Abstract
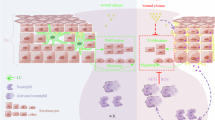
Defence from environmental threats is provided by physical barriers that confer mechanical protection and prevent the entry of microorganisms1. If microorganisms overcome those barriers, however, innate immune cells use toxic chemicals to kill the invading cells2,3. Here we examine immune diversity across tissues and identify a population of neutrophils in the skin that expresses a broad repertoire of proteins and enzymes needed to build the extracellular matrix. In the naive skin, these matrix-producing neutrophils contribute to the composition and structure of the extracellular matrix, reinforce its mechanical properties and promote barrier function. After injury, these neutrophils build ‘rings’ of matrix around wounds, which shield against foreign molecules and bacteria. This structural program relies on TGFβ signalling; disabling the TGFβ receptor in neutrophils impaired ring formation around wounds and facilitated bacterial invasion. We infer that the innate immune system has evolved diverse strategies for defence, including one that physically shields the host from the outside world.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All of the transcriptomics data are available in the Gene Expression Omnibus (GEO) and proteomics data in the Proteomics Identification Database (PRIDE). The individual GEO accession numbers are as follows: bulk RNA-seq from neutrophils in parabiotic tissues (GSE141745); bulk RNA-seq from tissues at varying circadian time points (GSE198770); bulk RNA-seq from TgfbrΔN neutrophils (GSE198654); bulk RNA-seq from skin fibroblasts (GSE202738); single-cell RNA-seq from skin tissue (GSE229493 and GSE271969); and ATAC-seq from tissue neutrophils (GSE141285). For proteomics data, they have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with dataset identifiers as follows: blood and lung cell lysates (PXD031943); neutropenic tissues (PXD031950); and wounded skin tissue (PXD031952). We also made use of the following datasets for our analysis: collagen-containing ECM (GO:0062023); ECM structural constituent (GO:0005201); collagen fibril organization (GO:0030199); and collagen metabolic process (GO:0032963); the Tabula Muris dataset to identify cell signatures; the GRCm38 Ensembl gene-build 84 as the mouse genome reference; and GENCODE vM22 to annotate genes. Source data are provided with this paper.
Code availability
No original software and/or algorithms were developed in the present study; however, code used for data analysis can be provided upon request. Any additional information required to reanalyse the data reported in this paper is available from the corresponding authors upon request. No original software and/or algorithms were developed in the present study, and all code used is available to any researcher for purposes of reproducing or extending these analyses.
Change history
07 May 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41586-025-09082-z
References
De Veer, M. J., Kemp, J. M. & Meeusen, E. N. T. The innate host defence against nematode parasites. Parasite Immunol. 29, 1–9 (2007).
Hickey, M. J. & Kubes, P. Intravascular immunity: the host–pathogen encounter in blood vessels. Nat. Rev. Immunol. 9, 364–375 (2009).
Hohl, T. M., Rivera, A. & Pamer, E. G. Immunity to fungi. Curr. Opin. Immunol. 18, 465–472 (2006).
Erin Chen, Y., Fischbach, M. A. & Belkaid, Y. Skin microbiota–host interactions. Nature 553, 427–436 (2018).
Driskell, R. R. et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504, 277–281 (2013).
Krausgruber, T. et al. Structural cells are key regulators of organ-specific immune responses. Nature 583, 296–302 (2020).
Nourshargh, S. & Alon, R. Leukocyte migration into inflamed tissues. Immunity 41, 694–707 (2014).
Burn, G. L., Foti, A., Marsman, G., Patel, D. F. & Zychlinsky, A. The neutrophil. Immunity 54, 1377–1391 (2021).
Phillipson, M. & Kubes, P. The neutrophil in vascular inflammation. Nat. Med. 17, 1381–1390 (2011).
Casanova-Acebes, M. et al. Neutrophils instruct homeostatic and pathological states in naive tissues. J. Exp. Med. 215, 2778–2795 (2018).
Casanova-Acebes, M. et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 153, 1025–1035 (2013).
Ballesteros, I. et al. Co-option of neutrophil fates by tissue environments. Cell 183, 1282–1297 (2020).
Becher, B. et al. High-dimensional analysis of the murine myeloid cell system. Nat. Immunol. 15, 1181–1191 (2014).
Theocharis, A. D., Manou, D. & Karamanos, N. K. The extracellular matrix as a multitasking player in disease. FEBS J. 286, 2830–2869 (2019).
Rubio-Ponce, A. et al. Combined statistical modeling enables accurate mining of circadian transcription. NAR Genom. Bioinform. 3, lqab031 (2021).
Csepregi, J. Z. et al. Myeloid-specific deletion of Mcl-1 yields severely neutropenic mice that survive and breed in homozygous form. J. Immunol. 201, 3793–3803 (2018).
Puente, A. et al. LOXL2—a new target in antifibrogenic therapy? Int. J. Mol. Sci. 20, 1634 (2019).
