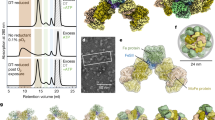
Abstract
The low reduction potentials required for the reduction of dinitrogen (N2) render metal-based nitrogen-fixation catalysts vulnerable to irreversible damage by dioxygen (O2)1,2,3. Such O2 sensitivity represents a major conundrum for the enzyme nitrogenase, as a large fraction of nitrogen-fixing organisms are either obligate aerobes or closely associated with O2-respiring organisms to support the high energy demand of catalytic N2 reduction4. To counter O2 damage to nitrogenase, diazotrophs use O2 scavengers, exploit compartmentalization or maintain high respiration rates to minimize intracellular O2 concentrations4,5,6,7,8. A last line of damage control is provided by the ‘conformational protection’ mechanism9, in which a [2Fe:2S] ferredoxin-family protein termed FeSII (ref. 10) is activated under O2 stress to form an O2-resistant complex with the nitrogenase component proteins11,12. Despite previous insights, the molecular basis for the conformational O2 protection of nitrogenase and the mechanism of FeSII activation are not understood. Here we report the structural characterization of the Azotobacter vinelandii FeSII–nitrogenase complex by cryo-electron microscopy. Our studies reveal a core complex consisting of two molybdenum–iron proteins (MoFePs), two iron proteins (FePs) and one FeSII homodimer, which polymerize into extended filaments. In this three-protein complex, FeSII mediates an extensive network of interactions with MoFeP and FeP to position their iron–sulphur clusters in catalytically inactive but O2-protected states. The architecture of the FeSII–nitrogenase complex is confirmed by solution studies, which further indicate that the activation of FeSII involves an oxidation-induced conformational change.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The principal data supporting the findings of this work are available within the figures and the Supplementary Information. Structural models have been deposited in the Protein Data Bank (PDB) with the following accession codes: 9CTZ (MoFeP), 9CU0 (1:1:1 MoFeP:FeP:FeSIImonomer complex), 9CU1 (terminal 2:2:1 MoFeP:FeP:FeSIIdimer complex) and 9CU2 (2:2:1 MoFeP:FeP:FeSIIdimer complex). The corresponding cryo-EM maps are available at the Electron Microscopy Data Bank (www.ebi.ac.uk/emdb/) with the following accession codes: EMD-45923 (MoFeP), EMD-45924 (1:1:1 MoFeP:FeP:FeSIImonomer complex, EMD-45925 (terminal-2:2:1 MoFeP:FeP:FeSIIdimer complex) and EMD-45926 (2:2:1 MoFeP:FeP:FeSIIdimer complex). We used UniProtKB to locate the sequence of the gene for A. vinelandii FeSII (Q44501). Source data are provided with this paper.
Change history
15 January 2025
In the version of the article initially published, the line in Fig. 4, Oxidizing conditions panel, between Complex association and FePox and MoFeP appeared originally as an arrow pointing from Complex association. The figure has been amended in the HTML and PDF versions of the article.
References
Chalkley, M. J., Drover, M. W. & Peters, J. C. Catalytic N2-to-NH3 (or -N2H4) conversion by well-defined molecular coordination complexes. Chem. Rev. 120, 5582–5636 (2020).
Rohr, B. A., Singh, A. R. & Nørskov, J. K. A theoretical explanation of the effect of oxygen poisoning on industrial Haber–Bosch catalysts. J. Catal. 372, 33–38 (2019).
Gallon, J. R. The oxygen sensitivity of nitrogenase: a problem for biochemists and micro-organisms. Trends Biochem. Sci. 6, 19–23 (1981).
Robson, R. L. & Postgate, J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu. Rev. Microbiol. 34, 183–207 (1980).
Wittenberg, J. B., Bergersen, F. J., Appleby, C. A. & Turner, G. L. Facilitated oxygen diffusion: the role of leghemoglobin in nitrogen fixation by bacteroids isolated from soybean root nodules. J. Biol. Chem. 249, 4057–4066 (1974).
