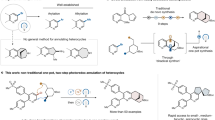
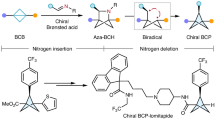
Abstract
The development of new synthetic methodologies is instrumental for enabling the discovery of new medicines. The methods that provide efficient access to structural alternatives for aromatic compounds (that is, saturated arene bioisosteres) have become highly coveted1,2,3,4. The incorporation of these bioisosteres typically leads to favourable drug-like properties and represents an emerging field of research. Here we report a new synthetic method that furnishes a coveted motif, the bicyclo[2.1.1]hexane scaffold5,6, using mild reaction conditions and an operationally simple protocol. The methodology proceeds through the uncommon coupling of two strained fragments: transiently generated cyclic allenes and bicyclo[1.1.0]butanes, which possess considerable strain energies of about 30 kcal mol−1 (ref. 7) and about 60 kcal mol−1 (ref. 6), respectively. The reaction is thought to proceed by a σ-bond insertion through a diradical pathway. However, rather than requiring an external stimulus to generate radical species, reactivity is thought to arise as a result of innate diradical character present in each reactant. This diradicaloid character8, an underused parameter in reaction design, arises from the severe geometric distortions of each reactant. Our studies provide a means to access functionalized bicyclo[2.1.1]hexanes of value for drug discovery, underscore how geometric distortion of reactants can be used to enable uncommon modes of reactivity and should encourage the further exploration and strategic use of diradicaloids in chemical synthesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Experimental procedures, characterization data, computational methods and computational data are provided in the Supplementary Information.
References
Tsien, J., Hu, C., Merchant, R. R. & Qin, T. Three-dimensional saturated C(sp3)-rich bioisosteres for benzene. Nat. Rev. Chem. 8, 605–627 (2024).
Denisenko, A. et al. 1,2-disubstituted bicyclo[2.1.1]hexanes as saturated bioisosteres of ortho-substituted benzene. Chem. Sci. 14, 14092–14099 (2023).
Subbaiah, M. A. M. & Meanwell, N. A. Bioisosteres of the phenyl ring: recent strategic applications in lead optimization and drug design. J. Med. Chem. 64, 14046–14128 (2021).
Lazzara, P. R. & Moore, T. W. Scaffold-hopping as a strategy to address metabolic liabilities of aromatic compounds. RSC Med. Chem. 11, 18–29 (2020).
Golfmann, M. & Walker, J. C. L. Bicyclobutanes as unusual building blocks for complexity generation in organic synthesis. Commun. Chem. 6, 9 (2023).
Kelly, C. B., Milligan, J. A., Tilley, L. J. & Sodano, T. M. Bicyclobutanes: from curiosities to versatile reagents and covalent warheads. Chem. Sci. 13, 11721–11737 (2022).
Johnson, R. P. Strained cyclic cumulenes. Chem. Rev. 89, 1111–1124 (1989).
Stuyver, T. et al. Do diradicals behave like radicals? Chem. Rev. 119, 11291–11351 (2019).
Buskes, M. J. & Blanco, M.-J. Impact of cross-coupling reactions in drug discovery and development. Molecules 25, 3493 (2020).
Magano, J. & Dunetz, J. R. Large-scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals. Chem. Rev. 111, 2177–2250 (2011).
Oehlrich, D. et al. 4-Alkoxy-6-oxo-pyridazine derivatives modulating NLRP3 and their preparation. US patent WO2022184843A1 (2009).
Liu, Y. et al. Pyridine-boryl radical-catalyzed [2π + 2σ] cycloaddition of bicyclo[1.1.0]butanes with alkenes. ACS Catal. 13, 5096–5103 (2023).
Guo, R. et al. Strain-release [2π + 2σ] cycloadditions for the synthesis of bicyclo[2.1.1]hexanes initiated by energy transfer. J. Am. Chem. Soc. 144, 7988–7994 (2022).
Ni, D. et al. Intermolecular formal cycloaddition of indoles with bicyclo[1.1.0]butanes by Lewis Acid Catalysis. Angew. Chem. Int. Ed. 62, e202308606 (2023).
Tang, L. et al. Silver‐catalyzed dearomative [2π+2σ] cycloadditions of indoles with bicyclobutanes: Access to Indoline fused bicyclo[2.1.1]hexanes. Angew. Chem. Int. Ed. 135, e202310066 (2023).
Dutta, S. et al. Double strain-release [2π+2σ]-photocycloaddition. J. Am. Chem. Soc. 146, 5232–5241 (2024).
Kleinmans, R. et al. Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer. Nature 605, 477–482 (2022).
Kleinmans, R. et al. ortho-Selective dearomative [2π + 2σ] photocycloadditions of bicyclic aza-arenes. J. Am. Chem. Soc. 145, 12324–12332 (2023).
Wang, H. et al. Dearomative ring expansion of thiophenes by bicyclobutane insertion. Science 381, 75–81 (2023).