Bredfeldt, J. S. et al. Computational segmentation of collagen fibers from second-harmonic generation images of breast cancer. J. Biomed. Opt. 19, 16007 (2014).
Chaudhuri, O., Cooper-White, J., Janmey, P. A., Mooney, D. J. & Shenoy, V. B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546 (2020).
Gomariz, A. et al. Quantitative spatial analysis of haematopoiesis-regulating stromal cells in the bone marrow microenvironment by 3D microscopy. Nat. Commun. 9, 2532 (2018).
Fischer, A. et al. Neutrophils direct preexisting matrix to initiate repair in damaged tissues. Nat. Immunol. 23, 518–531 (2022).
Adrover, J. M. et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity 50, 390–402 (2019).
Martin, C. et al. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 19, 583–593 (2003).
Buckley, C. D. et al. Persistent induction of the chemokine receptor CXCR4 by TGF-β1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J. Immunol. 165, 3423–3429 (2000).
Feng, X.-H. & Derynck, R. Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 21, 659–693 (2005).
Wang, S. et al. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J. Exp. Med. 203, 1519–1532 (2006).
Meng, X. M., Nikolic-Paterson, D. J. & Lan, H. Y. TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol. 12, 325–338 (2016).
Borkowski, T. A., Letterio, J. J., Farr, A. G. & Udey, M. C. A role for endogenous transforming growth factor β1 in Langerhans cell biology: the skin of transforming growth factor β1 null mice is devoid of epidermal Langerhans cells. J. Exp. Med. 184, 2417–2422 (1996).
Mangan, P. R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Yu, X. et al. The cytokine TGF-β promotes the development and homeostasis of alveolar macrophages. Immunity 47, 903–912 (2017).
Chikina, A. S. et al. Macrophages maintain epithelium integrity by limiting fungal product absorption. Cell 183, 411–428 (2020).
Heimroth, R. D. et al. The lungfish cocoon is a living tissue with antimicrobial functions. Sci. Adv. 7, eabj0829 (2021).
Zindel, J. et al. Primordial GATA6 macrophages function as extravascular platelets in sterile injury. Science 371, eabe0595 (2021).
Vega-Pérez, A. et al. Resident macrophage-dependent immune cell scaffolds drive anti-bacterial defense in the peritoneal cavity. Immunity 54, 2578–2594 (2021).
Lim, H. Y. et al. Hyaluronan receptor LYVE-1-expressing macrophages maintain arterial tone through hyaluronan-mediated regulation of smooth muscle cell collagen. Immunity 49, 326–341 (2018).
Ryu, S. et al. Siglec-F-expressing neutrophils are essential for creating a profibrotic microenvironment in renal fibrosis. J. Clin. Invest. 132, e156876 (2022).
Di Martino, J. S. et al. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat. Cancer 3, 90–107 (2022).
Bonnans, C., Chou, J. & Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 (2014).
Hasenberg, A. et al. Catchup: a mouse model for imaging-based tracking and modulation of neutrophil granulocytes. Nat. Methods 12, 445–452 (2015).
Tauriello, D. V. F. et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543 (2018).
Adrover, J. M. et al. Programmed ‘disarming’ of the neutrophil proteome reduces the magnitude of inflammation. Nat. Immunol. 21, 135–144 (2020).
Li, J. L. et al. Intravital multiphoton imaging of immune responses in the mouse ear skin. Nat. Protoc. 7, 221–234 (2012).
Picelli, S. et al. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat. Methods 10, 1096–1098 (2013).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Bonzon-Kulichenko, E., Garcia-Marques, F., Trevisan-Herraz, M. & Vázquez, J. Revisiting peptide identification by high-accuracy mass spectrometry: problems associated with the use of narrow mass precursor windows. J. Proteome Res. 12, 700–710 (2015).
Navarro, P. et al. General statistical framework for quantitative proteomics by stable isotope labeling. J. Proteome Res. 13, 1234–1247 (2014).
García-Marqués, F. et al. A novel systems-biology algorithm for the analysis of coordinated protein responses using quantitative proteomics. Mol. Cell. Proteomics 15, 1740–1760 (2016).
Uderhardt, S., Martins, A. J., Tsang, J. S., Lämmermann, T. & Germain, R. N. Resident macrophages cloak tissue microlesions to prevent neutrophil-driven inflammatory damage. Cell 177, 541–555 (2019).
Li, W., Germain, R. N. & Gerner, M. Y. High-dimensional cell-level analysis of tissues with Ce3D multiplex volume imaging. Nat. Protoc. 14, 1708–1733 (2019).
Lämmermann, T. et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498, 371–375 (2013).
Li, X. et al. Reinforcing neuron extraction and spike inference in calcium imaging using deep self-supervised denoising. Nat. Methods 18, 1395–1400 (2021).
Frangi, A. F., Niessen, W. J., Vincken, K. L. & Viergever, M. A. Multiscale vessel enhancement filtering. In Medical Image Computing and Computer-Assisted Intervention—MICCAI’98 (eds Wells, W. M. et al) 130–137 (Springer, 1998).
