Abstract
Hexameric helicases are nucleotide-driven molecular machines that unwind DNA to initiate replication across all domains of life. Despite decades of intensive study, several critical aspects of their function remain unresolved1: the site and mechanism of DNA strand separation, the mechanics of unwinding propagation, and the dynamic relationship between nucleotide hydrolysis and DNA movement. Here, using cryo-electron microscopy (cryo-EM), we show that the simian virus 40 large tumour antigen (LTag) helicase assembles in the form of head-to-head hexamers at replication origins, melting DNA at two symmetrically positioned sites to establish bidirectional replication forks. Through continuous heterogeneity analysis2, we characterize the conformational landscape of LTag on forked DNA under catalytic conditions, demonstrating coordinated motions that drive DNA translocation and unwinding. We show that the helicase pulls the tracking strand through DNA-binding loops lining the central channel, while directing the non-tracking strand out of the rear, in a cyclic process. ATP hydrolysis functions as an ‘entropy switch’, removing blocks to translocation rather than directly powering DNA movement. Our structures show the allosteric couplings between nucleotide turnover and subunit motions that enable DNA unwinding while maintaining dedicated exit paths for the separated strands. These findings provide a comprehensive model for replication fork establishment and progression that extends from viral to eukaryotic systems. More broadly, they introduce fundamental principles of the mechanism by which ATP-dependent enzymes achieve efficient mechanical work through entropy-driven allostery.
Similar content being viewed by others
Main
Genome unwinding is an essential process driven by ring-shaped hexameric helicases, enzymes that are critical for DNA replication, as well as for recombination and repair across all domains of life1,3. The simian virus 40 (SV40) large tumour antigen (LTag), a model AAA+ helicase, facilitates both initiation and progression of viral replication4,5,6, paralleling the roles of eukaryotic CDC45–MCM–GINS (CMG)7,8,9. The architecture of LTag comprises an amino-terminal origin-binding domain (OBD) and a carboxy-terminal helicase domain with an ATPase motor for translocation10,11,12,13. Replication is initiated when dual helicases assemble head-to-head at the origin, melt DNA locally and establish bidirectional replication forks through ATP-hydrolysis-dependent unwinding, with each hexamer moving 3′ to 5′ along its tracking strand14,15,16,17,18,19,20. Despite extensive study, the molecular details of fork establishment, hydrolysis-coupled DNA translocation and unwinding have remained largely elusive1. Current mechanistic models are derived from crystal and cryo-electron microscopy (cryo-EM) structures reconstituted under inactive conditions11,12,21,22,23,24,25 or from fragmented enzymatic snapshots obtained under hydrolysing conditions26,27, often at limited resolutions owing to inherent limitations of discrete classification of this fundamentally continuous process. Specific aspects that remain poorly understood include the site and mechanism of strand separation, the basis for consistent discrimination between tracking and non-tracking strands and, critically, the allosteric couplings that relay nucleotide status between active sites to coordinate nucleotide hydrolysis and DNA movement.
Here, we combine cutting-edge cryo-EM approaches, including continuous heterogeneity analysis2, deep-learning-based map postprocessing28 and molecular dynamics flexible fitting29, to resolve a seamless spectrum of conformations of LTag actively translocating on forked DNA, linking nucleotide hydrolysis with DNA progression through the helicase. We rigorously characterize a total of 15 conformational states encompassing the concurrent processes of ATP hydrolysis, DNA translocation, ADP/ATP exchange and interface reset to restart the cycle. Alongside further structures of LTag bound to forked or origin DNA under non-hydrolytic conditions, we present a comprehensive, empirically derived mechanism for replication fork establishment and hydrolysis-coupled unwinding that may extend to helicases across domains. We show that the helicase encircles forked DNA, engaging one strand while directing the other through its rear—a configuration mirrored during origin melting as dual helicases create bidirectional replication forks. Crucially, we reveal that rather than directly powering translocation, ATP functions as a block by maintaining the DNA-binding tier in tension relative to the collar tier, which is released upon hydrolysis, thereby ratcheting the DNA through the helicase. As such, we demonstrate that this coupling of nucleotide state to DNA movement occurs through trans-allosteric effects, in which hydrolysis at a single competent interface triggers correlated rigid-body motions of the ATPase domains.
Prehydrolytic helicase primed at a fork junction
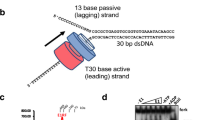
To capture the pretranslocation state, we reconstituted LTag with forked DNA and ATP in the absence of Mg2+, restricting nucleotide hydrolysis. The 3.1 Å cryo-EM reconstruction (Fig. 1a, Extended Data Fig. 1 and Extended Data Table 1) showed the helicase core to be structured as a two-tiered hexameric ring. Its six oligomerization domains (N tiers) formed a collar, with the bulkier ATPase motor domains (C tiers) situated beneath. Two-dimensional class averages indicated significant flexibility in the OBD and J domains at the N termini, manifesting as diffuse density (Extended Data Fig. 1). The DNA fork junction nestled in the central channel, with the tracking strand secured by six DNA-binding domains from the C tiers. The unpaired passive strand was disordered and not modelled (Fig. 1b–d). The N tiers exhibited slight six-fold symmetry deviations, whereas the C tiers showed pronounced asymmetry, in contrast to the C6 symmetry of DNA-absent (apo) LTag particles (Supplementary Figs. 1 and 2 and Extended Data Table 1). Unlike the symmetric apo structure, in which DNA-binding loops were equiplanar and partially flexible (Supplementary Fig. 2), DNA binding induced asymmetry through specific histidine–nucleobase and lysine– or arginine–phosphate interactions, driving C-tier rotations and creating a staircase arrangement of DNA-binding domains (Fig. 1c). ATP occupied all subunits in both forms, although density was reduced at subunit F in the DNA-bound state owing to there being fewer stabilizing contacts (Fig. 1d).
