Abstract
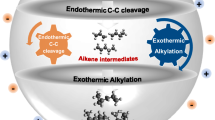
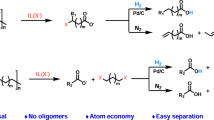
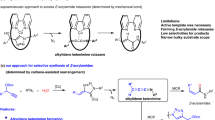
Carbohydrates are an abundant, inexpensive and renewable biomass feedstock that could be a cornerstone for sustainable chemical manufacturing, but scalable and environmentally friendly methods that leverage these feedstocks are lacking. For example, 1-allyl sorbitol is the foundational building block for the polypropylene clarifying agent Millad NX 8000, which is produced on the multi-metric ton scale annually, but the manufacturing process at present requires superstoichiometric amounts of tin1,2. The NX 8000 additives dominate about 80% of the global clarified polypropylene market3 and are used in concentrations of 0.01–1% during polypropylene production to improve its transparency and resistance to high temperatures, translating to 300–30,000 metric tons annually. The market volume of polypropylene in 2022 was approximately 79.01 million metric tons (MMT), with demand expected to rise by nearly 33% to 105 MMT by 2030 (ref. 4). The cost and sustainability benefits of clarified polypropylene are driving this demand, necessitating more clarifying agents5. Here we report a high-yielding allylation of unprotected carbohydrates in water using a catalytic amount of indium metal and either allylboronic acid or the pinacol ester (allylBpin) as donors. Aldohexoses, aminohexoses, ketohexoses and aldopentoses are all allylated in high yield under mild conditions and the indium metal is recoverable and reusable with no loss of catalytic activity. Leveraging these features, this process was translated to a scalable continuous synthesis of 1-allyl sorbitol in flow6 with high yield and productivity through Bayesian optimization of reaction parameters.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All experimental and spectroscopic data are available in the paper and Supplementary Information.
Code availability
The code for the Bayesian optimization presented in this paper is available at GitHub (https://github.com/fflorit/Millad).
References
Hernández-Fernández, J., Puello-Polo, E. & Marquez, E. Identifying, quantifying, and recovering a sorbitol-type petrochemical additive in industrial wastewater and its subsequent application in a polymeric matrix as a nucleating agent. Molecules 28, 4948 (2023).
Milliken invests to meet global demand for PP clarifier. Addit. Polym. 2014, 7 (2014).
Paben, J. APR gives recyclability thumbs up to PP additive. Resource Recycling (5 September 2019); https://resource-recycling.com/plastics/2019/09/05/apr-gives-recyclability-thumbs-up-to-pp-additive/
Market volume of polypropylene worldwide from 2015 to 2022, with a forecast for 2023 to 2030 (in million metric tons). Statista https://www.statista.com/statistics/1245169/polypropylene-market-volume-worldwide (2023).
Tullo, A. H. Making clearer polypropylene. Chem. Eng. News 88, 34–36 (2010).
Lin, M.-H. et al. Enabling technologies in carbohydrate chemistry: automated glycan assembly, flow chemistry and data science. Chem. Bio. Chem. 24, e202200607 (2023).
Zhou, F. & Li, C.-J. En route to metal-mediated and metal-catalysed reactions in water. Chem. Sci. 10, 34–46 (2019).
Hanessian, S. Total Synthesis of Natural Products: The ‘Chiron’ Approach (Pergamon Press, 1983).
Hanessian, S., Giroux, S. & Merner, B. L. Design and Strategy in Organic Synthesis: From the Chiron Approach to Catalysis (Wiley-VCH, 2013).
Newhouse, T., Baran, P. S. & Hoffmann, R. W. The economies of synthesis. Chem. Soc. Rev. 38, 3010–3021 (2009).
Trost, B. M. The atom economy—a search for synthetic efficiency. Science 254, 1471–1477 (1991).
Wender, P. A., Verma, V. A., Paxton, T. J. & Pillow, T. H. Function-oriented synthesis, step economy, and drug design. Acc. Chem. Res. 41, 40–49 (2008).
Wei, X.-F., Shimizu, Y. & Kanai, M. An expeditious synthesis of sialic acid derivatives by copper(I)-catalyzed stereodivergent propargylation of unprotected aldoses. ACS Cent. Sci. 2, 21–26 (2016).
Chung, K. & Waymouth, R. M. Selective catalytic oxidation of unprotected carbohydrates. ACS Catal. 6, 4653–4659 (2016).
Kan, J. et al. Umpolung carbonyls enable direct allylation and olefination of carbohydrates. Sci. Adv. 8, eabm6840 (2022).
Schmid, W. & Whitesides, G. M. Carbon-carbon bond formation in aqueous ethanol: diastereoselective transformation of unprotected carbohydrates to higher carbon sugars using allyl bromide and tin metal. J. Am. Chem. Soc. 113, 6674–6675 (1991).
Kim, E., Gordon, D. M., Schmid, W. & Whitesides, G. M. Tin- and indium-mediated allylation in aqueous media: application to unprotected carbohydrates. J. Org. Chem. 58, 5500–5507 (1993).
Petrier, C. & Luche, J. L. Allylzinc reagents additions in aqueous media. J. Org. Chem. 50, 910–912 (1985).
Nokami, J., Okawara, R., Otera, J. & Sudo, T. Allylation of aldehydes and ketones in the presence of water by allylic bromides, metallic tin, and aluminum. Organometallics 2, 191–193 (1983).