Saqlain, F., Popa, I., Fernández, J. M. & Alegre-Cebollada, J. A novel strategy for utilizing voice coil servoactuators in tensile tests of low volume protein hydrogels. Macromol. Mater. Eng. 300, 369–376 (2015).
Butt, H. J. & Jaschke, M. Calculation of thermal noise in atomic force microscopy. Nanotechnology 6, 1 (1995).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 7, 16878 (2017).
Chua, R. Y. R. & Wong, S. H. SNX3 recruits to phagosomes and negatively regulates phagocytosis in dendritic cells. Immunology 139, 30–47 (2013).
Acknowledgements
We thank M. Dueñas, L. Montesino, M. Vara, J. Nieto, Y. Qian, T. Khoyratty, S. Callejas, D. Cibrián and L. Morales for technical and analytical support with NanoString analysis, biomechanical and proteomic experiments; members of the A.H. laboratory for animal husbandry and discussion; the SIgN mouse core facility for technical help and support; the SIgN flow cytometry team for assistance with cell sorting; the SIgN functional genomics and SIgN computational immunology team (N. Ang, L. M. Chan and D. Kaibo) for expertise on and assistance with transcriptomics; D. Lopez for help with the infection experiments; and the Center for Cellular and Molecular Imaging, Electron Microscopy Facility at Yale Medical School for assistance. C. J. Yu-Shen provided material assistance with our in vitro bacterial killing assays. Dr. Mück-Häusl and Dr. Rinkevich at Helmholtz Zentrum München for help designing the CNA35-reporter mice. T.V. received the support of a fellowship from La Caixa Foundation (ID 100010434) with fellowship code LCF/BQ/DR21/11880022 and from Boehringer Ingelheim Fonds; A.Ö. was supported by the Swiss National Science Foundation (P500PB-206852); J.A.N.-A. was supported by fellowship SVP-2014-068595; J.L.L. was supported by A*STAR and a Juan de la Cierva JCI-2017-33136 fellowship from Ministerio de Ciencia e Innovación (MCIN); C.H.-L. was the recipient of an FPI predoctoral fellowship (BES-2015-073191); A.C.D. was supported by a Marie Curie Individual Fellowship DILEMMA 101065552 and from La Caixa Foundation (ID 100010434) under the agreement LCF/BQ/PI22/11910029; A.H. was supported by grant R01AI165661 from the NIH/NIAD, RTI2018-095497- B-I00 from MCIN, HR17_00527 from Fundación La Caixa, the Transatlantic Network of Excellence (TNE-18CVD04) from the Leducq Foundation, and FET-OPEN (no. 861878) from the European Commission; L.G.N. was supported by the Ministry of Science and Technology of China (grant 2023YFC2306302) and the National Natural Science Foundation of China (grants W2431020 and 92374205); G.R.P., R.G. and J.A.-C. were supported by Grant Tec4Bio-CM/P2018/NMT-4443 from the Comunidad de Madrid; K.K.K. was supported by grants NHLBI R01HL156998 and NHLBI R01HL153056 from the NIH; I.A.U. was supported by the Wellcome Trust Investigator Award 209422/Z/17/Z; J.V. was supported by grants PGC2018-097019-B-I00 and PID2021-122348NB-I00 from MCIN, and HR17-00247 and HR22/52420019 from Fundación La Caixa; S.U. was supported by the Hightech Agenda Bayern, the European Research Council (101039438) and the Deutsche Forschungsgesellschaft (DFG; 447268119, 448121430 and 405969122); I.B. was supported by grants from MCIN (RYC2020-029563-I and PID2022-140534NB-I00); and J.L.L and S.Z.C. were supported by the Singapore Immunology Network (SIgN) and the Agency for Science, Technology and Research (A*STAR). Volumetric imaging was performed at the Optical Imaging Centre Erlangen (OICE, FAU) funded by DFG (project 261193037). The CNIC is supported by the MCIN and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (CEX2020-001041-S).
Author information
Authors and Affiliations
Contributions
Conceptualization: A.H. Data curation: T.V., A.Ö., J.L.L., C.H.-L., I.B., A.R.-P., A.C.D., M.M.-M., E.C. and E.B.-K. Formal analysis: T.V., A.Ö., J.L.L., C.H.-L., I.B., A.R.-P., A.C.D. and M.M.-M. Funding acquisition: A.C.D., A.E.-A., K.K.K., I.A.U., J.V., G.R.P., J.A.-C., S.U., L.G.N. and A.H. Investigation: T.V., A.Ö., J.L.L., C.H.-L., I.B., A.C.D., E.C., J.A.N.-A., P.R.-G., A.D.J., C.M., F.M.E., A.Q.-A., E.B.-K., R.A.-V., E.G., S.Z.C., M.Y.C., I.K., B.G.-B., B.M. and G.R.P. Methodology: T.V., A.Ö., J.L.L., C.H.-L., A.C.D. and E.C. Project administration and supervision: A.H. Resources: M.Z., Y.-L.W., D.S., V.G., R.G., A.E.-A., K.K.K., I.A.U., A.D., S.Z.C., G.R.P., J.V., J.A.-C., S.U., L.N.G. and A.H. Software: A.R.-P. and M.M.-M. Validation: T.V., A.Ö., J.L.L. and A.H. Visualization: T.V., A.Ö., J.L.L. and A.H. Writing: T.V., A.Ö., J.L.L. and A.H. wrote the original draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Christopher Buckley, Paul Kubes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Matrix-producing neutrophils in barrier organs.