a, Cryo-EM reconstruction of the LTag hexamer bound to forked DNA and ATP viewed from below, coloured by subunit. Domain organization of LTag monomer shown above. b, Cartoon representation sliced to show DNA-binding loops intruding into minor groove at fork junction. Inset compares extended DNA density in consensus and variability maps. DNA substrate sequence is shown below with modelled region shaded. c, Close-up of the DNA fork highlighting key interactions. H513 residues (labelled) form hydrogen bonds with nucleobases (green dotted lines), whereas K512 and R456 contact the phosphate backbone. Loops from subunits D, E and F create an internal separation wedge below base P2 of the passive strand. d, Surface representation sectioned to reveal internal architecture. The tracking (pink) and passive (blue) strands are shown as sticks. Dashed line indicates the predicted path of the 5′ tail of the passive strand, which is not modelled in the map, through the gap between the C tiers of subunits A and F. e, Interface analysis. Left, bottom view highlighting ATP density (blue) at subunit interfaces. Right, close-up views of nucleotide-binding pockets showing distinct ATP type (BA, CB) and ADP type (DC, ED, FE) configurations on the basis of buried surface area (BSA) and residue interactions (dotted lines). Crystal structure overlays (transparent) showing chain C from the LTag–ATP structure (PDB 1SVM)11 superimposed on chain A of the cryo-EM model at the AB interface, with chain B from the LTag–ADP structure (PDB 1SVL)11 overlaying chain C at the CD interface.
At the fork junction, the map showed defined DNA base pairs (Fig. 1b), with limited density beyond, indicating flexibility in the DNA passing through the N-tier collar. Three-dimensional variability analysis (3DVA)2 better resolved duplex DNA features by reducing density averaging (Fig. 1b). The structure showed distinct DNA conformations: a B-form duplex (approximately 19-Å diameter) and compacted tracking strand (approximately 12 Å diameter). Six H513 residues follow the helical pattern of the tracking strand, establishing hydrogen bonds with successive bases, whereas K512 and R456 form salt bridges with backbone phosphates positioned two nucleotides downstream (Fig. 1c). Similar to E1 helicase25, the single-stranded DNA (ssDNA) nucleotides were organized with an increment of one nucleotide per subunit. Owing to the mixed composition of the DNA substrate (Fig. 1b), the tracking and passive strand nucleotides were modelled as thymidines and adenines, respectively. However, both purine and pyrimidine bases featured a polar atom (N or O) positioned to interact with the protonated (ε) nitrogen of H513. At the nexus, H513 from the top three loops penetrated the minor groove, where nucleobases T1 and T2 from the tracking strand maintained both H513 interaction and Watson–Crick pairing with their complementary bases P1 and P2 on the passive strand (Fig. 1c). Pairing of T3 was prevented by adjacent loop residues, beyond which the DNA-binding loops created an internal separation wedge directing the passive strand towards an exit between the top and bottom C tiers (Fig. 1d).
Nucleotide and interface coordination
The DNA-bound complex showed distinct interface patterns linked to DNA-binding loop positions, unlike the homogeneous interfaces in the apo state (Fig. 1e). Crystallographic studies11 have shown that whereas cis-residues interacting with ATP maintain their conformation when bound to ADP, trans-acting ‘finger’ residues (K418, R498, R540) from adjacent subunits define interface types through their interactions. As such, the top AB and BC interfaces in our structure corresponded to an ‘ATP-type’ profile, with K418 engaging β- or γ-phosphates, R540 binding γ-phosphate and R498 contacting cis-D474, potentially coordinating γ-phosphate through a water molecule11 (Fig. 1e). CD adopted an ADP-type configuration characterized by interaction between R498 and β-phosphate, whereas the DE and EF interfaces also seemed to be ADP type but lacked direct R498–nucleotide contact. The F subunit formed minimal contacts with A, completing the staircase. The interface buried surface area was correlated with nucleotide state, with ATP-type interfaces being more compact (Fig. 1e).
For comparison, we determined a structure of LTag bound to ADP and forked DNA (Supplementary Figs. 3 and 4). Although the overall architecture and DNA-binding loops were similar, double-stranded DNA density was absent from the ADP state, probably owing to the N tier channel being approximately 5 Å wider, preventing the transient interactions that would stabilize the duplex. This structural change explains the results of previous protection assays showing ATP-specific fork stabilization30. The ADP structure showed weaker AB and BC interfaces owing to lost γ-phosphate interactions, whereas the CD, DE and EF interfaces were similar to those in the ATP state. The latter interfaces showed γ-phosphate position density, potentially representing a chloride ion, hydrolysed inorganic phosphate (Pi) or ordered solvent (Supplementary Fig. 4c). The structure with AMP-PNP-Mg2+ closely resembled the ATP state, with minor deviations (Supplementary Figs. 5 and 6). Together, these non-hydrolytic structures indicate that ATP hydrolysis may occur near the top of the staircase, with ADP release at the base, as has been proposed for the related E1 helicase25.