Poole, R. K. & Hill, S. Respiratory protection of nitrogenase activity in Azotobacter vinelandii—roles of the terminal oxidases. Biosci. Rep. 17, 303–317 (1997).
Postgate, J. R. Biological nitrogen fixation: fundamentals. Phil. Trans. R. Soc. Lond. B Biol. Sci. 296, 375–385 (1982).
Takimoto, R. et al. A critical role of an oxygen-responsive gene for aerobic nitrogenase activity in Azotobacter vinelandii and its application to Escherichia coli. Sci. Rep. 12, 4182 (2022).
Dalton, H. & Postgate, J. Effect of oxygen on growth of Azotobacter chroococcum in batch and continuous cultures. J. Gen. Microbiol. 54, 463–473 (1968).
Shethna, Y., DerVartanian, D. & Beinert, H. Non heme (iron-sulfur) proteins of Azotobacter vinelandii. Biochem. Biophys. Res. Commun. 31, 862–868 (1968).
Robson, R. Characterization of an oxygen-stable nitrogenase complex isolated from Azotobacter chroococcum. Biochem. J. 181, 569–575 (1979).
Scherings, G., Haaker, H., Wassink, H. & Veeger, C. On the formation of an oxygen‐tolerant three-component nitrogenase complex from Azotobacter vinelandii. Eur. J. Biochem. 135, 591–599 (1983).
Hoffman, B. M., Lukoyanov, D., Yang, Z.-Y., Dean, D. R. & Seefeldt, L. C. Mechanism of nitrogen fixation by nitrogenase: the next stage. Chem. Rev. 114, 4041–4062 (2014).
Fowler, D. et al. The global nitrogen cycle in the twenty-first century. Phil. Trans. R. Soc. Lond. B Biol. Sci. 368, 20130164 (2013).
Seefeldt, L. C., Hoffman, B. M. & Dean, D. R. Mechanism of Mo-dependent nitrogenase. Annu. Rev. Biochem. 78, 701–722 (2009).
Einsle, O. & Rees, D. C. Structural enzymology of nitrogenase enzymes. Chem. Rev. 120, 4969–5004 (2020).
Spatzal, T. et al. Evidence for interstitial carbon in nitrogenase FeMo cofactor. Science 334, 940–940 (2011).
Lancaster, K. M. et al. X-ray emission spectroscopy evidences a central carbon in the nitrogenase iron-molybdenum cofactor. Science 334, 974–977 (2011).
Georgiadis, M. et al. Crystallographic structure of the nitrogenase iron protein from Azotobacter vinelandii. Science 257, 1653–1659 (1992).
Rutledge, H. L. & Tezcan, F. A. Electron transfer in nitrogenase. Chem. Rev. 120, 5158–5193 (2020).
Seefeldt, L. C. et al. Reduction of substrates by nitrogenases. Chem. Rev. 120, 5082–5106 (2020).
Yates, M. G. & Planque, K. Nitrogenase from Azotobacter chroococcum: purification and properties of the component proteins. Eur. J. Biochem. 60, 467–476 (1975).
Eady, R., Smith, B., Cook, K. & Postgate, J. Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem. J. 128, 655–675 (1972).
Wong, P. P. & Burris, R. Nature of oxygen inhibition of nitrogenase from Azotobacter vinelandii. Proc. Natl Acad. Sci. USA 69, 672–675 (1972).
Bulen, W., Burns, R. & LeComte, J. Nitrogen fixation: hydrosulfite as electron donor with cell-free preparations of Azotobacter vinelandii and Rhodospirillum rubrum. Proc. Natl Acad. Sci. USA 53, 532–539 (1965).
Kelly, M. Some properties of purified nitrogenase of Azotobacter chroococcum. Biochim. Biophys. Acta Enzymol. 171, 9–22 (1969).