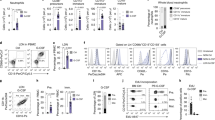
a, Isolation gates for sorting Ly6GHI neutrophils from the indicated tissues, with representative images of cytospins of the sorted cells and purities from a representative experiment; scale bar, 10 μm. b, Volcano plots showing transcript enrichment in lung, skin and intestine relative to the levels found in the three sterile tissues. Data from the transcriptome shown in Fig. 1a. c, Computational validation in single cell datasets showing that cells sorted for the analyses shown in Fig. 1a retain a neutrophil transcriptional identity. A signature score of the cells sorted and sequenced in Fig. 1a (top 500 genes) was projected over a map of multiple cell types from the Tabula muris dataset and show strongest identity with the neutrophil cluster. d, Left, production of reactive oxygen species (ROS) after vehicle or PMA stimulation by blood or skin neutrophils, measured by fluorescence of the probe DHR123. Right, frequency of neutrophils from blood and skin that phagocytose fluorescent beads, using CD11bNEG cells as negative control. Skin data are from 7 mice and blood data are from 8 mice and shown as mean ± s.e.m. P-value determined by two-sided two-tailed Student’s t-test (ROS) or one-way ANOVA with multiple comparison test (phagocytosis). e, Immunofluorescence analysis of barrier tissues to score the abundance of Col3a1+ (green) neutrophils in Ly6GTomato mice (red) stained for vessels with L. esculentum lectin (grey). Left, representative images of neutrophils in lungs; right, 3D reconstructions showing the presence of Col3a1 inside the neutrophils. f, Pie charts show the frequencies of Col3a1+ Mrp14+ cells in the indicated tissues; data from 8-9 images per tissue from 4 mice. g, Number of all neutrophils or only Col3a1+ neutrophils per imaged volume area in the indicated tissues; data from 8-9 areas from 4 mice. Note that in lung the calculated neutrophil densities were lower due to the much greater tissue volumes compared to skin and gut, despite having higher absolute total counts. h, Representative immunofluorescence micrographs of the indicated matrix proteins in neutrophils from blood and lungs. Images are representative of 300 cells from 3 mice. i, Production of collagen by neutrophils ex vivo. Neutrophils sort-purified from blood, lung or skin of wild-type and Col3ΔN (Mrp8Cre; Col3a1fl/fl) mice were set in culture for the indicated times and stained for the presence of Col3a1. Graphs show the kinetics of the frequency of Col3a1+ neutrophils. Data are shown as mean ± s.e.m. and from 3 independent experiments; n = 4 wild-type and n = 3 Col3ΔN mice. Statistical comparison of every time point versus time zero was calculated by a two-sided unpaired Student’s t-test. j, Percentage of Col3a1+ neutrophils extracted from the indicated skin regions. Data from 3 mice. Bars show mean ± s.e.m. analysed by two-way ANOVA with Tukey’s multiple-comparisons test.
Extended Data Fig. 2 Human neutrophils and neutropenia mouse models.
a, Cytometry plots showing the frequency of COL3+ neutrophils of human skin and blood. Right, quantification of percentages. Bars show mean ± s.e.m. from 5 blood and 6 skin samples. P-value determined by two-sided unpaired Student’s t-test. b, Image of a cleared human skin sample stained for neutrophils and Col3a1. White arrowheads show COL3-negative neutrophils, and yellow arrowheads COL3+ neutrophils. The image is representative of 3 donors. c, Representative images of Col3a1 staining in sorted neutrophils from human blood, spleen, lungs, and skin. Negative control IgG staining is shown for human skin neutrophils. Right, per cent Col3a1+ neutrophils in barrier and non-barrier human tissues. Data are from 3 human specimens per tissue. P-value determined by one-way ANOVA with multigroup comparison test. d, Representative cytometry plots and gating strategy for neutrophils across the indicated tissues (iDTR model). e, Neutrophil numbers in tissues by flow cytometry of the three neutropenia mouse models and their respective controls (IgG, MRP8WT; iDTR and LyzMWT; Mcl1flox, respectively). Mice were treated intraperitoneally with either anti-Ly6G (1A8, 3 µg/g) or control IgG (3 µg/g) antibodies or diphtheria toxin (DT, 10 μg/kg) for two consecutive days and tissues were collected on the third day. The number of mice (n) is displayed in the figure. P-values were determined by a two-sided unpaired Student’s t-test.
Extended Data Fig. 3 Circadian patterns of the matrix transcriptome in the skin.