Reconstruction of the helicase in action
To visualize hydrolysis-coupled DNA translocation and unwinding, we reconstituted LTag with a forked substrate and ATP, in the presence of Mg²+, and analysed the vitrified mixture using cryo-EM. The 3 Å consensus reconstruction (Extended Data Fig. 2 and Extended Data Tables 2–4) showed increased disorder compared with non-hydrolytic states, particularly in DNA and subunits A and F, reflecting an averaged superposition of conformational states arising from ATP hydrolysis (Extended Data Fig. 2). Fork junction density was discernible yet weak, probably owing to averaging across various hexamer positions around the nexus and instances of LTag translocating along fully unwound strands. To separate these states, the dataset was subjected to 3DVA2 with specification of three principal components (Extended Data Fig. 2). Five volumes spanning approximately equally spaced points along each trajectory (Supplementary Videos 1–3) were robustly modelled using interactive molecular dynamics flexible fitting29 following deep-learning-based map postprocessing28 (Extended Data Tables 2–4). The trajectories were interpreted along a temporal direction consistent with the known 3′–5′ translocation polarity of LTag, with the N tier oriented towards the 5′ end of the tracking strand31,32. Comprehensive validations, including global atom resolvability (Q-scores33), model geometry criteria and alternative refinement protocols confirmed the high quality and consistency of the structures. Validation by means of back-projected reconstructions further supported the findings (Supplementary Fig. 7 and Supplementary Table 1).
ATP-hydrolysis-coupled DNA translocation
The first variability component showed that the N-tier collar served as a fixed platform while the C tiers underwent coordinated rotational movements (Fig. 2a) These C-tier rotations, occurring as rigid units of the tiers with their DNA-binding loops, guided the linear traversal of the DNA through the helicase channel (Fig. 2c,d). In the initial phase of the trajectory, subunits A to E formed concerted DNA contacts through H513–nucleobase and K512–phosphate interactions (Fig. 2b,c). As the trajectory proceeded, the A subunit loop released from DNA, while the interaction of subunit B was weakened, reflected by increasing B factors (from 104 Å2 to 134 Å2 for B-subunit loop residues 509–517 between the initial and final frames) (Fig. 2c). Simultaneously, the F subunit loop established stronger DNA contacts with the nucleotide at the bottom of the staircase (Fig. 2c), as evidenced by its increased resolvability and decreasing B factors (from 163 Å2 to 131 Å2 for the same residues over the trajectory). This sequence of coordinated changes collectively drove DNA translocation by one base per hydrolysis cycle (Supplementary Video 4).
a, Overlay of initial (frame 1) and final (frame 5) states from the first variability component, showing the magnitude and direction of C-tier rotations in each subunit. b, Overlay of DNA conformations and DNA-binding residues K512 and/or H513 across the trajectory, demonstrating coordinated movements. c, Side-by-side comparison of initial and final DNA states, including H513 interactions. Nucleotides are coloured by associated subunit; hydrogen bonds are shown as red dotted lines. Green arrows indicate backbone phosphate bound to the loop of subunit D, highlighting the net translocation of one base along the trajectory. d, Superposition of subunit B from initial and final frames of the trajectory showing C-tier rotation, resulting in a downward shift of both the DNA-binding loop and bound DNA. e, Top, interactions at nucleotide-binding pocket between subunits B and C in the initial and final frames. The calculated BSA is shown, alongside the assigned interface type. Hydrogen bonds are shown as black dotted lines. The analysis shows disruption at the BC interface, indicating a catalytic event at the hydrolysis site. Middle, map density around nucleotide and DNA. Bottom, cryo-EM maps without postprocessing, depicting the helicase channel region, showing weak but persistent density for the passive strand at the DNA nexus. f, Atomic models coloured by B factor, showing increased overall mobility in the final state.
Analysis of interface changes demonstrated coupling between ATP hydrolysis and DNA movement (Fig. 2e). The initially most stable BC interface underwent substantial remodelling, losing 25% of interface area and 40% of contacts as R540 dissociated from the γ-phosphate of ATP and transitioning to an ADP-like state (Fig. 2e). By contrast, the AB interface showed more modest changes: a 9% reduction in buried surface area and one fewer contact point, with partial disruption of ATP contacts through R540 and the R498–D474 salt bridge (Supplementary Fig. 8a). Although other interfaces maintained their nucleotide coordination, they rotated in response to motions in upstream subunits (Supplementary Fig. 9a). The divergence in dynamics between the AB and BC interfaces reflected their C-tier motions: A and B rotated together, whereas B and C moved asynchronously, breaking the BC interface (Fig. 2a). The BC interface retained γ-phosphate-like density throughout the trajectory, despite changes in residue coordination (Supplementary Fig. 8a). This density may represent either replacement of the γ-phosphate by another ion after ATP hydrolysis, as seen in the ADP-bound structure, or retention of hydrolysed phosphate in the pocket through interactions with both cis and trans residues, as supported by molecular dynamics simulations (Supplementary Video 5). Similarly, gp4 helicase27 has consistently shown γ-phosphate density at active sites under hydrolytic conditions. In addition, a recent investigation of the AAA+ enzyme p97, combining cryo-EM, nuclear magnetic resonance spectroscopy and molecular dynamics simulations, directly identified a metastable ADP-Pi reaction intermediate, demonstrating that Pi can remain confined within nucleotide pockets after hydrolysis34. Reinforcing the broader relevance of this mechanism, biophysical studies of Rho and T7 helicases35,36 have demonstrated a consistent lag between ATP hydrolysis and Pi release, indicating that Pi retention may represent a general feature of helicase translocation. ADP + Pi would eventually be released at the staircase bottom, where interactions are minimal.
