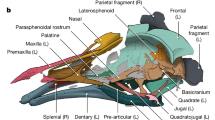
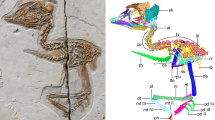
Abstract
Fossils representing Cretaceous lineages of crown clade birds (Aves) are exceptionally rare but are crucial to elucidating major ecological shifts across early avian divergences. Among the earliest known putative crown birds is Vegavis iaai1,2,3,4,5, a foot-propelled diver from the latest Cretaceous (69.2–68.4 million years ago)6 of Antarctica with controversial phylogenetic affinities2,7,8,9,10. Initially recovered by phylogenetic analyses as a stem anatid (ducks and closely related species)1,2,11, Vegavis has since been recovered as a stem member of Anseriformes (waterfowl)7,8,9, or outside Aves altogether10. Here we report a new, nearly complete skull of Vegavis that provides new insight into its feeding ecology and exhibits morphologies that support placement among waterfowl within crown-group birds. Vegavis has an avian beak (absence of teeth and reduced maxilla) and brain shape (hyperinflated cerebrum and ventrally shifted optic lobes). The temporal fossa is well excavated and expansive, indicating that this bird had hypertrophied jaw musculature. The beak is narrow and pointed, and the mandible lacks retroarticular processes. Together, these features comprise a feeding apparatus unlike that of any other known anseriform but like that of other extant birds that capture prey underwater (for example, grebes and loons). The Cretaceous occurrence of Vegavis, with a feeding ecology unique among known Galloanserae (waterfowl and landfowl), is further indication that the earliest anseriform divergences were marked by evolutionary experiments unrepresented in the extant diversity3,11,12,13.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
AMNH FARB 30899 is catalogued into the Fossil Amphibians, Reptiles, and Birds collection at the American Museum of Natural History, New York, NY, USA. Details regarding the generation of digital files and their derivatives in this study are included in Supplementary Information and are archived on MorphoSource at https://www.morphosource.org/projects/000545411. Morphological character matrices, output files from phylogenetic analyses, coordinates corresponding to aligned scan data and derivative polygons, a three-dimensional PDF of aligned scan data and derivative polygons, and surface files of retrodeformed skeletal elements are available at Dryad (https://doi.org/10.5061/dryad.n02v6wx3k)77.
References
Noriega, J. I. & Tambussi, C. P. A Late Cretaceous Presbyornithidae (Aves: Anseriformes) from Vega Island, Antarctic Peninsula: paleobiogeographic implications. Ameghiniana 32, 57–61 (1995).
Clarke, J. A., Tambussi, C. P., Noriega, J. I., Erickson, G. M. & Ketcham, R. A. Definitive fossil evidence for the extant avian radiation in the Cretaceous. Nature 433, 305–308 (2005).
Clarke, J. A. et al. Fossil evidence of the avian vocal organ from the Mesozoic. Nature 538, 502–505 (2016).
West, A. R. et al. An avian femur from the Late Cretaceous of Vega Island, Antarctic Peninsula: removing the record of cursorial landbirds from the Mesozoic of Antarctica. PeerJ 7, e7231 (2019).
Acosta Hospitaleche, C. & Worthy, T. H. New data on the Vegavis iaai holotype from the Maastrichtian of Antarctica. Cretaceous Res. 124, 104818 (2021).
Roberts, E. M. et al. New age constraints support a K/Pg boundary interval on Vega Island, Antarctica: implications for latest Cretaceous vertebrates and paleoenvironments. GSA Bull. 135, 867–885 (2022).
Agnolín, F. L., Brissón Egli, F., Chatterjee, S., Garcia Marsà, J. A. & Novas, F. E. Vegaviidae, a new clade of southern diving birds that survived the K/T boundary. Sci. Nat. 104, 87 (2017).
Worthy, T. H., Degrange, F. J., Handley, W. D. & Lee, M. S. Y. The evolution of giant flightless birds and novel phylogenetic relationships for extinct fowl (Aves, Galloanseres). R. Soc. Open Sci. 4, 170975 (2017).
Tambussi, C. P., Degrange, F. J., De Mendoza, R. S., Sferco, E. & Santillana, S. A stem anseriform from the early Palaeocene of Antarctica provides new key evidence in the early evolution of waterfowl. Zool. J. Linn. Soc. 186, 673–700 (2019).
