Abstract
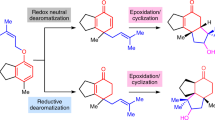
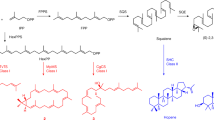
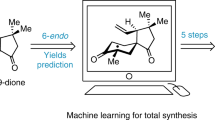
The synthesis of a complex molecule begins from an initial design stage1,2,3,4 in which possible routes are triaged by strategy and feasibility, on the basis of analogy to similar reactions2,3. However, as molecular complexity increases, predictability decreases5; inevitably, even experienced chemists resort to trial and error to identify viable intermediates en route to the target molecule. We encountered such a problem in the synthesis of picrotoxane sesquiterpenes in which pattern-recognition methods anticipated success, but small variations in structure led to failure. Here, to solve this problem but avoid tedious guess-and-check experimentation, we built a virtual library of elusive late-stage intermediate analogues that were triaged by reactivity and altered the synthesis pathway. The efficiency of this method led to concise routes to 25 naturally occurring picrotoxanes. Costly density-functional-theory transition-state calculations were replaced with faster reactant parameterizations to increase scalability and, in this case, inform the mechanism. This approach can serve as an add-on search to human or computer-assisted synthesis planning applicable to high-complexity targets and/or steps with little representation in the literature or reaction databases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are made available in the main text or Supplementary Information, including experimental procedures, copies of NMR spectra and X-ray structure reports. Structural parameters are available from the Cambridge Crystallographic Data Centre (CCDC) under the following reference numbers: des-Bz 19, 2250952; (+)-tutin (1), 2304983; 21, 2304987; SI-6′, 2303677.
Code availability
The code used in Figs. 4 and 5 is in the Supplementary Information (page 203). The source code for AutoDFT is available on Zenodo at https://doi.org/10.5281/zenodo.14366481 (ref. 49).
References
Corey, E. J. & Wipke, W. T. Computer-assisted design of complex organic syntheses. Science 166, 178–192 (1969).
Szymkuć, S. et al. Synthetic planning: the end of the beginning. Angew. Chem. Int. Ed. 55, 5904–5937 (2016).
Coley, C. W., Rogers, L., Green, W. H. & Jensen, K. F. Computer-assisted retrosynthesis based on molecular similarity. ACS Cent. Sci. 3, 1237–1245 (2017).
Cadeddu, A., Wylie, E. K., Jurczak, J., Wampler-Doty, M. & Grzybowski, B. A. Organic chemistry as a language and the implications of chemical linguistics for structural and retrosynthetic analyses. Angew. Chem. Int. Ed. 53, 8108–8112 (2014).
Strieth-Kalthoff, F. et al. Artificial intelligence for retrosynthetic planning needs both data and expert knowledge. J. Am. Chem. Soc. 146, 11005–11017 (2024).
Gössinger, E. in Progress in the Chemistry of Organic Natural Products Vol. 93 (eds Douglas Kinghorn, A., Falk, H. & Kobayashi, J.) 71–210 (Springer, 2010).
Olsen, R. W. & Sieghart, W. International union of pharmacology. LXX. Subtypes of γ-aminobutyric acid A receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 60, 243–260 (2008).
Ozoe, Y. et al. Picrodendrin and related terpenoid antagonists reveal structural differences between ionotropic GABA receptors of mammals and insects. Bioorg. Med. Chem. 6, 481–491 (1998).
Watanabe, I., Koike, K., Satou, T. & Nikaido, T. Nematocidal activity of picrodendrins against a species of Diplogastridae. Biol. Pharm. Bull. 22, 1310 (1999).
Fernandez, F. et al. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat. Neurosci. 10, 411 (2007).
Pressly, B. et al. Comparison of the toxicokinetics of the convulsants picrotoxinin and tetramethylenedisulfotetramine (TETS) in mice. Arch. Toxicol. 94, 1995–2007 (2020).
Wakamatsu, K., Kigoshi, H., Niiyama, K., Niwa, H. & Yamada, K. Stereocontrolled total synthesis of (+)-tutin and (+)-asteromurin A, toxic picrotoxane sesquiterpenes. Tetrahedron 42, 5551–5558 (1986).