Examination of maps without postprocessing showed persistent passive strand density at the fork nexus, while tracking-strand density weakened near subunit A as its loop disengaged (Fig. 2e), indicating that both strands may be drawn into the helicase chamber during translocation. A control 3DVA analysis of LTag bound to forked DNA with ADP showed no DNA or loop movements, confirming that ATP hydrolysis drove the observed movements (Supplementary Fig. 9). The first principal component showed that ATP hydrolysis at the BC interface acted as an ‘entropy switch’. Release of R540 from the γ-phosphate of ATP triggered transition from a more ordered strained state to a less ordered and more relaxed state on the part of the subunits, as characterized by a discernible increase in overall atomic displacement factors, particularly in subunits A and B (Fig. 2f). This entropic release drove C-tier rotations relative to the N-tier collar domains, underpinning DNA progression. Notably, DNA-binding loops themselves showed no allosteric changes during these movements, indicating that ATP hydrolysis may remove a block to translocation rather than directly powering it.
Dynamics of translocation cycle reset
The second principal component showed reconfiguration of the loop staircase, priming the system for the subsequent ATP hydrolysis and translocation cycle. Starting in a prehydrolysis phase (Fig. 3a,b), the trajectory progressed through three key events: first, subunit A released from DNA; second, DNA advanced through the channel; and, third, the loop of subunit F detached from DNA and subsequently moved upward along the staircase, coinciding with a narrowing of the distance between subunits F and A (Fig. 3a–d and Supplementary Video 6). The rotational axes of the C tiers differed from those of the first component. Subunits A and B showed reduced collinearity, and the F subunit rotated in the opposite direction, moving towards subunit A (Figs. 2a and 3a). Interfacial analysis indicated disruptions at both the AB and the BC interfaces. Although this might initially seem to indicate dual hydrolytic events, we deduced that AB interface disruption was a secondary consequence of hydrolysis at the BC interface, resulting from rotation of the C tier of the B subunit away from subunit A. Several lines of evidence support this interpretation. The AB interface inherently tended towards motion owing to the limited stabilizing contacts of subunit A with the previous F subunit. This was reflected in higher B factors for the C tier of subunit A compared with that of subunit B (114 Å2 versus 88 Å2). Furthermore, unlike that of subunit C, the ATP-coordinating residue R498 of subunit B showed no side-chain density in the initial frame of the trajectory and failed to form a stable salt bridge with D474 (Supplementary Fig. 8b). In the crystal structure of LTag bound to ATP without DNA11, R498 was critical for positioning the catalytic water molecule at the active site. This water molecule, which was not directly resolved in our maps owing to limitations of cryo-EM in detecting ordered water in dynamic regions, was located at the apex of the metal ion coordination sphere and facilitated the nucleophilic attack on ATP γ-phosphate, catalysing its hydrolysis. The increased P value for the AB interface compared with that of the BC interface (Fig. 3e) further supports its inherently dynamic nature and reduced capability for ATP hydrolysis. Hence, it is highly likely that subunit A retains ATP while disengaging from B, with this interface gaining hydrolytic competence when it shifts to the BC position in the next cycle.
a, Overlay of initial and final states of the second variability component, showing magnitude and direction of C-tier rotations in each subunit. b, Side-by-side comparison of initial and final DNA–H513 conformations from the second and third components. Component 2 shows one-base translocation between states, whereas component 3 shows no movement. Red dotted lines indicate hydrogen bonds; green arrows mark the phosphate group interacting with the DNA-binding loop of subunit D. c, Superposition of subunit F from initial and final frames of component 2, demonstrating upward C-tier and loop movement and DNA release. d, C-tier interfaces between subunits A and F over second principal trajectory, highlighting progressive narrowing of the AF gap, with the BC interface shown for comparison. e, Interface analysis showing concurrent BC and AB disruption in component 2, whereas BC remains intact in component 3, tying breakage of this interface to translocation. BSA, P values, interface types and hydrogen bonds (black dotted lines) are shown. f, Conformational changes at the fork nexus over a full translocation cycle. The duplex DNA segment is modelled as in the prehydrolytic structure (Fig. 1); the third panel is as the first but with LTag subunits rotated one step around the N-tier six-fold axis. Loop staircase restoration drives nexus rotation and base unpairing at the fork junction (black).
The preparatory phase for the next cycle involved the loop of the F subunit releasing DNA and moving upward while approaching subunit A. The final nucleobase capture by F probably occurred too rapidly to resolve, bringing the system back to its starting configuration. Analysis of the third component (Supplementary Video 7) reinforced the critical role of BC interface breakage in DNA translocation (Fig. 3b,e). During this trajectory, the DNA remained nearly static (Fig. 3b), with the BC interface retaining its ATP-like coordination. Subunit F seemed to be bound to ATP at either end of the trajectory. However, subunit A formed a stable interaction with DNA (Fig. 3b), and through ATP with B (Fig. 3e), when approached by an ascending ATP-bound subunit F.
