74, 531–544 (2018).
Acknowledgements
The authors thank the cryo-EM Facility of Westlake University for providing support on cryo-EM data collection; Westlake University HPC Center for computational resources and related assistance; the Mass Spectrometry and Metabolomics Core Facility of Westlake University for mass spectrometry analysis. This work was supported by National Natural Science Foundation of China (32271261 to J.W. and 32271239 to Z.Y.), Zhejiang Provincial Natural Science Foundation of China (LR22C050003 to J.W.), Westlake University (1011103860222B1 to J.W. and 1011103560222B1 to Z.Y.) and Westlake Education Foundation (101486021901 to J.W. and 101456021901 to Z.Y.). Research reported in this publication was also supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number P50NS053672 to K.P.C. K.P.C is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
J.W. and Z.Y. conceived and supervised the project. J.W., Z.Y. and L.W. designed the experiments. L.W. prepared the protein samples, collected cryo-EM datasets and performed all other biochemical experiments. X.G., Q.X. and G.H. performed the cryo-EM data processing. Q.X. and J.W. built the atomic model. T.Y. and K.P.C. advised on DGC protein preparation and contributed to manuscript discussions. All authors contributed to data analysis. J.W., Z.Y. and L.W. wrote the manuscript with input from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Jeffrey Chamberlain and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Endogenous purification of the DGC from mouse skeletal muscle.
a, A classic schematic showing the overall organization of the DGC. The LG4 and LG5 domains of laminin-α2 interact with the glycans on α-DG. Inset: crystal structure of the LG4 and LG5 domains of laminin-α2 in complex with glycans (PDB: 5IK5). ECM: extracellular matrix; ABD: actin-binding domain; CR: cysteine-rich domain; CT: C-terminal domain. b, Size-exclusion chromatogram and corresponding SDS-PAGE analysis of the purified LG4 and LG5 domains of laminin-α2. Purifications were repeated independently at least three times with similar results. c, A diagram showing the purification procedure of the native DGC from mouse skeletal muscle. SEC: size exclusion chromatography; WB: western blot; MS: mass spectrometry. d, Size exclusion chromatography profile of the purified DGC sample. The shaded fractions were collected for cryo-EM and MS analysis. e, Western blot analysis of the gel filtration fractions against multiple DGC components. Each western blot was repeated at least twice with similar results. For gel source data, see Supplementary Fig. 1a. f, MS analysis of the purified DGC sample. Potential DGC components are listed in the order of decreasing peptide-spectrum match (PSM) scores. The DGC components observed in our model are highlighted in green. g, Peptide identification of dystrophin by MS analysis of the purified DGC sample. The identified regions are shaded in cyan, which account for a total of 67% sequence coverage.
Extended Data Fig. 2 Cryo-EM data analysis of the DGC.
a, A raw cryo-EM image of the purified DGC sample out of a dataset of 40,736 images. Representative particles are highlighted by green circles. Scale bar: 50 nm. b, Two-dimensional class averages. Box size: 469.6 Å. c, Gold-standard Fourier shell correlation (FSC) curves of the final maps. d, A flowchart of cryo-EM data processing. For details, see ‘Image processing’ in the Methods. Map 1 (EMDB-39569) is the map with the highest overall resolution and Map 2 (EMDB-39568) is the map with the clearest intracellular densities. Both maps were used to guide model building. The local resolutions of the two maps were estimated by cryoSPARC.
Extended Data Fig. 3 Density maps of the DGC components.
a, Density maps of selected segments of each DGC component. The density of dystrobrevin is presented in Fig. 4f. The boundaries of each segment and some bulky residues are labelled. b, The glycan densities of all identified glycosylation sites and the densities of three cholesterol-like lipids. The density maps were generated in ChimeraX.
Extended Data Fig. 4 Structural topology and sequence alignment of β-, γ-, and δ-sarcoglycans.
a, Topological diagram of β-, γ-, and δ-sarcoglycans. The β strands on face (a), (b), and (c) of the ECD tower are coloured in purple, green, and cyan, respectively. The anti-parallel β strands within the same face of the ECD tower are boxed. The β14 strand of β-sarcoglycan, which differs from the other two sarcoglycans, is highlighted by a red box. The β strands on each face of the ECD tower are connected by coloured dashed lines. b, Sequence alignment among β-, γ-, and δ-sarcoglycans. Secondary structure elements of β-sarcoglycan are labelled above the sequence and that of γ- and δ-sarcoglycans are labelled below the sequence. The N-terminal regions that are not modelled are indicated by dashed lines. Glycosylation sites are indicated by green boxes and asterisks. Disulfide bonds are labelled by orange lines. The UniProt IDs for each sequence are as follows: β-sarcoglycan: P82349, γ-sarcoglycan: P82348, δ-sarcoglycan: P82347. c, Structural comparison among several β-helical-containing proteins. The structures presented include the β-helical domains of the ECD tower of the DGC, VgrG (PDB: 6SK0), Pdp-VgrG (PDB: 6U9E), gp5 from the T4 bacteriophage (PDB: 1K28), and gpJ in closed/apo (PDB: 8XCK) and open/receptor-bound (PDB: 8XCJ) states. The relative rotational and overall height changes of gpJ between the apo and receptor-bound states are indicated by grey arrows and dashed lines, respectively.