Krische, M. J. & Trost, B. M. Transformations of the picrotoxanes: the synthesis of corianin and structural analogues from picrotoxinin. Tetrahedron 54, 7109–7120 (1998).
Trost, B. & Krische, M. J. Palladium-catalyzed enyne cyclo-isomerization reaction in an asymmetric approach to the picrotoxane sesquiterpenes. 2. Second-generation total syntheses of corianin, picrotoxinin, picrotin, and methyl picrotoxate. J. Am. Chem. Soc. 121, 6131 (1999).
Niwa, H. et al. Stereocontrolled total synthesis of (−)-picrotoxinin and (+)-coriamyrtin via a common isotwistane intermediate. J. Am. Chem. Soc. 106, 4547–4552 (1984).
Tanaka, K., Uchiyama, F., Sakamoto, K. & Inubushi, Y. Stereocontrolled total synthesis of (±)-coriamyrtin. J. Am. Chem. Soc. 104, 4965–4967 (1982).
Ikeuchi, K. et al. Total synthesis of (+)-coriamyrtin via a desymmetrizing strategy involving a 1,3-cyclopentanedione moiety. Org. Lett. 25, 2751–2755 (2023).
Tong, G. et al. C5 methylation confers accessibility, stability and selectivity to picrotoxinin. Nat. Commun. 14, 8308 (2023).
Tong, G. & Shenvi, R. A. Revision of the unstable picrotoxinin hydrolysis product. Angew. Chem. Int. Ed. 60, 19113 (2021).
Fields, B. A., Reeve, J., Bartholomaeus, A. & Mueller, U. Human pharmacokinetic study of tutin in honey; a plant-derived neurotoxin. Food Chem. Toxicol. 72, 234–241 (2014).
Dorigo, A. E. & Houk, K. N. Transition structures for intramolecular hydrogen atom transfers: the energetic advantage of seven-membered over six-membered transition structures. J. Am. Chem. Soc. 109, 2195–2197 (1987).
Dorigo, A. E. & Houk, K. N. On the relationship between proximity and reactivity. An ab initio study of the flexibility of the OH· + CH4 hydrogen abstraction transition state and a force-field model for the transition states of intramolecular hydrogen abstractions. J. Org. Chem. 53, 1650–1664 (1988).
Crossley, S. W. M., Tong, G., Lambrecht, M., Burdge, H. E. & Shenvi, R. A. Synthesis of picrotoxinin via late-stage strong bond activations. J. Am. Chem. Soc. 142, 11376–11381 (2020).
Boto, A., Hernández, D., Hernández, R. & Suárez, E. β-Fragmentation of primary alkoxyl radicals versus hydrogen abstraction: synthesis of polyols and α,ω-differently substituted cyclic ethers from carbohydrates. J. Org. Chem. 68, 5310–5319 (2003).
Gutekunst, W. R. & Baran, P. S. C–H functionalization logic in total synthesis. Chem. Soc. Rev. 40, 1976–1991 (2011).
Bakanas, I., Lusi, R. F., Wiesler, S., Cooke, J. H. & Sarpong, R. Strategic application of C–H oxidation in natural product total synthesis. Nat. Rev. Chem. 7, 783–799 (2023).
Salatin, T. D. & Jorgensen, W. L. Computer-assisted mechanistic evaluation of organic reactions. 1. Overview. J. Org. Chem. 45, 2043–2051 (1980).
Wei, J. N., Duvenaud, D. & Aspuru-Guzik, A. Neural networks for the prediction of organic chemistry reactions. ACS Cent. Sci. 2, 725–732 (2016).
Zhang, P. et al. A neural network model informs the total synthesis of clovane sesquiterpenoids. Nat. Synth. 2, 527–534 (2023).
Lin, Y., Zhang, R., Wang, D. & Cernak, T. Computer-aided key step generation in alkaloid total synthesis. Science 379, 453–457 (2023).
Murphy, S. K., Park, J.-W., Cruz, F. A. & Dong, V. M. Rh-catalyzed C–C bond cleavage by transfer hydroformylation. Science 347, 56–60 (2015).
Eckart, C. The theory of collisions. Phys. Rev. 35, 1303 (1930).
Garrett, B. C. & Truhlar, D. G. Semiclassical tunneling calculations. J. Phys. Chem. 83, 2921–2926 (1979).
Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 3, 107–115 (1935).
Truhlar, D. G., Hase, W. L. & Hynes, J. T. Current status of transition-state theory. J. Phys. Chem. 87, 2664–2682 (1983).
Brun, P. & Waegell, B. Heterocyclisation intramoleculaire d’alcools possedant un squelette cedranique. Oxydation par le tetraacetate de plomb et par l’oxyde de mercure et le brome. Tetrahedron 32, 1137–1145 (1976).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Meng, S.-S. et al. Chiral phosphoric acid catalyzed highly enantioselective desymmetrization of 2-substituted and 2,2-disubstituted 1,3-diols via oxidative cleavage of benzylidene acetals. J. Am. Chem. Soc. 136, 12249–12252 (2014).
Forbes, C. P., Goosen, A. & Laue, H. A. H. Hypoiodite reaction: kinetic study of the reaction of 1,1-diphenylethylene with mercury(II) oxide-iodine. J. Chem. Soc. Perkin I 2350–2353 (1974).
Wang, J. B. & Suginome, H. Intramolecular β, γ-addition of allylic alkoxyl radicals. A new general synthesis of α-lodoepoxides by photolysis of allylic alcohol hypoiodites in the presence of mercury(II) oxide, iodine and pyridine in benzene. J. Chem. Soc. Chem. Commun. 1629–1631 (1990).
Frisch, M. J., Pople, J. A. & Binkley, J. S. Self‐consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 80, 3265–3269 (1984).
Liu, F. et al. Hydrogen abstraction by alkoxyl radicals: computational studies of thermodynamic and polarity effects on reactivities and selectivities. J. Am. Chem. Soc. 144, 6802–6812 (2022).
Shen, Y. et al. Automation and computer-assisted planning for chemical synthesis. Nat. Rev. Methods Primers 1, 23 (2021).
Betinol, I. O., Kuang, Y. & Reid, J. P. Guiding target synthesis with statistical modeling tools: a case study in organocatalysis. Org. Lett. 24, 1429–1433 (2022).
Boger, D. L. et al. Total synthesis of the vancomycin aglycon. J. Am. Chem. Soc. 121, 10004–10011 (1999).
Zhong, W., Yang, Z. & Chen, C. Y.-C. Retrosynthesis prediction using an end-to-end graph generative architecture for molecular graph editing. Nat. Commun. 14, 3009 (2023).
Ihlenfeldt, W.-D. & Gasteiger, J. Computer-assisted planning of organic syntheses: the second generation of programs. Angew. Chem. Int. Ed. 34, 2613–2633 (1995).
Ting, S. I. shenvilab/autodft: AutoDFT. Zenodo https://doi.org/10.5281/zenodo.14366481 (2024).
Acknowledgements
We acknowledge J. S. Chen, B. Sanchez, Q. N. Wang, J. Lee and B. Orzolek for analysis; L. Pasternack, G. J. Kroon and D.-H. Huang for assistance with NMR spectroscopy; M. Gembicky, J. Bailey and the entire UCSD Crystallography Facility for X-ray crystallographic analysis; and J.-C. Ducom and L. Dong for assistance with the computing cluster. We thank S. Ting for conversations about statistical modelling; and K. Engle, T. Cernak and D. Wang for computational suggestions. Funding was provided by the National Institutes of Health (GM122606) and the Skaggs Graduate School of Chemical and Biological Sciences (Dale Boger Endowed Graduate Fellowship to C.L.).
Author information
Authors and Affiliations
Contributions
R.A.S. and C.L. conceived the project. C.L. executed the combined computational and experimental work with guidance and verification by R.A.S., who also oversaw the research. R.A.S. and C.L. composed the paper, C.L. compiled the supplementary materials and R.A.S. provided editorial feedback and oversight.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Text, Figures, Tables and Data – see Contents page for details.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Shenvi, R.A. Total synthesis of 25 picrotoxanes by virtual library selection. Nature 638, 980–986 (2025). https://doi.org/10.1038/s41586-024-08538-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-08538-y
This article is cited by
-
Divergent synthesis of Haplomitrium diterpenoids via a late-stage biomimetic skeletal reorganization approach
Nature Communications (2025)