Unified model of fork unwinding
Combining insights from our prehydrolysis structure (Fig. 1) with the dynamic analyses above enabled us to map the conformational changes at the DNA fork nexus during a complete hydrolysis cycle (Fig. 3f and Supplementary Video 8). Upon hydrolysis, the nexus shifts within the helicase as the DNA-binding loops pull the tracking strand. Subunit F and its loop move upward, restoring the staircase structure and causing the nexus to rotate. This rotation results from the free nucleobase at the top of the staircase integrating into the compacted helix of the tracking strand. Meanwhile, the segment of the tracking strand held by the loops translates without rotation, disrupting the base pair at the fork junction. Critically, the terminal base of the passive strand can no longer pair with the tracking strand, which is coordinated by H513 of subunit B (Fig. 3f). This differential movement—rotation at the top and translation below—promotes base pair melting at the fork junction.
Fork establishment on origin DNA
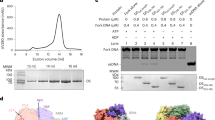
We next explored how replication forks were established at the SV40 origin, a 64-base-pair core region containing early palindrome (EP) and AT-rich halves, each with two GAGGC pentanucleotides (PEN) and AT-rich sequences recognized by OBDs37 (Fig. 4a). We determined cryo-EM structures of LTag bound to AMP-PNP-Mg²+ and either the EP or the AT-rich half of the origin core (Extended Data Figs. 3–5 and Extended Data Table 5). Notably, the EP- and AT-bound reconstructions were nearly identical to each other (root mean square deviation of approximately 0.25 Å), and both were very similar to LTag bound to forked DNA (root mean square deviation of approximately 0.60 Å) (Fig. 4b). This conservation supports a unified mechanism of DNA engagement across different stages of fork establishment and progression. In both origin structures, the OBDs were flexible and disengaged from the DNA (Fig. 4b), probably owing to competition for limited binding sites among the six OBDs, as well as structural constraints (Fig. 4c). The F subunit showed an ATP-like configuration with its DNA-binding loop elevated to the staircase top, ready to capture a nucleobase. Alignment with crystal structures of LTag dimer-EP10 and OBD tetramer-PEN37 highlighted a strong correlation between the fork nexus and DNA-binding loops, indicating that origin melting may begin through stepwise addition of LTag monomers, in which D and E subunit loops insert into the minor groove to encircle the tracking strand (Fig. 4c). Previous studies have shown that LTag assembly on the origin initiates melting at the EP region, disrupting eight base pairs38. These bases precisely match the DNA segment displaced by the D and E loops in the fully assembled LTag hexamer on the EP half (Fig. 4c). Alignment with the AT structure identified melting inside the AT-rich region known to undergo structural distortions during double hexamer assembly38. Thus, melting starts symmetrically at two sites flanking the origin centre, disrupting at least five base pairs per end. Our demonstration that LTag efficiently unwinds duplex DNA containing the EP half of the origin core (Fig. 4d), combined with its ability to unwind forked substrates lacking a 5′ tail32, supports internal strand separation with rear exit.
a, SV40 replication origin sequence highlighting EP and AT-rich regions with PEN elements. b, Cryo-EM reconstructions of LTag bound to EP and AT half origins with atomic models. The bottom-left panel shows DNA-binding loop interactions with the nexus. Hydrogen bonds are shown as black dotted lines. Loops of D and E create a wedge, disrupting base-pairing below P2, promoting local DNA melting. In the next panel, the three-dimensional class of LTag–EP shows double-stranded DNA (dsDNA) not engaged to flexible OBDs. The bottom-right panels show alignments of LTag–EP or LTag–AT models with fork-DNA-bound LTag (this work). c, Model of LTag dimer bound to EP (PDB 4GDF10) showing the predicted region of initial melting, aligned with the model of LTag–EP (this work) to show the matching base pairs at the nexus. The alignment predicts local melting in the EP sequence and shows that OBD binding to two PEN sequences hinders hexamer assembly. Inset illustrates loops of subunits D and E intruding into the minor groove to prevent base-pairing. The EP sequence shows the predicted melted bases. d, Assay of EP half-origin DNA unwinding by LTag. DNA was incubated with increasing LTag concentrations (50–500 nM) and ATP for 45 min. Controls included unboiled (first lane) and boiled (second lane) substrates. Substrate mobility shifts indicate unwinding. For gel source data, see Supplementary Fig. 10. e, Cryo-EM analysis of LTag bound to the full-origin core. The top panel shows two-dimensional class averages of the double hexameric assembly. The bottom-left panel shows a Gaussian-filtered 3.7 Å-resolution map of the assembly, showing an ordered hexamer, flexible OBDs and DNA, and a second low-resolution hexamer. Duplex DNA density near OBDs is visible at higher map thresholds. The bottom-right panel shows the LTag double hexamer model, sliced to reveal the DNA substrate (yellow) in the helicase channel. r.m.s.d., root mean square deviation.