Haaker, H. & Veeger, C. Involvement of the cytoplasmic membrane in nitrogen fixation by Azotobacter vinelandii. Eur. J. Biochem. 77, 1–10 (1977).
Yates, M. Effect of non-haem iron proteins and cytochrome C from Azotobacter upon the activity and oxygen sensitivity of Azobacter nitrogenase. FEBS Lett. 8, 281–285 (1970).
Maier, R. & Moshiri, F. Role of the Azotobacter vinelandii nitrogenase-protective Shethna protein in preventing oxygen-mediated cell death. J. Bacteriol. 182, 3854–3857 (2000).
Lery, L. M., Bitar, M., Costa, M. G., Rössle, S. C. & Bisch, P. M. Unraveling the molecular mechanisms of nitrogenase conformational protection against oxygen in diazotrophic bacteria. BMC Genom. 11, S7 (2010).
Schlesier, J., Rohde, M., Gerhardt, S. & Einsle, O. A conformational switch triggers nitrogenase protection from oxygen damage by Shethna protein II (FeSII). J. Am. Chem. Soc. 138, 239–247 (2016).
Wang, Z., Burns, A. & Watt, G. Complex formation and oxygen sensitivity of Azotobacter vinelandii nitrogenase and its component proteins. Biochemistry 24, 214–221 (1985).
Veeger, C. et al. in Nitrogen Fixation Vol. 1 (eds Newton, W. E. & Orme-Johnson, W. H.) 111–137 (University Park Press, 1980).
Kabasakal, B. V. et al. The crystal structure of Shethna protein II (FeSII) from Azotobacter vinelandii suggests a domain swap. Acta Crystallogr. D Struct. Biol. 80, 599–604 (2024).
Rutledge, H. L., Cook, B. D., Nguyen, H. P. M., Herzik, M. A. & Tezcan, F. A. Structures of the nitrogenase complex prepared under catalytic turnover conditions. Science 377, 865–869 (2022).
Cook, B. D. et al. Preparation of oxygen-sensitive proteins for high-resolution cryoEM structure determination using (an)aerobic blot-free vitrification. Preprint at bioRxiv https://doi.org/10.1101/2024.07.19.604374 (2024).
Tezcan, F. A. et al. Nitrogenase complexes: multiple docking sites for a nucleotide switch protein. Science 309, 1377–1380 (2005).
Lou, J. et al. Mutagenesis studies of the FeSII protein of Azotobacter vinelandii: roles of histidine and lysine residues in the protection of nitrogenase from oxygen damage. Biochemistry 38, 5563–5571 (1999).
Dey, A. et al. Solvent tuning of electrochemical potentials in the active sites of HiPIP versus ferredoxin. Science 318, 1464–1468 (2007).
Lanzilotta, W. N. & Seefeldt, L. C. Changes in the midpoint potentials of the nitrogenase metal centers as a result of iron protein molybdenum-iron protein complex formation. Biochemistry 36, 12976–12983 (1997).
Moshiri, F., Crouse, B. R., Johnson, M. K. & Maier, R. J. The “nitrogenase-protective” FeSII protein of Azotobacter vinelandii: overexpression, characterization, and crystallization. Biochemistry 34, 12973–12982 (1995).
Alleman, A. B. & Peters, J. W. Mechanisms for generating low potential electrons across the metabolic diversity of nitrogen-fixing bacteria. Appl. Env. Microbiol. 89, e00378–00323 (2023).
Ribbe, M. W., Hu, Y., Hodgson, K. O. & Hedman, B. Biosynthesis of nitrogenase metalloclusters. Chem. Rev. 114, 4063–4080 (2014).
Rubio, L. M. & Ludden, P. W. Biosynthesis of the iron-molybdenum cofactor of nitrogenase. Annu. Rev. Microbiol. 62, 93–111 (2008).