Circadian expression pattern of total skin mRNA in neutrophil-depleted (iDTR, orange) or control (grey) mice. For each gene, total tissue RNA levels were smoothed and normalized to the ZT where control curve was at minimum as baseline to focus on showing the variations in gene expression. (raw data available through this paper). Empty boxes represent undetected genes. See Fig. 2a,b for the experimental scheme and general representation of the data.
Extended Data Fig. 4 Structure and mechanics of the skin.
a, Second harmonic generation (SHG) in the ear skin to determine average fibre width using CT-FIRE (see Methods). b, Measure of fibre “vesselness” to detect potential changes in fibre structure using the Frangi score (coloured as shown in the scale). More signal in the control group (Cre-negative Rosa26iDTR littermates) shows loss of fibrous matrix structure in the iDTR and TgfbrΔN mice, as shown in the quantifications below. The number of replicates is indicated in the plot. P-value determined by two-sided multiple t-test. c, Experimental design and schematics of the tensile tester used to measure the passive force of tissues at defined strains (see Methods). d, Skin stiffness at different times of day (ZT), which display marked circadian variations; the numbers of replicates (n) are displayed in the figure; p-value was calculated using the amplitude vs. zero test22. e,f, Neutrophil depletion using anti-Ly6G antibody (1A8) (e) caused loss of diurnal variations in skin stiffness (f) measured at the trough and peak times (ZT9 and ZT17). The number of replicates for each group is displayed in the figure. P-value determined by one-way ANOVA with multiple comparison test (e,f). Box plots show median ± interquartile; whiskers show the range from minimum to maximum.
Extended Data Fig. 5 Collagen-producing neutrophils localize near subepidermal fibres.
a, Multiphoton sectional imaging of ear skin explants of LysMGFP mice with anti-Ly6G (for neutrophils) and wheat germ agglutinin (WGA, for vasculature), combined with SHG to show the position of the subepidermal collagen layer and, at right, SHG imaging of this layer above looser collagen in the rest of dermis, with several neutrophils shown from a side view. b, Reconstruction of a 1.5 × 1.5 mm region of the ear skin imaged from the top by two-photon imaging showing the network of CD31+ vessels as well as intravascular (yellow) and extravasated (pink) neutrophils as also shown in the inset at right. Images are representative of 4 mice. c, Distribution of distances for neutrophils to sup-epidermal and dermal collagen compared with random dots, illustrating the preferential localization of neutrophils near the subepidermal layer of collagen. Data are from 193 cells and 3 mice. P values were calculated using the two sample Kolmogorov–Smirnov tests comparing the sample distributions of the empirical distances against the simulated randomly generated dots. d, Single-cell RNA-seq of the ear skin, with UMAP showing the main identified clusters corresponding to the indicated cell types and distribution of the cells in control and neutrophil-depleted (anti-Ly6G, 2 days) skins. e,f, Violin plots showing expression of the Col3a1 gene across the different cell types in the skin (e) and expression of the matrix signature (f; genes from Fig. 1a) in the different clusters, showing the strongest expression in fibroblasts. g, Representative images of one neutrophil and one fibroblast stained for COL3 and specific markers (Mrp14 and Podoplanin, respectively). Right, quantification of COL3+ frequencies and COL3 signal intensity per cell area or in total cells from the immunofluorescence images. Data are shown as mean ± s.e.m. from 3 mice, with the number of cells (n) displayed in the figure. P-value determined by unpaired two-sided Student’s t-test.
Extended Data Fig. 6 Neutrophils deposit collagen and regulate collagen fibres in the skin.
a, Mechanism of collagen tagging by CNA35–mCherry inside cells. This strategy was used to generate the Rosa26LSL; CNA35-mCherry reporter model, which allows Cre-dependent expression of a fluorescent form of CNA35, a collagen-binding protein of bacterial origin. Cell-specificity in this model is permitted because the fluorescent protein lacks an export signal and cannot be sorted out of the endoplasmic reticulum unless it binds trimeric collagen, thereby preventing unspecific labelling of collagen produced by other cells. b, Neutrophils sorted from naive lungs were cultured overnight for 16 h on fibronectin-coated glass coverslips. Left, representative images (top) and reconstructions (bottom) of a Col+ lung neutrophil stained for DAPI, Col1/3, and wheat germ agglutinin (WGA). White arrows show deposits of extracellular collagen. In contrast ColNEG neutrophils do not show extracellular deposits (middle panels). Right, quantification of extracellular collagen signal area (top) and the number of collagen specks, within a 100 μm perimeter of individual neutrophils (n = 5 ColNEG cells, 7 Col+ cells). Data are presented as mean values ± s.e.m. c, Representative images (top) and reconstructions (bottom) of a Col+ lung neutrophil (determined by positive CNA35–GFP signal). White arrowheads indicate the extracellular deposits positive for CNA35–mCherry and CNA35–GFP, indicating collagen deposition by the cell. These deposits are not found around CNA35–GFPNEG neutrophils (middle panels). Right, quantification of extracellular CNA35–mCherry+ area (top) and number of specks in a 100 µm x 100 µm perimeter around individual neutrophils. Data show mean ± s.e.m. from 4 GFPNEG cells and 4 GFP+ cells. P-values were determined by a two-sided unpaired Student’s t-test. d, Representative image from the skin of Ly6GCre; Rosa26LSL/CNA35-mCherry mice showing extracellular deposits of CNA35–mCherry. e, Top and side views of the skin from Rosa26LSL/CNA35-mCherry reporter mice crossed with CreNEG control mice (n = 7), and Ly6GCre (n = 11) or DptCreERT2 (n = 4) driver lines to induce CNA35–mCherry expression in neutrophils and fibroblasts. Ly6GCre targets mature neutrophils, while DptCreERT2 is expressed by 60% of fibroblasts. Arrows show distances of CNA35-collagen to the epidermis. f, Volume per cent of CNA35–mCherry in the naive skin of the neutrophil (n = 3) and fibroblast (n = 3) reporter lines. g, Distance to the epidermis of collagen produced by neutrophils or fibroblasts. P-value determined by two-way ANOVA.