Cryo-EM analysis of LTag with AMP-PNP-Mg2+ and the full-origin core (Fig. 4e, Extended Data Figs. 6 and 7 and Extended Data Table 5) showed two head-to-head hexamers, separated by two layers of flexible OBDs (Fig. 4e). Three-dimensional reconstruction at 3.7 Å resolution showed one well-ordered hexamer, aligned with the structures bound to the EP or AT half of the origin, as well as a stretch of duplex DNA density emerging from the hexamer N tiers (Fig. 4e). At a lower threshold, density for the OBD layers and the second hexamer becomes apparent. Despite 3D classification and variability analysis, the second hexamer’s resolution could not be improved; this indicated significant positional heterogeneity between the lobes of the double hexamer, consistent with earlier studies14,39. However, symmetry in LTag binding to each origin half (Fig. 4b) enabled us to construct an empirical model of the double hexamer bound to the full origin (Fig. 4e). By extending the DNA from the ordered hexamer and placing a second hexamer bound to the locally melted end, we generated a model that fits the experimental density (Fig. 4e). These structures demonstrate the stepwise process of origin recognition, DNA melting and establishment of bidirectional replication forks.
Discussion
Entropy-switch-driven substrate translocation
We have demonstrated that LTag translocates on ssDNA by means of a trans-allosteric, entropy-driven mechanism regulated by ATP binding and hydrolysis (Fig. 5a). Hydrolysis occurs at a stable ATP-type interface near the staircase top, whereas nucleotide exchange happens at the bottom. The cycle completes with the formation of an ATP interface between the bottom and top subunits and the loop of the bottom subunit capturing the free DNA nucleobase at the top. This mechanism is likely to apply broadly to hexameric helicases using rotary cycling, despite variations in step sizes and polarity. These include viral E1 (ref. 25), bacteriophage T7 (ref. 27), bacterial DnaB21 and Rho23, as well as archaeal MCM24. By contrast, the heterohexameric eukaryotic CMG has inspired models of asymmetrical translocation26,40. Initial studies identified fewer conformers than expected for symmetric translocation26, but recent cryo-EM studies have shown further conformers41, indicating that CMG may also translocate through a universal, symmetric rotary mechanism24. In addition, our ‘block release’ model could explain why disabling specific ATPase subunits in CMG does not markedly impair its DNA unwinding capabilities. For instance, a Drosophila CMG mutant with a Walker A mutation in MCM6 retains 70–80% of wild-type unwinding efficiency, whereas the same mutation in MCM3 reduces activity to 1–5% (ref. 8). Structural data indicate that the MCM6–MCM4 interface possesses strong intrinsic stability22, allowing the staircase to reconstitute even without ATP41. This would enable translocation to progress in mutant MCM6, as the molecular ‘block’ is naturally absent. AlphaFold3 (ref. 42) predictions support this observation, showing that KA mutations in Walker A significantly affect ATP binding in MCM3 but not in MCM6.
a, Mechanism of internal fork unwinding by LTag, combining insights from the prehydrolytic structure of LTag bound to forked DNA and ATP and an actively translocating LTag incorporating Mg2+. The model outlines a single translocation cycle whereby ATP hydrolysis at the tightest hexamer interface (BC) acts as an ‘entropy switch’. Hydrolysis initiates synchronized rotations of the C tiers across different subunits and rearrangements of the DNA-binding loops, facilitating longitudinal movement of the tracking strand and DNA nexus within the helicase inner chamber. Following exchange of ADP–Pi for ATP by the F subunit, a new series of C-tier rotations occurs. This shifts the DNA-binding loop of the F subunit to the top of the staircase, concurrently establishing the ATP interface between the F and A subunits. Reconstitution of the staircase structure leads to melting of one base pair at the nexus, progressing the unwinding process. b, Model of bidirectional origin unwinding facilitated by dual LTag helicases: assembly of two hexamers in a head-to-head configuration at the origin causes melting at the EP and AT-rich regions. ATP-hydrolysis-driven traction on the tracking strand by each hexamer leads to shearing of the DNA between the hexamers and creation of single-stranded loops at the rear of the helicase. When the unwound DNA strands spanning the hexamers are fully elongated, they exit their respective hexamers. This results in the passive strands being routed around the exterior of the hexamers with concurrent release of the tracking-strand loops formed at the back of the helicase, culminating in separation of the helicases.
The entropy-switch principle may extend beyond helicases to other AAA+ translocases, such as VAT or ClpXP43,44, suggesting a potentially general mechanism for molecular motor function.
Model for bidirectional fork unwinding
Our data show that LTag internally unwinds DNA forks by engulfing the fork nexus, pulling the tracking strand and rotating the nexus, with the unwound strands exiting through the helicase rear (Fig. 5a and Supplementary Video 8). We demonstrate that dual LTag hexamer assembly at the replication origin drives local melting at both ends, establishing opposing fork structures poised for bidirectional unwinding. At the origin, we surmise that each hexamer uses ATP hydrolysis to pull its respective tracking strand in opposite directions, progressively destabilizing and shearing intervening DNA base pairs while twisting strands beyond the DNA-binding loops (Fig. 5b). Multiple hydrolysis cycles generate single-stranded loops extending from the C tiers, consistent with previous biochemical suggestions31. Once the unwound DNA strands between hexamers are fully stretched, they are expelled from their corresponding helicase cores, with the release of tracking-strand loops formed at the rear and passive strands running outside (Fig. 5b). The structural flexibility of the LTag double hexamer probably facilitates lateral pulling and strand expulsion, with non-tracking strands exiting through C-tier openings and transient N-tier gaps—a process supported by the ability of LTag to oligomerize from dimers to hexamers16,17. Once the hexamers have uncoupled, they are primed to continue unwinding by so-called steric exclusion19,20, as proposed for both E1 (ref. 45) and CMG helicases46,47,48, but they may reinternalize the fork upon encountering a tracking-strand nick. However, our structural and previous biochemical data38 indicate that LTag inherently internalizes the fork junction, including the passive strand, within its inner core. This indicates the helicase may naturally re-engulf the fork after initial passive strand extrusion, even in the absence of DNA nicks. Importantly, the fundamental mechanism of DNA translocation relies on tracking-strand interactions with the DNA-binding loops, independent of passive strand positioning relative to the helicase core. The helicase can effectively process DNA in either configuration, as the motive force acts exclusively through the tracking strand. This mechanistic flexibility helps to explain why strand separation has been suggested to occur at different positions within various helicases45,46,47,48,49.