Berman-Frank, I., Lundgren, P. & Falkowski, P. Nitrogen fixation and photosynthetic oxygen evolution in cyanobacteria. Res. Microbiol. 154, 157–164 (2003).
Santi, C., Bogusz, D. & Franche, C. Biological nitrogen fixation in non-legume plants. Ann. Bot. 111, 743–767 (2013).
Ryu, M.-H. et al. Control of nitrogen fixation in bacteria that associate with cereals. Nat. Microbiol. 5, 314–330 (2020).
Quechol, R. et al. Heterologous synthesis of the complex homometallic cores of nitrogenase P- and M-clusters in Escherichia coli. Proc. Natl Acad. Sci. USA 120, e2314788120 (2023).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Jamali, K. et al. Automated model building and protein identification in cryo-EM maps. Nature 628, 450–457 (2024).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019).
Wojdyr, M. Fityk: a general-purpose peak fitting program. J. Appl. Crystallogr. 43, 1126–1128 (2010).
Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 78, 1606–1619 (2000).
Stoll, S. & Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 178, 42–55 (2006).
Manalastas-Cantos, K. et al. ATSAS 3.0: expanded functionality and new tools for small-angle scattering data analysis. J. Appl. Crystallogr. 54, 343–355 (2021).
Svergun, D., Barberato, C. & Koch, M. H. J. CRYSOL—a program to evaluate X-ray solution scattering of biological macromolecules from atomic coordinates. J. Appl. Crystallogr. 28, 768–773 (1995).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Honorato, R. V. et al. The HADDOCK2.4 web server for integrative modeling of biomolecular complexes. Nat. Protoc. 19, 3219–3241 (2024).
Acknowledgements
We thank members of the Tezcan and Herzik Labs, in particular, T. Ho, T. Choi and R. Herold for helpful discussions. The authors acknowledge the UCSD cryo-EM facility and the scientific and technical assistance of M. Matyszewski and I. Kuschnerus. We thank B. Dennis, K. Smith and the UCSD Physics Computing Facility for their support. This work was funded by the National Institutes of Health (NIH) (grant no. R01-GM148607 to F.A.T, grant no. R35-GM138206 to M.A.H., grant no. R35-GM126961 to R.D.B., a UCSD Interfaces Traineeship grant no. T32 EB009380 to S.M.N.), NASA (grant no. 80NSSC18M0093; ENIGMA: Evolution of Nanomachines in Geospheres and Microbial Ancestors (NASA Astrobiology Institute Cycle 8)), the National Science Foundation (Graduate Research Fellowship grant no. DGE-2038238 to V.H.E.) and the Searle Scholars Program (M.A.H.). B.D.C. is supported as a Goeddel Technology Sandbox Fellow. SAXS experiments were performed at the Stanford Synchrotron Radiation Lightsource (supported by the DOE, Office of Basic Energy Sciences contract no. DE-AC02-76SF00515 and NIH grant no. P30-GM133894). Molecular graphics and analyses were performed with UCSF ChimeraX, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from NIH grant no. R01-GM129325 and the Office of Cyber Infrastructure and Computational Biology, National Institute of Allergy and Infectious Diseases.
Author information
Authors and Affiliations
Contributions
S.M.N. conceived the project, designed experiments, performed protein isolation/characterization, cryo-EM and AUC experiments and analysis, and co-wrote the manuscript. B.D.C. performed cryo-EM experiments and analysis, and co-wrote the manuscript. S.S. performed protein isolation/characterization, EPR, circular dichroism and AUC experiments and analysis, and co-wrote the manuscript. V.H.E. performed protein isolation/characterization, SAXS, circular dichroism and AUC experiments and analysis, and co-wrote the manuscript. A.A.S. performed EPR experiments. K.L.M. conducted protein docking analysis. R.D.B. oversaw EPR experiments and analysis. M.A.H. conceived and directed the project, designed experiments, oversaw cryo-EM experiments and performed data analysis, and co-wrote the manuscript. F.A.T. conceived and directed the project, designed experiments, performed data analysis and wrote the manuscript. All authors participated in the editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Justin North and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 CryoEM data processing workflow for the FeSII-nitrogenase complex.