Field, D. J., Benito, J., Chen, A., Jagt, J. W. M. & Ksepka, D. T. Late Cretaceous neornithine from Europe illuminates the origins of crown birds. Nature 579, 397–401 (2020).
Musser, G. & Clarke, J. A. A new Paleogene fossil and a new dataset for waterfowl (Aves: Anseriformes) clarify phylogeny, ecological evolution, and avian evolution at the K-Pg Boundary. PLoS ONE 19, e0278737 (2024).
Mayr, G. Paleogene Fossil Birds (Springer International Publishing, 2022).
Houde, P., Dickson, M. & Camarena, D. Basal Anseriformes from the early Paleogene of North America and Europe. Diversity 15, 233 (2023).
Ericson, P. G. P. et al. Diversification of Neoaves: integration of molecular sequence data and fossils. Biol. Lett. 2, 543–547 (2006).
Prum, R. O. et al. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 526, 569–573 (2015).
Kuhl, H. et al. An unbiased molecular approach using 3′-UTRs resolves the avian family-level tree of life. Mol. Biol. Evol. 38, 108–127 (2021).
Brocklehurst, N. & Field, D. J. Tip dating and Bayes factors provide insight into the divergences of crown bird clades across the end-Cretaceous mass extinction. Proc. R. Soc. Lond. B. Biol. Sci. 291, 20232618 (2024).
Torres, C. R., Norell, M. A. & Clarke, J. A. Bird neurocranial and body mass evolution across the end-Cretaceous mass extinction: the avian brain shape left other dinosaurs behind. Sci. Adv. 7, eabg7099 (2021).
Crane, A. et al. Taphonomic damage obfuscates interpretation of the retroarticular region of the Asteriornis mandible. Geobios https://doi.org/10.1016/j.geobios.2024.03.003 (2024).
Chatterjee, S. The morphology and systematics of Polarornis, a Cretaceous loon (Aves: Gaviidae) from Antarctica. In Proc. 5th Symposium of the Society of Avian Paleontology and Evolution, Beijing 2000 (eds. Zhou, Z. & Zhang, F.) 125–155 (Science Press, Beijing, 2002).
Mayr, G. A partial skeleton of a new fossil loon (Aves, Gaviiformes) from the early Oligocene of Germany with preserved stomach content. J. Ornithol. 145, 281–286 (2004).
Mayr, G., De Pietri, V. L., Love, L., Mannering, A. & Scofield, R. P. Oldest, smallest and phylogenetically most basal pelagornithid, from the early Paleocene of New Zealand, sheds light on the evolutionary history of the largest flying birds. Pap. Palaeontol. 7, 217–233 (2021).
Benito, J., Kuo, P.-C., Widrig, K. E., Jagt, J. W. M. & Field, D. J. Cretaceous ornithurine supports a neognathous crown bird ancestor. Nature 612, 100–105 (2022).
Álvarez-Herrera, G. P., Rozadilla, S., Agnolín, F. L. & Novas, F. E. Jaw anatomy of Vegavis iaai (Clarke et al., 2005) from the Late Cretaceous Antarctica, and its phylogenetic implications. Geobios 83, 11–20 (2024).
Elzanowski, A. & Stidham, T. A. A galloanserine quadrate from the Late Cretaceous Lance Formation of Wyoming. Auk 128, 138–145 (2011).
Zusi, R. L. A functional and evolutionary analysis of rhynchokinesis in birds. Smithsonian Contrib. Zool. 395 (1984); https://doi.org/10.5479/si.00810282.395.
Elzanowski, A. New observations of the skull of Hesperornis with reconstructions of the bony palate and otic region. Postilla 207 (1991); https://elischolar.library.yale.edu/peabody_museum_natural_history_postilla/207/.
Field, D. J. et al. Complete Ichthyornis skull illuminates mosaic assembly of the avian head. Nature 557, 96–100 (2018).
Alonso, P. D., Milner, A. C., Ketcham, R. A., Cookson, M. J. & Rowe, T. B. The avian nature of the brain and inner ear of Archaeopteryx. Nature 430, 666–669 (2004).
Kurochkin, E. N., Saveliev, S. V., Postnov, A. A., Pervushov, E. M. & Popov, E. V. On the brain of a primitive bird from the Upper Cretaceous of European Russia. Paleontol. J. 40, 655–667 (2006).