In eukaryotic systems, as in the SV40 system, CMG helicases arranged head-to-head at origins lead to formation of bidirectional replication forks7,50. The structure of the yeast CMG double hexamer bound to duplex DNA51 and that pertaining to the initial phase of origin melting52 indicate that origin melting is fundamentally driven by ATP binding, formation of the DNA-binding loop staircase and tracking-strand engagement by the loops (Extended Data Fig. 8). These factors, as proposed for the SV40 system, contribute to strand separation within the helicase inner chamber and enable each hexamer to acquire a configuration primed for ATP-driven translocation46. As such, principles underlying origin melting seem to be conserved in both domains (Extended Data Fig. 8). Considering that CMG probably translocates on DNA by means of a general rotary mechanism24,41, it is plausible that the DNA shearing and helicase uncoupling proposed here remain applicable.
Methods
Protein cloning, expression and purification
Full-length SV40 LTag (accession number: P03070) N-terminally double 6× histidine-tagged, together with the TEV cleavage site sequence, was cloned into a pFastBac1 plasmid by GenScript. The recombinant expression plasmid was introduced into MultiBac baculoviral DNA in DH10MultiBac, and bacmid DNA was isolated. To prepare the baculovirus, the bacmid DNA of LTag was transfected into Sf9 cells using FuGENE HD (Promega) according to the manufacturer’s instructions. The resulting supernatant was used as the P1 virus stock and amplified to obtain P2 virus stock. The P2 virus stock was then further amplified to obtain P3 virus stock for large-scale expression. LTag was expressed by transfecting 4 l of Sf9 suspension culture at a density of 2 × 106 cells ml−1 with LTag P3 virus for 55–60 h. Cells were collected by centrifugation at 5,500g for 10 min and resuspended in lysis buffer (20 mM Tris (pH 8.0), 50 mM imidazole, 500 mM KCl, 1 mM dithiothreitol (DTT), 5% (v/v) glycerol and EDTA-free protease inhibitor cocktail tablet per 50 ml (Roche, UK)). The cell lysate was clarified by centrifugation at 95,834g for 1 h at 4 °C. The supernatant was directly loaded on to a 5-ml HisTrap affinity column (Cytiva) pre-equilibrated with buffer A (20 mM Tris (pH 8.0), 50 mM imidazole, 500 mM KCl, 1 mM DTT and 10% glycerol). The loaded column was washed with 50 ml of buffer A containing 50 mM imidazole followed by 50 ml of buffer A containing 100 mM imidazole to remove non-specific binding of the protein to the column. Finally, the protein was eluted with a 20-ml gradient to 500 mM imidazole using buffer B containing low salt (20 mM Tris (pH 8.0), 500 mM imidazole, 250 mM KCl, 1 mM DTT, 10 % glycerol). The pooled fractions of LTag were treated with TEV protease to remove the His-tag and dialysed overnight in dialysis buffer (20 mM Tris (pH 8.0), 100 mM KCl, 1 mM DTT and 10% glycerol). The cleaved protein was then loaded into a HisTrap HP 1-ml affinity column (Cytiva) pre-equilibrated with buffer C (20 mM Tris (pH 8), 250 mM KCl, 1 mM DTT and 10% glycerol). The loaded column was then washed with 10 ml of buffer D followed by a 10-ml linear gradient of buffer B. Flow-through fractions that contained LTag were concentrated and loaded on to a HiLoad 16/600 Superdex 200 pg (Cytiva) pre-equilibrated with gel filtration buffer (20 mM Tris (pH 8.0), 100 mM KCl, 1 mM DTT and 10% glycerol). Collected fractions were flash-frozen and stored at −80 °C.
DNA substrates
The forked DNA substrate for reconstitution with LTag and ATP in the absence of Mg2+ was generated by annealing the 5′-GGTGATCGTTCGCTACATGTCGTCAGGATTCCAGGCAG-3′ and 5′-AGACACATGGATGTAGCGAACGATCACC-3′ oligonucleotides, purchased from Eurofins. The forked DNA substrate for reconstitution with LTag and ADP, LTag and ATP in the presence of Mg2+, or LTag and AMP-PNP in the presence of Mg2+ was adapted from SenGupta and Borowiec30, with unpaired arms changed to poly-Ts. The constituent oligonucleotides were therefore 5′-TTCTGTGACTACCTGG ACGACCGGGTTTTTTTTTTTTTTTTTTTTTTTTTTTTTT-3′ and 5′-TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTCCCGGTCGTCCAGGTAGTCACAGA-3′. The annealed product was purchased from Eurogentec. The gap DNA substrate for reconstitution of the LTag and ATP apo complex was created by annealing oligonucleotides 5′-AGCTATGACCATGATTACGAATTG[23ddC]-3′ and 5′-TTTTTCGGAGTCGTTTCGACTCCGATTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTGCA ATTCGTAATCATGGTCATAGCT-3′. LTag showed no binding to this substrate without a free 3′ ssDNA tail.