Single-particle cryoEM data processing workflow for (left to right) 2:2:1 complex (2.27 Å, C2 symmetry), as-isolated MoFeP (2.67 Å, C2 symmetry), 1:1:1 complex (3.94 Å, C1), and terminal end-piece of 2:2:1 complex (2.83 Å, C1) obtained from the same O2-exposed sample prepared with 10 μM MoFeP, 20 μM FeP, 20 μM FeSII, 5 mM DT, and 5 mM MgATP. Briefly, particle coordinates were obtained via template picking either with MoFeP or ternary complex. Picked particles were subject to 2-D classification before re-extraction based on the desired box size of the targeted structure. Ab initio model generation as well as 3-D classification were used to sort heterogeneity in-between multiple rounds of NU-refinement. The 2:2:1 complex (far left) also utilized RBMC before final refinement. The terminal 2:2:1 complex (far right) utilized local masking and refinement to sort only terminal complex particles. Each of the final structures is colored by local resolution and the 3-D FSC plots are shown.
Extended Data Fig. 2 CryoEM and activity analyses of FeSII-nitrogenase samples that are O2-exposed or under reduced/anaerobic conditions.
a, Four denoised micrographs from the O2-exposed sample (top) and 50 class averages obtained from 2-D classification (bottom) illustrating the presence of the 2:2:1 complex and extended polymers. b, Four denoised micrographs from the anaerobic sample (top) and 50 class averages from 2-D classification (bottom) indicating lack of 2:2:1 complexes (or polymers) and that most classes contain free MoFeP and FeP. c, Relative catalytic activities of MoFeP and FeP samples that have been prepared using the cryoEM sample preparation conditions, as monitored by acetylene reduction experiments. In these experiments, FeP and MoFeP mixtures were made using the cryoEM sample preparation conditions in the presence or absence of FeSII and with or without O2 exposure for 2 min. After this treatment, FeP and MoFeP mixtures were tested for acetylene reduction activity under anaerobic conditions after being transferred into a solution containing DT, MgATP and an enzymatic ATP regeneration system (see Methods). Blue dots: MoFeP + FeP + FeSII – O2. Green dots: MoFeP + FeP + FeSII + O2. Red dots: MoFeP + FeP – FeSII + O2. Error bars correspond to standard deviation from the mean of three biological replicates.
Extended Data Fig. 3 Solution characterization of the 2:2:1 complex and FeSII homodimer.
a, Sedimentation velocity/analytical ultracentrifugation (AUC) profiles of a 1MoFeP:2FeP:2FeSII mixture containing reduced MoFeP, reduced FeP, and oxidized FeSII at different concentrations of total protein (25 µM, 50 µM, and 100 µM). The data suggest that the formation of the 2:2:1 complex and higher order oligomers is concentration dependent. b, AUC profile of the all-oxidized MoFeP:FeP:FeSII mixture (50 µM total concentration), highlighting that the protective complex/polymer can form without the need of electron transfer between FeP and FeSII. c, AUC profiles of reduced (grey) and oxidized (magenta) FeSII (80 μM). Both display the same sedimentation peak and molecular weight estimates representative of a dimeric species. Compared to the sedimentation profile of the oxidized FeSII, the AUC profile of the reduced FeSII displays a broader peak, suggesting the conformationally dynamic nature of the FeSII homodimer in the reduced state in solution. d, Circular dichroism spectra of reduced (grey) and oxidized (magenta) FeSII at 10 μM concentration, highlighting the differences in secondary structure observed between these samples.