Walsh, S. A., Milner, A. C. & Bourdon, E. A reappraisal of Cerebavis cenomanica (Aves, Ornithurae), from Melovatka, Russia. J. Anat. 229, 215–227 (2016).
Chiappe, L. M., Navalón, G., Martinelli, A. G., Nava, W. & Field, D. J. Fossil basicranium clarifies the origin of the avian central nervous system and inner ear. Proc. R. Soc. Lond. B Biol. Sci. 289, 20021398 (2022).
Milner, A. C. & Walsh, S. A. Avian brain evolution: new data from Palaeogene birds (Lower Eocene) from England. Zool. J. Linn. Soc. 155, 198–219 (2009).
Zelenitsky, D. K., Therrien, F., Ridgely, R. C., McGee, A. R. & Witmer, L. M. Evolution of olfaction in non-avian theropod dinosaurs and birds. Proc. R. Soc. Lond. B Biol. Sci. 278, 3625–3634 (2011).
Smith, N. A. & Clarke, J. A. Endocranial anatomy of the Charadriiformes: sensory system variation and the evolution of wing-propelled diving. PLoS ONE 7, e49584 (2012).
Kawabe, S., Ando, T. & Endo, H. Enigmatic affinity in the brain morphology between plotopterids and penguins, with a comprehensive comparison among water birds: neuroanatomy of Plotopteridae. Zool. J. Linn. Soc. 170, 467–493 (2014).
Proffitt, J. V., Clarke, J. A. & Scofield, R. P. Novel insights into early neuroanatomical evolution in penguins from the oldest described penguin brain endocast. J. Anat. 229, 228–238 (2016).
Handley, W. D. & Worthy, T. H. Endocranial anatomy of the giant extinct Australian mihirung birds (Aves, Dromornithidae). Diversity 13, 124 (2021).
Riamon, S. et al. The endocast of the insular and extinct Sylviornis neocaledoniae (Aves, Galliformes), reveals insights into its sensory specializations and its twilight ecology. Sci. Rep. 12, 21185 (2022).
Howard, H. A new wading bird from the Eocene of Patagonia. Am. Mus. Novitates 1710 (American Museum of Natural History, 1955); https://digitallibrary.amnh.org/items/bc030d2f-1df2-425b-aebc-aabf8519c29d.
Mayr, G., De Pietri, V. L., Scofield, R. P. & Worthy, T. H. On the taxonomic composition and phylogenetic affinities of the recently proposed clade Vegaviidae Agnolín et al., 2017 – neornithine birds from the Upper Cretaceous of the Southern Hemisphere. Cretac. Res. 86, 178–185 (2018).
Mayr, G., Carrió, V. & Kitchener, A. On the “screamer-like” birds from the British London Clay: an archaic anseriform-galliform mosaic and a non-galloanserine “barb-necked” species of Perplexicervix. Palaeontol. Electron. https://doi.org/10.26879/1301 (2023).
Mayr, G. A new avian species with tubercle-bearing cervical vertebrae from the Middle Eocene of Messel (Germany). In Proc. VII International Meeting of the Society of Avian Paleontology and Evolution (eds Boles, W. E. & Wrthy, T. H.). Rec. Aust. Mus. 62, 21–28 (2010).
Bhattacharyya, B. N. Avian jaw function: adaptation of the seven–muscle system and a review. Proc. Zool. Soc. 66, 75–85 (2013).
Sun, Z. et al. Rapid and recent diversification patterns in Anseriformes birds: inferred from molecular phylogeny and diversification analyses. PLoS ONE 12, e0184529 (2017).
Garcia Marsà, J. A., Agnolín, F. L. & Novas, F. Bone microstructure of Vegavis iaai (Aves, Anseriformes) from the Upper Cretaceous of Vega Island, Antarctic Peninsula. Hist. Biol. 31, 163–167 (2019).
Beecher, W. J. Adaptations for food-getting in the American blackbirds. Auk 68, 411–440 (1951).
Bock, W. J. Kinetics of the avian skull. J. Morphol. 114, 1–41 (1964).
Zusi, R. L. The role of the depressor mandibulae muscle in kinesis of the avian skull. Proc. U S Natl Mus. 123, 1–28 (1967).
Zweers, G. A. & Vanden Berge, J. C. Evolutionary transitions in the trophic system of the wader-waterfowl complex. Neth. J. Zool. 47, 255–287 (1996).