Extended Data Fig. 7 Characterization of TgfbrΔN mice.
a, Blood counts in control and TgfbrΔN mice showing normal values for mice lacking neutrophil-specific TGFβ signalling. Data from 10 mice per group. b, Characterization of granulopoiesis in TgfbrΔN mice showing segmented, banded and metamyelocytic stages as percentages defined by imaging of Giemsa stains of bone-marrow samples. c, Differential expression of genes associated with blood, bone marrow (BM), spleen and lung signatures as defined in (3), as well as genes associated with the matrix signature (ECM) as defined in Fig. 1a. Comparisons are for neutrophils extracted from blood and lungs of wild-type control and TgfbrΔN mice. Data are from 3 mice per group. d, Representative reconstruction of neutrophils from the different tissues (top), and frequency of Col3a1+ neutrophils (bottom) extracted from tissues of wild-type control (n = 3) and TgfbrΔN mice (n = 3). e, Col3a1 expression in neutrophils imaged directly in the skin of wild-type control (822 cells from 5 images, 2 mice) and TgfbrΔN mice (1419 cells from 9 images, 3 mice). Representative images (left), showing neutrophils detected using Mrp14 which was used to normalize Col3a1 expression across samples (normalized intensity, middle dot plot). Using relative Col3a1+ levels >2,000 arbitrary units we stratified cells in tissues as Col3a1lo/hi and calculated the per cent of Col3a1hi cells in each group, where bars show median values. f, Representative images of Col3a1 expression inside and outside neutrophils imaged in the steady-state skin of Ly6GCreNEG control and Ly6G Cre+; Tgfbrfl/fl mice, showing neutrophils detected using Mrp14 (green), Col3a1 inside neutrophils (red) and Col3a1 outside neutrophils (magenta). g, Quantification of Col3a1 protein inside Tgfbr2fl/fl (143 cells, 2 mice) and Ly6GCre; Tgfbr2fl/fl neutrophils (275 cells, 3 mice); bars show median values. Right, box plots show extracellular Col3a1 protein signal in the same images from Tgfbr2fl/fl (143 cells from 11 images, 2 mice) and Ly6GCre; Tgfbr2fl/fl mice (275 cells from 17 images, 3 mice). P-values determined by two-tailed Student’s t-test. All controls here are Cre-negative Tgfbrflox littermates. Box plots show median ± interquartile; whiskers show the range from minimum to maximum.
Extended Data Fig. 8 Characterization of the skin of TgfbrΔN mice.