Extended Data Fig. 4 CryoEM volumes of the different oligomeric forms of the FeSII-nitrogenase complex.
a, Two views of as-isolated MoFeP (2.67 Å, C2 symmetry). b, Two views of 1:1:1 MoFeP:FeP:FeSIImonomer (3.94 Å, C1 symmetry) c, Two views of 2:2:1 MoFeP:FeP:FeSIIdimer-Terminal (2.83 Å, C1 symmetry) d, Two views of 2:2:1 MoFeP:FeP:FeSIIdimer (2.27 Å, C2 symmetry).
Extended Data Fig. 5 Comparison of FeP, MoFeP, and FeSII cryoEM structures from the 2:2:1 complex with corresponding crystal structures.
a, ADP-bound FeP from the 2:2:1 complex aligned with the following X-ray crystal structures: AMPPCP-bound FeP (PDB ID: 4WZA), nucleotide-free FeP (PDB ID: 2AFH), and ADP-bound FeP (PDB ID: 2AFI). b, MoFeP from the 2:2:1 complex aligned with MoFeP from the crystal structure of the ADP-bound 2:1 FeP:MoFeP complex (PDB ID: 2AFI). c, FeSII from the 2:2:1 complex aligned with the symmetry mates of the “closed” FeSII subunit X-ray crystal structure (PDB ID: 6YAV).
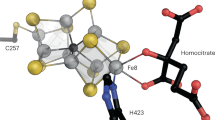
Extended Data Fig. 6 Close-up views of key residues in FeSII that were previously investigated for mutational effects in literature.
a, ε¹H56 and ε2K16 (in grey) exhibit intramolecular hydrogen bonding between ε¹E109 and ε¹D110, respectively. b, Interaction of ε¹H13 with MoFeP’ α1’E288 accompanied by ε¹H56, ε¹K15, ε¹K16, ε2K16 (in grey) shown in the background. c, ε¹K15 and ε¹K16 (in grey) and the interaction of the latter with ε2D110. d, ε¹K70 and ε¹K71 (in grey) and the interaction of the former with the backbone carbonyl of ε¹D44 and the latter with ε¹R99.
Extended Data Fig. 7 EPR spectra of FeSII and the FeSII-nitrogenase mixtures.
a, X-band EPR spectrum of chemically reduced FeSII collected at 40 K, 1 mW (black, top) and its simulated spectrum (blue, bottom) showing the S = 1/2 EPR signal of the [2Fe:2S]+-cluster with g = 2.04, 1.95, 1.89, which match those previously reported by Moshiri et al. (ref. 41). b, Overlaid X-band EPR spectra of chemically reduced FeSII (black trace) and the mixture of reduced MoFeP, reduced FeP, and oxidized FeSII (red trace) collected at 15 K, 63 µW. The difference spectrum (gray trace) demonstrates that both spectra are identical. Therefore, the EPR spectrum of the protein mixture does not have any contribution from reduced FeP. c, X-band EPR spectra (15 K) of a mixture of reduced FeP and oxidized FeSII in different incubation conditions: 15-s incubation prior to 90-s O2 exposure (top), 15-s incubation prior to 90-s O2 exposure in the presence of reduced MoFeP (middle), and 5-min incubation prior to 90-s O2 exposure (bottom). The spectra show that (1) electron transfer (ET) from FeP to FeSII can occur in the absence of MoFeP, and (2) MoFeP enhances ET between FeP and FeSII, allowing it to happen with minimal incubation time, in agreement with the formation of a ternary complex. The S = 3/2 signal in the bottom spectrum is assigned to “junk” Fe(III) which likely originates from O2 exposure of the oxidized FeP. d, The low-field region of EPR spectra (15 K) of a sample containing reduced MoFeP and oxidized FeSII (top), a separate sample containing reduced MoFeP, reduced FeP, and oxidized FeSII (middle), and the difference spectrum (grey, bottom) showing the presence of “junk” Fe(III) that originates from the O2 damage to MoFeP clusters when all three protein components are not present (i.e., the ternary complex cannot be formed).