Chan, T.-H. & Li, C.-J. A concise chemical synthesis of (+)-3-deoxy-d-glycero-d-galacto-nonulosonic acid (KDN). J. Chem. Soc. Chem. Commun. 1992, 747–748 (1992).
Li, C.-J. & Chan, T. H. Organometallic reactions in aqueous media with indium. Tetrahedron Lett. 32, 7017–7020 (1991).
Tan, K.-T., Chng, S.-S., Cheng, H.-S. & Loh, T.-P. Development of a highly α-regioselective metal-mediated allylation reaction in aqueous media: new mechanistic proposal for the origin of α-homoallylic alcohols. J. Am. Chem. Soc. 125, 2958–2963 (2003).
Kumar, D., Vemula, S. R., Balasubramanian, N. & Cook, G. R. Indium-mediated stereoselective allylation. Acc. Chem. Res. 49, 2169–2178 (2016).
Xie, C. et al. Dibenzylidene sorbitol (DBS)-based compounds, compositions and methods for using such compounds. US patent 2007/0249850 (2007).
Xie, C. Substituted alditol compounds, compositions, and methods. US patent 7,888,454 (2011).
Yus, M., González-Gómez, J. C. & Foubelo, F. Catalytic enantioselective allylation of carbonyl compounds and imines. Chem. Rev. 111, 7774–7854 (2011).
Mlynarski, S. N., Schuster, C. H. & Morken, J. P. Asymmetric synthesis from terminal alkenes by cascades of diboration and cross-coupling. Nature 505, 386–390 (2014).
Denmark, S. E. & Fu, J. Catalytic enantioselective addition of allylic organometallic reagents to aldehydes and ketones. Chem. Rev. 103, 2763–2794 (2003).
Schneider, U., Ueno, M. & Kobayashi, S. Catalytic use of indium(0) for carbon−carbon bond transformations in water: general catalytic allylations of ketones with allylboronates. J. Am. Chem. Soc. 130, 13824–13825 (2008).
Molander, G. A., Cavalcanti, L. N., Canturk, B., Pan, P. S. & Kennedy, L. E. Efficient Hydrolysis of Organotrifluoroborates via Silica Gel and Water. J. Org. Chem. 74, 7364–7369 (2009).
Saloranta, T. et al. From mannose to small amphiphilic polyol: perfect linearity leads to spontaneous aggregation. Cryst. Growth Des. 16, 655–661 (2016).
Paquette, L. A. & Mitzel, T. M. Addition of allylindium reagents to aldehydes substituted at Cα or Cβ with heteroatomic functional groups. Analysis of the modulation in diastereoselectivity attainable in aqueous, organic, and mixed solvent systems. J. Am. Chem. Soc. 118, 1931–1937 (1996).
Gordon, D. M. & Whitesides, G. M. Indium-mediated allylations of unprotected carbohydrates in aqueous media: a short synthesis of sialic acid. J. Org. Chem. 58, 7937–7938 (1993).
Coley, C. W. et al. A robotic platform for flow synthesis of organic compounds informed by AI planning. Science 365, eaax1566 (2019).
Nambiar, A. M. K. et al. Bayesian optimization of computer-proposed multistep synthetic routes on an automated robotic flow platform. ACS Cent. Sci. 8, 825–836 (2022).
Shields, B. J. et al. Bayesian reaction optimization as a tool for chemical synthesis. Nature 590, 89–96 (2021).
Hartman, R. L., McMullen, J. P. & Jensen, K. F. Deciding whether to go with the flow: evaluating the merits of flow reactors for synthesis. Angew. Chem. Int. Ed. 50, 7502–7519 (2011).
Johnson, M. D. et al. Continuous liquid vapor reactions part 1: design and characterization of a reactor for asymmetric hydroformylation. Org. Process Res. Dev. 20, 888–900 (2016).
Acknowledgements
We thank the technical support teams of NMR, mass spectrometry and microanalytical laboratories of the University of Illinois at Urbana-Champaign and Massachusetts Institute of Technology. This work was also supported by the Molecule Maker Lab Institute: an AI Research Institutes programme supported by the US National Science Foundation under grant no. CHE 2019897. We are also grateful to the National Institutes of Health (GM R35 127010) for generous financial support.
Author information
Authors and Affiliations
Contributions
T.A. conceived and contributed to the allylBpin reaction and flow chemistry, including experimental analytical setup, reaction development, DOE design, data analysis, product characterization and manuscript writing. T.M. and M.A. contributed to the allylBF3K reaction, including experimental analytical setup, reaction development, data analysis, product characterization and manuscript writing. M.D.B. discussed the results, provided suggestions, supervised and revised the paper. F.F. developed the Bayesian algorithm to flow and contributed to writing the paper. K.F.J. supervised the flow chemistry and revised the paper. S.E.D. conceptualized, supervised the project and revised the paper. All authors contributed to assembling the final draft of the paper.
Corresponding authors
Ethics declarations
Competing interests
T.A., T.M., M.A., F.F., M.D.B., K.F.J. and S.E.D. are inventors on a patent application related to this work (US patent application no. 63/737,683) filed by the University of Illinois at Urbana-Champaign.
Peer review
Peer review information
Nature thanks Dawen Niu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data, including Supplementary Tables 1–10 and Supplementary Figs. 1–15, see contents for details.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adak, T., Menard, T., Albritton, M. et al. Catalytic allylation of native hexoses and pentoses in water with indium. Nature 640, 94–99 (2025). https://doi.org/10.1038/s41586-025-08690-z
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-08690-z