Ericson, P. G. P. Systematic relationships of the palaeogene family Presbyornithidae (Aves: Anseriformes). Zool. J. Linn. Soc. 121, 429–483 (1997).
Mayr, G. Late Oligocene mousebird converges on parrots in skull morphology. Ibis (Lond. 1859) 155, 384–396 (2013).
Clarke, J. A. Morphology, phylogenetic taxonomy, and systematics of Ichthyornis and Apatornis (Avialae: Ornithurae). Bull. Am. Mus. Nat. Hist. 286, 1–179 (2004).
Bell, A. & Chiappe, L. M. Anatomy of Parahesperornis: evolutionary mosaicism in the Cretaceous Hesperornithiformes (Aves). Life 10, 62 (2020).
Olson, S. L. The anseriform relationships of Anatalavis Olson and Parris (Anseranatidae), with a new species from the lower Eocene London Clay. Smithsonian Contrib. Paleobiol. 89, 231–243 (1999).
Olson, S. L. & Feduccia, A. Presbyornis and the origin of the Anseriformes (Aves: Charadriomorphae). Smithsonian Contrib. Zool. 323 (Smithsonian Institution Press, 1980); https://doi.org/10.5479/si.00810282.323.
Feduccia, A. The Age of Birds (Harvard Univ. Press, 1980).
De Pietri, V. L., Scofield, R. P., Zelenkov, N., Boles, W. E. & Worthy, T. H. The unexpected survival of an ancient lineage of anseriform birds into the Neogene of Australia: the youngest record of Presbyornithidae. R. Soc. Open Sci. 3, 150635 (2016).
Zelenkov, N. V. & Stidham, T. A. Possible filter-feeding in the extinct Presbyornis and the evolution of Anseriformes (Aves). Зоол. ж. 97, 943–956 (2018).
Berv, J. S. & Field, D. J. Genomic signature of an avian Lilliput Effect across the K–Pg extinction. Syst. Biol. 67, 1–13 (2018).
Feduccia, A. The Origin and Evolution of Birds (Yale Univ. Press, 1999).
Chiappe, L. M. Glorified Dinosaurs (Wiley, 2007).
Roberts, E. M. et al. Stratigraphy and vertebrate paleoecology of Upper Cretaceous–?lowest Paleogene strata on Vega Island, Antarctica. Palaeogeogr. Palaeoclimatol. Palaeoecol. 402, 55–72 (2014).
Cordes-Person, A., Acosta Hospitaleche, C., Case, J. & Martin, J. An enigmatic bird from the lower Maastrichtian of Vega Island, Antarctica. Cretac. Res. 108, 104314 (2020).
Longrich, N. An ornithurine-dominated avifauna from the Belly River Group (Campanian, Upper Cretaceous) of Alberta, Canada. Cretac. Res. 30, 161–177 (2009).
Longrich, N. R., Tokaryk, T. & Field, D. J. Mass extinction of birds at the Cretaceous–Paleogene (K–Pg) boundary. Proc. Natl. Acad. Sci. USA 108, 15253–15257 (2011).
Davis, S. N. et al. New records of Theropoda from a Late Cretaceous (Campanian-Maastrichtian) locality in the Magallanes-Austral Basin, Patagonia, and insights into end Cretaceous theropod diversity. J. South Am. Earth Sci. 122, 104163 (2023).
Clarke, J. & Norell, M. The morphology and phylogenetic position of Apsaravis ukhaana from the Late Cretaceous of Mongolia. Am. Mus. Novitates 3387, 1–47 (2002).
Clarke, J. A., Zhou, Z. & Zhang, F. Insight into the evolution of avian flight from a new clade of Early Cretaceous ornithurines from China and the morphology of Yixianornis grabaui. J. Anat. 208, 287–308 (2006).
Li, Z., Zhou, Z., Wang, M. & Clarke, J. A. A new specimen of large-bodied basal enantiornithine Bohaiornis from the Early Cretaceous of China and the inference of feeding ecology in Mesozoic birds. J. Paleontol. 88, 99–108 (2014).
Huang, J. et al. A new ornithurine from the Early Cretaceous of China sheds light on the evolution of early ecological and cranial diversity in birds. PeerJ 4, e1765 (2016).
Miller, M. A., Pfeiffer, W. & Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In 2010 Gateway Computing Environments Workshop (GCE) https://doi.org/10.1109/GCE.2010.5676129 (IEEE, 2010).