Extended Data Fig. 8 Experimental small-angle X-ray scattering (SAXS) profiles of reduced and oxidized FeSII.
a, Guinier analysis of the scattering profiles of reduced (left, grey) and oxidized (right, pink) FeSII (30 μM) and the associated residual plots. b, Log-scale plots of the experimental scattering curves of reduced (left) and oxidized (right) FeSII compared to CRYSOL-generated theoretical scattering curves of the FeSII crystallographic structure (PDB ID: 6YAV) in the closed state (magenta), proposed open/domain-swapped state (yellow), and the FeSII cryoEM structure (blue) from this work. c, Kratky plots of reduced (left, grey) and oxidized (right, pink) FeSII each compared to the theoretical scattering profiles of the FeSII crystal structure (PDB ID: 6YAV) in the closed state (magenta), proposed open/domain-swapped state (yellow), and the FeSII cryoEM structure (blue) from this work.
Extended Data Fig. 9 Potential interactions between the 2:2:1 nitrogenase-FeSII complex, Av Ferredoxin (Fdx) and Av Flavodoxin II (Fld).
The first column of panels shows the surface representation of FeSII-nitrogenase complex alone in a and interacting with Av Fdx (PDB ID: 7FD1) in d and g, and with Av Fld II (PDB ID: 5K9B) in j and m. The manually docked geometries for Fdx and Fld are shown in red (d, e, f) and light purple (j, k, l) respectively. The docking geometries with the best HADDOCK scores for Fdx and Fld are shown in red (g, h, i) and dark purple (m, n, o), respectively. The second column of panels depicts the side views of the docking orientations shown in the first column of panels. The third column of panels shows the close-up images of the second column panels, highlighting the relative positions of the redox cofactors.
Supplementary information
Supplementary Table 1
Key metrics for ferredoxin docking clusters. Key docking metrics for the top ten ferredoxin clusters obtained from HADDOCK. Cluster 3 ranks highest with the most favourable HADDOCK score (−123.4 ± 7.4), the smallest RMSD (0.9 ± 0.5 Å) and strong van der Waals and electrostatic energies, indicating a stable binding mode. Other clusters, such as Cluster 2 and Cluster 7, show higher RMSD values and less favourable interaction energy profiles, although still presenting viable docking configurations.
Supplementary Table 2
Key metrics for flavodoxin docking clusters. Key HADDOCK docking metrics for the top ten flavodoxin clusters. Cluster 2 ranks highest with a HADDOCK score of −184.7 ± 5.7, the smallest RMSD of 1.0 ± 0.6 Å and the strongest van der Waals and electrostatic interaction energies. Cluster 1 and Cluster 3 follow in rank but display higher RMSD values and less favourable interaction energy profiles compared with Cluster 2
Supplementary Table 3
Reliability and reproducibility checklist. Further information on reliability and reproducibility regarding HADDOCK docking simulations.
Supplementary Video 1
Overview of the FeSII–nitrogenase complex. CryoEM structure of 2:2:1 FeSII–nitrogenase complex with a break down in subunits of FeP, MoFeP and FeSII displayed as cartoon ribbon structures.
Supplementary Video 2
Proposed FeSII–nitrogenase mechanism. Formation of FeSII–nitrogenase complex under oxidizing conditions and its subsequent dissociation under reducing conditions.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Narehood, S.M., Cook, B.D., Srisantitham, S. et al. Structural basis for the conformational protection of nitrogenase from O2. Nature 637, 991–997 (2025). https://doi.org/10.1038/s41586-024-08311-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-08311-1
This article is cited by
-
Preparation of oxygen-sensitive proteins for high-resolution cryoEM structure determination using blot-free vitrification
Nature Communications (2025)
-
How a small but mighty protein protects a life-sustaining enzyme
Nature (2025)
-
Architecture, catalysis and regulation of methylthio-alkane reductase for bacterial sulfur acquisition from volatile organic compounds
Nature Catalysis (2025)