Wang, J.-J. et al. Switching between the [2π+2σ] and hetero‐[4π+2σ] cycloaddition reactivity of bicyclobutanes with Lewis acid catalysts enables the synthesis of spirocycles and bridged heterocycles. Angew. Chem. Int. Ed. 63, e202405222 (2024).
Yan, H., Liu, Y., Feng, X. & Shi, L. Hantzsch esters enabled [2π+2σ] cycloadditions of bicyclo [1.1.0] butanes and alkenes under photo conditions. Org. Lett. 25, 8116–8120 (2023).
Agasti, S. et al. A catalytic alkene insertion approach to bicyclo[2.1.1]hexane bioisosteres. Nat. Chem. 15, 535–541 (2023).
Meijere, A.De et al. Cycloadditions of methylenecyclopropanes and strained bicyclo[n.1.0]alkanes to radicophilic olefins. Tetrahedron 42, 1291–1297 (1986).
Dutta, S. et al. Photoredox-enabled dearomative [2π + 2σ] cycloaddition of phenols. J. Am. Chem. Soc. 146, 2789–2797 (2024).
Wittig, G. & Fritze, P. On the intermediate occurrence of 1,2-cyclohexadiene. Angew. Chem. Int. Ed. 5, 846 (1966).
Moser, W. R. The Reactions of gem-Dihalocyclopropanes with Organometallic Reagents. PhD dissertation, Massachusetts Institute of Technology (1964).
Shi, J., Li, L. & Li, Y. o-Silylaryl triflates: a journey of Kobayashi aryne precursors. Chem. Rev. 121, 3892–4044 (2021).
Tadross, P. M. & Stoltz, B. M. A comprehensive history of arynes in natural product total synthesis. Chem. Rev. 112, 3550–3577 (2012).
Roberts, C. C. Strained allenes for heterocycle difunctionalization. Nat. Synth. 3, 286–287 (2024).
Wang, B. et al. Generation and trapping of electron-deficient 1,2-cyclohexadienes. Unexpected hetero-Diels–Alder reactivity. Org. Biomol. Chem. 19, 399–405 (2021).
Lofstrand, V. A., McIntosh, K. C., Almehmadi, Y. A. & West, F. G. Strain-activated Diels–Alder trapping of 1,2-cyclohexadienes: intramolecular capture by pendent furans. Org. Lett. 21, 6231–6234 (2019).
Lofstrand, V. A. & West, F. G. Efficient trapping of 1,2‐cyclohexadienes with 1,3‐dipoles. Chem. Eur. J. 22, 10763–10767 (2016).
Kelleghan, A. V., Tena Meza, A. & Garg, N. K. Generation and reactivity of unsymmetrical strained heterocyclic allenes. Nat. Synth. 3, 329–336 (2024).
Yamano, M. M. et al. Intercepting fleeting cyclic allenes with asymmetric nickel catalysis. Nature 586, 242–247 (2020).
McVeigh, M. S., Sorrentino, J. P., Hands, A. T. & Garg, N. K. Access to complex scaffolds through [2 + 2] cycloadditions of strained cyclic allenes. J. Am. Chem. Soc. 146, 15420–15427 (2024).
Westphal, M. V. et al. Water-compatible cycloadditions of oligonucleotide-conjugated strained allenes for DNA-encoded library synthesis. J. Am. Chem. Soc. 142, 7776–7782 (2020).
Ippoliti, F. M. et al. Total synthesis of lissodendoric acid A via stereospecific trapping of a strained cyclic allene. Science 379, 261–265 (2023).
Pomerantz, M., Gruber, G. W. & Wilke, R. N. Electronic structure and reactivity of small-ring compounds. III. mechanistic studies of the bicyclobutane-benzyne reaction. J. Am. Chem. Soc. 90, 5040–5041 (1968).
Pomerantz, M., Wilke, R. N., Gruber, G. W. & Roy, U. Electronic structure and reactivity of small ring compounds. V. reaction of some bicyclobutanes with various dienophiles. J. Am. Chem. Soc. 94, 2752–2758 (1972).
Dasgupta, A. et al. Stereoselective Alder-ene reactions of bicyclo[1.1.0]butanes: facile synthesis of cyclopropyl- and aryl-substituted cyclobutenes. J. Am. Chem. Soc. 146, 1196–1203 (2023).
Borden, W. T. Pyramidalized alkenes. Chem. Rev. 89, 1095–1109 (1989).
Vázquez, S. & Camps, P. Chemistry of pyramidalized alkenes. Tetrahedron 61, 5147–5208 (2005).
Haddon, R. C. Comment on the relationship of the pyramidalization angle at a conjugated carbon atom to the σ bond angles. J. Phys. Chem. A 105, 4164–4165 (2001).
Sterling, A. J., Smith, R. C., Anderson, E. A. & Duarte, F. Beyond strain release: delocalization-enabled organic reactivity. J. Org. Chem. 89, 9979–9989 (2024).