a, Representative images of neutrophils (green) stained for Col3a1 (red) in the skin of WT control (CreNEG Tgfbrflox littermates, 5 images from 2 mice) and TgfbrΔN mice (9 images from 3 mice), which we used to quantify the number of neutrophils per volume area is shown at right. Data are presented as mean values ± s.e.m., compared by two-tailed unpaired Student’s t-test. b, Histological characterization of the skin of wild-type control (n = 3) and TgfbrΔN mice (n = 3) by hematoxylin-eosin staining for cell and tissue structure, Masson’s trichrome for collagen-rich structures, and Ki67 staining for dermal and epidermal proliferation. Images are quantified in the dot plot graphs. P-values were determined by a two-sided unpaired Student’s t-test. c, Examples of SHG and fibre reconstruction using CT-FIRE for estimation of fibre width in control and TgfbrΔN mice also shown as distribution of widths in the histogram below (and in Extended Data Fig. 4A for control vs. iDTR mice). P-values were determined by a two-sided unpaired Student’s t-tests. d, Schematics of the atomic force microscopy (AFM) set-up used to measure the stiffness of tissue samples (left) and its quantification in the form of elastic Young’s modulus in lung, intestine and skin of CreNEG;Tgfbrflox control (referred to here as WT; n = 5) and TgfbrΔN littermates (n = 5 mice). Each dot represents the median Young’s modulus value calculated from ∼250 individual force–distance analysed curves per mouse. Data were compared by two-sided unpaired Student’s t-test. Right, representative height images and corresponding Young’s modulus maps of skin from control (n = 5) and TgfbrΔN littermates (n = 5) acquired by AFM indentation experiments. e, TEM images of the ear skin transversal sections showing collagen fibres in the subepidermal and lower dermis regions, which were automatically segmented for analysis (coloured fibres). Yellow circles highlight large collagen fibres (>0.2 μm2) in the subepidermal region. f, Quantification of fibre size in the skin of TgfbrΔN mice (19825 fibres) and CreNEG littermate controls (62279 fibres); data are from 2 independent experiments. P-values determined by Kruskal-Wallis non-parametric test. g, Per cent of ‘large’ fibres in the subepidermis and lower dermis from the images in f. Data are presented as mean values ± s.e.m., and p-values were determined by Kruskal-Wallis non-parametric test. h, Permeability assays in control (n = 7) and TgfbrΔN mice (n = 7) measured by FITC–dextran injected either intratracheally (for lung), or by oral gavage (for gut) in CreNEG control (n = 8) and TgfbrΔN mice (n = 8). Evans blue given intravenously to control (n = 11) and TgfbrΔN mice (n = 5) and then measured in the indicated tissues. All controls here were Cre-negative Tgfbrflox littermates. i,j, Stiffness (i) and permeability assay (j) using Evans blue in the ear skin of Mrp8Cre; Tgfbr2flox and Ly6GCre; Tgfbr2flox mice, as well as CreNEG; Tgfbr2flox littermates, or mice treated with anti-Ly6G to deplete neutrophils or isotype control antibody. The numbers of replicates are displayed in the figure. P-values determined by two-sided Student’s t-test comparing each depletion method with their controls (left panels), or one-way ANOVA with multiple comparison test (right panels). Box plots in (d,h–j) show median ± interquartile; whiskers show the range from minimum to maximum. Box plots in f show median ± interquartile; whiskers are defined with percentiles and IQR (interquartile range, P75–P25); the points outside this range are the outliers.
Extended Data Fig. 9 Dynamics of the skin needle wound model.
a, Imaging of puncture-induced wound healing (days 0 to 9; D0-D9) as visualized by SHG acquired by multiphoton imaging of the ear skin from control (WT), iDTR and TgfbrΔN mice. Wound area is highlighted with yellow dotted lines. Note the different stages indicated on top. For simplicity, only Cre-negative Tgfbr2flox littermates are shown as representative controls. b, Kinetics of neutrophil counts in the wound estimated by confocal imaging and wound size (dotted areas in a) in control mice. c, Kinetics of wound healing in controls and matched iDTR (left) or TgfbrΔN mice (right). Controls refer to the respective Cre-negative floxed littermates. Data from 2 ears per mouse and 3 mice per group, analysed by two-sided unpaired t-test between the same time points. d, Left, scheme of in vivo neutrophil labelling in iLy6G mice to assess transit between tissues. Right, frequencies of CD11b+Ly6G+Tomato+ neutrophils of iLy6GtdTom mice at the indicated times after tamoxifen injection (days 0 to 3; D0-D3) in the bone marrow (BM) (n of mice: D0 = 6, D1 = 8, D2 = 2, D3 = 7), blood (n of mice: D0 = 8, D1 = 9, D2 = 2, D3 = 7), and wounded skin(n of mice: D0 = 5, D1 = 8, D2 = 2, D3 = 7). Data are mean ± s.e.m. and p values are 0.015 for BM vs blood comparison and p = 0.029 for BM vs skin comparison at day 1, determined by mixed effect model with Tukey’s multiple comparison test. e, Representative images of the skin at different times after generating a needle wound, showing the kinetics of neutrophils that express or not COL3. The quantification of these images are shown in Fig. 4b. f, Staining for the indicated matrix proteins around wounds on day 3. Images representative of 4 mice per group. g, Representative images and quantification of COL3+ matrix rings around skin wounds in CreNEG control and Ly6GCre; Tgfbr2fl/fl mice at day 3, with quantification of COL3+ areas shown in the box and whisker plot at right. Data are from 4 CreNEG control and 3 Ly6GCre; Tgfbr2fl/fl mice. h, Image of a day 3 needle wound from the skin of iDTR mice with incomplete neutrophil depletion, showing the spatial correlation between Col3a1 deposition and the presence of residual neutrophils. i, Heat map of proteomic analysis of wounded skin from control, iDTR and TgfbrΔN mice, with colour scales showing z-scores. Control represents combined data from 2 Cre-negative floxed littermates for iDTR and TgfbrΔN mice, for a total of 4 mice per group. P-values determined by two-tailed Student’s t-test comparing the same time points between groups (c) or the two experimental groups (g).