Huelsenbeck, J. P. & Ronquist, F. MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17, 754–755 (2001).
Ronquist, F. et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012).
Worthy, T. H. et al. Osteology supports a stem-galliform affinity for the giant extinct flightless bird Sylviornis neocaledoniae (Sylviornithidae, Galloanseres). PLoS ONE 11, e0150871 (2016).
Swofford, D. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods) (Sinauer Associates, 2003).
Torres, C. R. et al. Data from: Cretaceous Antarctic bird skull elucidates early avian ecological diversity [dataset]. Dryad https://doi.org/10.5061/dryad.n02v6wx3k (2024).
Acknowledgements
We thank the United States Antarctic Program for field support; the US National Science Foundation (NSF) for funding (nos. NSF DBI-2010996 to C.R.T., NSF ANT-1141820 to J.A.C., NSF ANT-1142129 to M.C.L., NSF ANT-0636639 and NSF ANT-1142052 to R.D.E.M. and NSF ANT-1142104 to P.M.O.); C. Mehling, M. Norell and R. O’Leary (AMNH) and S. Brady (Carnegie Museum of Natural History) for specimen access; D. Pickering (Carnegie Museum of Natural History) for mechanical preparation; M. Colbert and J. Maisano (UTCT) and R. Ridgely and L. Witmer (Ohio University) for computed tomography scanning; S. Aftabizadeh (Ohio University) and E. Gorscak (Ohio University) for preliminary segmentation of computed tomography scan data; Oxford University Press for permission to use Conflicto reconstruction (Fig. 4c); and F. Degrange (CONICET) for access to Conflicto computed tomography scan data.
Author information
Authors and Affiliations
Contributions
C.R.T., J.A.C., M.C.L. and P.M.O. designed the project. R.D.E.M., E.M.R., M.C.L. and J.A.C. conducted the fieldwork when AMNH FARB 30899 was collected in 2011, and E.M.R. discovered the specimen. M.C.L. supervised mechanical preparation of the specimen. J.R.G., P.M.O. and C.R.T. completed digital preparation and interpretation of the specimen using µCT, developed digital models of the specimen and provided primary description and comparisons. E.M.R. established geological context and taphonomic interpretations. C.R.T., G.M.M., J.A.C., M.C.L. and P.M.O. contributed to specimen comparison and character coding. C.R.T. conducted phylogenetic analyses. C.R.T., P.M.O., J.R.G. and J.A.C. developed the manuscript, with contributions and/or editing from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Daniel Field, Vanesa De Pietri, Claudia Tambussi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Additional photographs of new Vegavis skull (AMNH FARB 30899).
a, detailed view of palatal region of ‘block A’ in ventral view (see main text Fig. 1b). b–c, detailed views of the caudal portion of the dorsolateral margin of the left mandible of ‘block B’ (see main text Fig. 1d) in rostrolateral (b) and caudolateral (c) views. Dashed lines indicate the margins of the lateral (blue) and medial (green) cotyles. Arrowheads indicate areas of taphonomic damage to the dorsolateral margin of the caudal mandible; asterisks (*) indicate area where the hooked dorsal process has been lost (see Extended Data Fig. 4). Scale bars, 1 cm (a–b) and 0.5 cm (c).
Extended Data Fig. 2 Digital renderings of the braincase of Vegavis iaai (AMNH FARB 30899).
a–c, Basicranium in ventral (a), dorsal/interior (b), and caudal (c) views. d–g, Left braincase in lateral (d), internal (e), dorsal (f), and caudal (g) views. Colours: brown, braincase; yellow, nasal. Numbers correspond to autapomorphies of V. iaai: 1, the presence of paired foramina on the parasphenoid perforating the endocranial cavity; 2, the unique conformation of the temporal fossa. Arrows indicate anatomical orientations: D, dorsal; L, left; R, rostral. Scale bars, 1 cm.
Extended Data Fig. 3 Digital renderings of the rostrum and palate of Vegavis iaai (AMNH FARB 30899).
a–c, Right lacrimal in caudal (a), lateral (b), and internal/medial (c) views. d–g, Right side of the rostrum in lateral (d), internal/medial (e), dorsal (f), and ventral (g) views. h–k, Palate in dorsal (h), ventral (i), right lateral (j), and left lateral (k) views. Colours: green, maxilla; orange, jugal; pink, palatines; purple, lacrimal; red, premaxilla; teal, vomers (fused); yellow, nasal. Arrows indicate anatomical orientations: D, dorsal; L, left; M, medial; R, rostral. Scale bars, 1 cm.