Carpino, L. A. & Sau, A. C. Convenient source of ‘naked’ fluoride: tetra-n-butylammonium chloride and potassium fluoride dihydrate. J. Chem. Soc., Chem. Commun. 1979, 514–515 (1979).
Garwood, J. J. A., Chen, A. D. & Nagib, D. A. Radical polarity. J. Am. Chem. Soc. 146, 28034–28059 (2024).
McVeigh, M. S. & Garg, N. K. Interception of 1,2-cyclohexadiene with TEMPO radical. Tetrahedron Lett. 87, 153539–153543 (2021).
Braslavsky, S. E. Glossary of terms used in Photochemistry, 3rd edition (IUPAC recommendations 2006). Pure Appl. Chem. 79, 293–465 (2007).
Wu, J. Diradicaloids (Jenny Stanford, 2022).
Dewar, M. J. S., Ford, G. P., McKee, M. L., Rzepa, H. S. & Wade, L. E. The Cope rearrangement. MINDO/3 studies of the rearrangements of 1,5-hexadiene and bicyclo[2.2.0]hexane. J. Am. Chem. Soc. 99, 5069–5073 (1977).
Salem, L. & Rowland, C. The electronic properties of diradicals. Angew. Chem. Int. Ed. 11, 92–111 (1972).
Doehnert, D. & Koutecky, J. Occupation numbers of natural orbitals as a criterion for biradical character. Different kinds of biradicals. J. Am. Chem. Soc. 102, 1789–1796 (1980).
Abe, M. Diradicals. Chem. Rev. 113, 7011–7088 (2013).
Bickelhaupt, F. M. & Houk, K. N. Analyzing reaction rates with the distortion/interaction‐activation strain model. Angew. Chem. Int. Ed. 56, 10070–10086 (2017).
Viesser, R. V., Donald, C. P., May, J. A. & Wu, J. I. Can twisted double bonds facilitate stepwise [2 + 2] cycloadditions? Org. Lett. 26, 3778–3783 (2024).
Acknowledgements
We thank the NIH-NIGMS (R35 GM139593 to N.K.G. and F31 GM149161 to A.T.M.), the NSF (CHE–2153972 to K.N.H. and DGE-2034835 to A.V.K.), the UCLA Cota Robles Fellowship program (C.A.R.), the Foote family (A.V.K. and A.T.M.) and the Trueblood family (N.K.G.). These studies were supported by shared instrumentation grants from the NSF (CHE-1048804), the NIH NCRR (S10RR025631) and the NIH ORIP (S10OD028644). Calculations were performed on the Hoffman2 cluster and the UCLA Institute of Digital Research and Education (IDRE) at UCLA and the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by the National Science Foundation (OCI-1053575). We thank Z. G. Walters (UCLA) and A. Wong (UCLA) for computational assistance. We are indebted to our recently departed colleague and dear friend, F. Stoddart (1942–2024), for inspiring us, by his example, to explore the unknown with curiosity and passion, and to support and cherish the next generation of brilliant scientific minds.
Author information
Authors and Affiliations
Contributions
A.T.M., C.A.R. and A.V.K. designed and performed the experiments and analysed the experimental data. H.S., A.T.M. and C.A.R. designed, performed and analysed the computational studies. K.N.H and N.K.G. directed the investigations and prepared the paper with contributions from all authors; all authors contributed to discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Computational study using BCB 21.
Reaction pathway and calculated transition states TS1a and TS1b for the σ-bond insertion reaction of strained cyclic allene 14 and monosubstituted BCB 21. ΔG and ΔH are calculated at ωΒ97X-D/def2TZVPP/SMD(DME)//ωΒ97X-D/def2SVP level of theory. Ph, phenyl; TS, transition state.
Extended Data Fig. 2 Computational study using BCB 18.
Reaction pathway and calculated transition states TS3a and TS3b for the σ-bond insertion reaction of strained cyclic allene 14 and disubstituted BCB 18. ΔG and ΔH are calculated at ωΒ97X-D/def2TZVPP/SMD(DME)//ωΒ97X-D/def2SVP level of theory. Me, methyl; Ph, phenyl; TS, transition state.
Supplementary information
Supplementary Information
This file contains two parts: part 1: Experimental Section and NMR Spectra; and part 2: Computational Section and References.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tena Meza, A., Rivera, C.A., Shao, H. et al. σ-Bond insertion reactions of two strained diradicaloids. Nature 640, 683–690 (2025). https://doi.org/10.1038/s41586-025-08745-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-08745-1
This article is cited by
-
Thermal [2+2] cycloaddition as a route to gem-difluoro heterobicyclo[n.1.1]alkanes
Nature Chemistry (2026)
-
Catalytic asymmetric activation of bicyclobutanes
Nature Synthesis (2026)
-
Hyperpyramidalized alkenes with bond orders near 1.5 as synthetic building blocks
Nature Chemistry (2026)