Extended Data Fig. 5 Domain organization of dystroglycan and α-sarcoglycan.
a, Schematic diagrams of dystroglycan and α-sarcoglycan. The resolved extracellular domains of the two components are shaded in colour. Glycosylation sites and disulfide bonds are labelled. SP: signal peptide. b, Structural comparisons among the extracellular domains of dystroglycan and α-sarcoglycan. The red arrow indicates the reported dividing point between α-DG and β-DG, situated in the loop region between the β2 and β3 strands of the P domain. c, Structural overlay among the extracellular domains of dystroglycan and α-sarcoglycan. The three models are superimposed by their P domains. The double-headed arrow indicates conformational variations of the immunoglobulin-like domains.
Extended Data Fig. 6 Interaction between dystrophin and dystrobrevin.
a, Schematic diagram of dystrophin and dystrobrevin. b, Structure of CR domain of dystrophin fitted onto the cryo-EM map. The map is shown as a transparent surface. The red arrow indicates extra unassigned density near the zinc-binding site of the ZZ domain. c, Overall structure of the resolved domains of dystrophin and dystrobrevin. The crystal structure of human dystrophin in complex with the C-terminus of β-DG peptide (PDB:1EG4) is also presented for comparison. The domains are coloured in the same scheme as in a. d, Comparison of the crystal structure and the cryo-EM structure of the WW and EF-hand domains of dystrophin. The cryo-EM density map of this region is shown as a transparent surface. The red arrow indicates the conformational deviation of the WW domain between the two structures. S3059 and T3074 on the WW domain of dystrophin, and two tyrosine residues on the C-terminus of β-DG, which have been reported as phosphorylation sites, are shown as sticks. e, Structural overlay of the EF-hand domains in dystrobrevin and dystrophin. f, Comparison between the cryo-EM structure and the predicted structure by Alphafold3. The predicted structure between dystrophin (3049–3678) and dystrobrevin (1–746) suggests the presence of two major interaction interfaces. g, Gel filtration binding assay to verify the interaction between dystrobrevin and dystrophin. Left: dystrobrevin co-migrates with the GST-tagged dystrophin. Right: dystrobrevin does not co-migrate with GST alone. The co-migration of dystrobrevin and GST-tagged dystrophin is highlighted by a red box. The assay was repeated independently three times with similar results. For gel source data, see Supplementary Fig. 1b.
Extended Data Fig. 7 Recombinant expression of the extracellular domains of α-sarcoglycan and dystroglycan.
a, Size-exclusion chromatogram of wild-type and 19 disease-related mutations of α-sarcoglycan (1–250). In addition to the monomeric peak, the recombinantly expressed protein also exhibits an oligomeric peak, possibly due to heterologous overexpression in HEK293 cells. b, SDS-PAGE analysis of the purified wild-type and disease-related mutations of α-sarcoglycan. For gel source data, see Supplementary Fig. 1c. c, Size-exclusion chromatogram of wild-type and the C667F mutation of dystroglycan (492–712). d, SDS-PAGE analysis of the purified wild-type and the C667F mutation of dystroglycan. For gel source data, see Supplementary Fig. 1d.
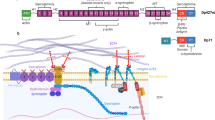
Extended Data Fig. 8 A modified schematic of the DGC based on our structure.
In this model, sarcoglycans play a central role in complex assembly. In the extracellular side, β-, γ-, and δ-sarcoglycans co-fold to form a large ECD tower, which serves as docking sites for multiple extracellular domains from other components. In the transmembrane region, the sarcoglycans and sarcospan flank two sides of the transmembrane of β-DG, thereby stabilizing the latter. In the cytoplasmic region, sarcoglycans and β-DG directly interact with the ZZ domain of dystrophin. The structural features of the DGC, including the characteristic tilt angle of the ECD tower, enable it to efficiently connect the two sides of sarcolemma and transmit both longitudinal and lateral forces.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wan, L., Ge, X., Xu, Q. et al. Structure and assembly of the dystrophin glycoprotein complex. Nature 637, 1252–1260 (2025). https://doi.org/10.1038/s41586-024-08310-2
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-08310-2
This article is cited by
-
Structure-guided design of a prefusion GPC trimer induces neutralizing responses against LASV
npj Vaccines (2025)
-
Deficient Astrocyte Homeostatic Support Contributes to Brain Impairment in Duchenne Muscular Dystrophy
Neurochemical Research (2025)