Extended Data Fig. 10 Characterization of Col3a1ΔN mice.
a, Schematic representation of the Col3a1fl/fl mouse gene construct. Exons 2-3-4 are flanked by loxP sites which are recognized as targets of DNA cleavage by Cre recombinase. Primers 1 and 2 are used for the genotyping. Primers 3 and 4 are used for detection of depletion of gene construct after crossing with the Mrp8Cre driver line. b, Genotypes of Mrp8Cre Col3a1fl/fl (Col3a1ΔN) mice determined by PCR of genomic DNA using primers 1 and 2 that target the inserted loxP site (left). Deletion of the Col3a1 gene was confirmed by PCR of genomic DNA of lung neutrophils using primers 3 and 4 (right). Controls are CreNEG floxed neutrophils. DNA from lung fibroblasts was used as control for driver specificity. Blots are from 3 experiments. c, Representative 3D reconstructions of MRP14+ neutrophils and PDPN+ fibroblasts stained for Col3a1, with Col3a1+ cells indicated with asterisks (left). Percentage of Col3a1+ neutrophils and fibroblasts extracted from lungs of Cre-negative controls and Mrp8Cre; Col3a1fl/fl (Col3a1ΔN) mice (right). Data are presented as mean values ± s.e.m. and from 4 Cre-negative mice (455 neutrophils and 106 fibroblasts) and 5 Col3a1ΔN mice (505 neutrophils and 142 fibroblasts). d, Representative images and quantification of COL3+ matrix rings around day 3 skin wounds in the same control and Col3a1ΔN mice. Right, quantification of COL3+ and laminin+ areas (from Fig. 4h) is shown in the box and whisker plot. The number of replicates are displayed in the figure. P-values determined by two-tailed Student’s t-test. Box plots show median ± interquartile; whiskers show the range from minimum to maximum.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1–4 and Supplementary Tables 1–6.
Supplementary Data (Supplementary Figure 2)
Source Data for Supplementary Figure 2.
Supplementary Data (Supplementary Figure 4)
Source Data for Supplementary Figure 4.
Supplementary Video 1
3D imaging of the naive skin. Whole-mount confocal imaging and 3D rendering of a representative immunofluorescence of the cleared naive skin of a Ly6GCre; Rosa26Tomato mouse, showing neutrophils (red), vessels (blue), Col3 protein (green) and nuclei (DAPI, grey). The video is from a representative ear skin of 5 mice analysed.
Supplementary Video 2
Spatiotemporal dynamics of neutrophils in live mouse skin. Representative intravital microscopy sequences depicting tdTomato+ neutrophils (CatchUp mice) exhibiting distinct motility patterns and compartmental distribution: (A) intravascular trafficking within blood vessels and (B) interstitial migration through the collagen-rich ECM. (C) Side-by-side comparison demonstrating the dual-compartment neutrophil distribution in naïve skin. Colours: CatchUp neutrophils (red), second harmonics (teal), anti-CD31 injected IV (grey).
Supplementary Video 3
Quantitative analysis workflow of neutrophil-induced collagen remodelling. Sequential high-resolution confocal microscopy (t0, t1) captures structural changes in the subepidermal collagen network, visualized through SHG. Initial volume acquisition was followed by deconvolution and 3D Frangi filtering to enhance fibrillar structures. TdTomato+ neutrophils (Ly6GTomato mice) were monitored using ultra-low, minimal-perturbation imaging excitation. Migration paths enabled comparative analysis of collagen reorganization by quantifying 3D Frangi filter intensities within and adjacent to neutrophil trajectories.
Supplementary Video 4
Time-lapse multiphoton intravital microscopy of Lyz2 GFP neutrophils around skin wounds. Ear skin wounds in albino Lyz2-GFP mice were imaged repeatedly for 10 minutes at the indicated days (D0-D7). Neutrophils are highly motile on D1 within the initial wound. After D2, however, only those at the periphery remain motile. Video is representative of 4 independent mice and is looped three times. Each image: 450 um x 450 um, time lapse of 1 min between frames, maximal intensity projection of approximately 72 µm in Z-axis with 4 µm step size. (Green: Lyz2-EGFP neutrophils, Blue: SHG).
Supplementary Video 5
Structure of the matrix ring around wounds. The wounded ear skin from a wild-type mouse was explanted on day 3, fixed and stained for COL3A1 staining. Multiphoton imaging allowed the capture of second harmonic signal (grey) together with the COL3A1+ areas (orange). Video is representative of 4 independent experiments. The image is 950 x 950 µm and has a depth of 250 µm.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vicanolo, T., Özcan, A., Li, J.L. et al. Matrix-producing neutrophils populate and shield the skin. Nature 641, 740–748 (2025). https://doi.org/10.1038/s41586-025-08741-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-08741-5
This article is cited by
-
Building “walls” to stop pathogens: neutrophils play a role in the repair of extracellular matrix
Military Medical Research (2025)
-
Neutrophils make matrix to fortify barrier immunity
Nature Reviews Immunology (2025)
-
Immune cells ‘bandage’ wounds with bacteria-trapping goo
Nature (2025)
-
A bioabsorbable body-coupling-electrotherapy suture
Nature Communications (2025)
-
Structural immunity: immune cells as architects of tissue barriers
Nature Reviews Immunology (2025)