Extended Data Fig. 4 Digital renderings of the lower jaw (mandible) and natural endocast of Vegavis iaai.
a–c, Comparison of the new (AMNH FARB 30899) left and previously described2 (MACN-PV 19.748) right (mirrored) caudal mandible of Vegavis in dorsal (a), internal/medial (b), and lateral (c) views. d–f, Right rostral mandible (AMNH FARB 30899) in lateral (d), internal/medial (e), and dorsal (f) views. g–k, Natural endocast (AMNH FARB 30899) in right lateral (g), rostral (h), dorsal (i), caudal (j), and ventral (k) views. Numbers correspond to autapomorphies of V. iaai: 3, the presence of a hooked dorsal process at, and contiguous with, the medial cotyle of the mandible; 4, the presence of a fossa at the rostromedial margin of the lateral cotyle of the mandible; 5, the position of the medial cotyle of the mandible caudomedial to the lateral cotyle, such that the medial cotyle approaches the lateral margin of the mandible; 6, the presence of a medially projecting tubercle on the dorsomedial edge of the mandible just caudal to the coronoid process; 7, presence of a well-developed lateral crest on the caudal mandible; 8, presence on the caudal face of the mandible of a shallow retroarticular fossa. Red dashed lines indicate areas of damage along medial margin. Arrows indicate anatomical orientations: D, dorsal; L, left; M, medial; R, rostral. Scale bars, 1 cm.
Extended Data Fig. 5 Digital rendering of retro-deformed skull of Vegavis iaai (AMNH FARB 30899).
a, Right lateral, b, dorsal, and c, ventral views. Colours: blue, mandible; brown, braincase; green, maxilla; grey, impression; orange, jugal; pink, palatine; purple, lacrimal; red, premaxilla; teal, vomers (fused); yellow, nasal. Scale bar, 1 cm.
Extended Data Fig. 6 Results of analyses of morphological character matrix focused on resolving relationships along the avian stem (‘avialan matrix’).
a–j, Simplified cladograms showing relationships among Aves resulting from the avialan matrix in our primary analyses (a–b), analyses of only data from previously reported Vegavis specimens (c–d), analyses of only data from the new Vegavis specimen (e–f), when data from new and previously reported specimens were treated as independent tips (g–h), and our primary analysis modified to include Gansus yumenensis (i–j) using Bayesian inference (BI) (a,c,e,g,i) and maximum parsimony (MP) (b,d,f,h,j) approaches. Red box indicates our primary analyses with Vegavis coded to include all known specimens (indicated by “total”). Dagger symbols indicate extinct taxa. Numbers correspond to posterior probabilities for BI and bootstrap support values for MP.
Extended Data Fig. 7 Results of our analyses of morphological character matrix focused on resolving relationships within Galloanserae (‘galloanserine matrix’).
a–h, Simplified cladograms showing relationships among Anseriformes (excluding Anachronornis) resulting from the galloanserine matrix in our primary analyses (a–b), analyses of only data from previously reported Vegavis specimens (c–d), analyses of only data from the new Vegavis specimen (e–f), and when data from new and previously reported specimens were treated as independent tips (g–h) using BI (a,c,e,g) and MP (b,d,f,h) methods. i–p, Simplified cladograms showing relationships among Anseriformes (including Anachronornis) resulting from the galloanserine matrix in our primary analyses (i–j), analyses of only data from previously reported Vegavis specimens (k–l), analyses of only data from the new Vegavis specimen (m–n), and when data from new and previously reported specimens were treated as independent tips (o–p) using BI (i,k,m,o) and MP (j,l,n,p) methods. Red box indicates our primary analyses with Vegavis coded to include all known specimens (indicated by “total”). Dagger symbols indicate extinct taxa. Numbers correspond to posterior probabilities for BI and bootstrap support values for MP.
Supplementary information
Supplementary Information
This file contains methods, systematic palaeontology, descriptions and comparisons, phylogenetic results, character support for clades of interest, character descriptions, data descriptions and references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Torres, C.R., Clarke, J.A., Groenke, J.R. et al. Cretaceous Antarctic bird skull elucidates early avian ecological diversity. Nature 638, 146–151 (2025). https://doi.org/10.1038/s41586-024-08390-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-08390-0