Full SV40 origin DNA (top 5′-CACTACTTCTGGAATAGCTCAGAGGCCGAGGCGGCCTCGGCCTCTGCATAAATAAAAAAAATTA-3′ and bottom 5′-TAATTTTTTTTATTTATGCAGAGGCCGAGGCCGCCTCGGCCTCTGAGCTATTCCAGAAGTAGTG-3′), EP (top 5′-CACTACTTCTGGAATAGCTCAGAGGCCGAGGCG-3′ and bottom 5′-CGCCTCGGCCTCTGAGCTATT CCAGAAGTAGTG-3′) and AT-rich (top 5′-GGCCTCGGCCTCTGCAT AAATAAAAAAAATTA-3′ and bottom 5′-TAATTTTTTTTATTTATGCAGAGGCCGAGGCC-3′) half-origin DNA substrates were synthesized by GenScript (Piscataway) and each annealed by heating at 95 °C for 2 min followed by gentle cooling at −1 °C min−1 to 25 °C. The annealed substrates were further treated with S1 nuclease (Fermentas Life Sciences) at room temperature for 1 h and purified using a PCR clean-up kit (Macherey-Nagel).
Buffers
The buffer used for the LTag–ATP–DNA, LTag–ADP–DNA and LTag–ATP (apo) samples was based on the DNA-binding assay of Li et al.12, comprising 1 mM ATP, 50 mM Tris-HCl (pH 8.0) and 200 mM NaCl. The buffer used for the LTag–ATP-Mg2+–DNA sample was based on the DNA helicase assay from ref. 12 and was composed of 50 mM HEPES (pH 7.5), 1 mM ATP, 3 mM MgCl2, 1 mM DTT and 50 mM NaCl. The buffer used for the LTag–AMP-PNP-Mg2+-DNA complex consisted of 50 mM Tris-HCl (pH 7.5), 1 mM DTT, 100 mM NaCl, 5 mM MgCl2 and 3 mM AMP-PMP. The buffer used for LTag with full- and half-origin DNA contained 50 mM Tris-HCl (pH 8), 100 mM NaCl, 1 mM DTT, 5 mM MgCl2, 5 mM KCl and 3 mM AMP-PNP.
Cryo-EM sample preparation
The LTag stock was preincubated with buffer before addition of DNA substrate (in equimolar ratio to the putative hexamer). The steps were performed in a 4 °C temperature-controlled room. The final protein concentration in samples applied to the grids was approximately 0.098 mg ml−1 (equivalent to 0.2 µM hexamer). R1.2/1.3 or R2/2 UltrAuFoil grids (Quantifoil Micro Tools) were glow-discharged with 40 mA for 5 min inside a Quorum GloQube Plus unit. A graphene oxide (Merck) coating was applied53. Then, 3 µl of sample was applied to the grid in a Vitrobot Mark IV at 4 °C and 100% relative humidity and, following a wait time of 30 s, blotted for 3 s with blot force 4 and plunged into liquid ethane. For the LTag–AMP-PNP-Mg2+–DNA complex, 2 μM LTag (with respect to the hexamer) was mixed in the corresponding buffer and incubated at 4 °C for 30 min. Following this, 2 μM DNA was added, and the reaction was incubated at 4 °C for a further 30 min. Then, 3 µl of sample was deposited on to a R2/2 Au 300 mesh grid previously glow-discharged at 40 mA for 60 s. The grid was frozen on a Vitrobot with a 3-s blotting time, 0-s wait time and a blot force of 5, with 4 °C and 100% humidity in the chamber. To reconstitute the origin melting complexes, LTag stock was mixed with full origin (2:1 molar ratio), EP or AT half origin in equimolar ratio to a final concentration of 1 µM in the aforementioned buffer, and the reaction was incubated at room temperature for 1 h before sample freezing. A Quantifoil Au holey grid (R2/2 300 mesh) was glow-discharged at 30 mA for 30 s using PELCO easiGlow (Ted Pella, Inc.). Then, 3 μl of sample was applied to the glow-discharged grid and blotted for 3 s using a blot force of 0 and no wait time. The sample was then plunge-frozen in liquid ethane using FEI Vitrobot Mark IV preset at 4 °C and 100% humidity.
Cryo-EM data acquisition
LTag–ATP, LTag–ATP–DNA, LTag–ADP–DNA, LTag–AMP-PNP-Mg2+–DNA, and LTag–ATP-Mg2+–DNA data were collected on a Titan Krios G3 transmission electron microscope (Thermo Fisher Scientific) at ×105,000 magnification in AFIS mode with 100-mm objective aperture, using a GATAN K3 direct detection camera at the end of a GATAN BioQuantum energy filter (20 eV slit width) in counted superresolution mode (binning 2), with EPU 2.12 software (Thermo Fisher Scientific), at the Leicester Institute of Structural and Chemical Biology, UK. Individual exposures were acquired at a dose rate of 18 e− px−1 s−1 in 50 equal fractions over 2 s, with a calibrated pixel size of 0.835 Å px−1 and an accumulated dose of 50 e− Å−2. The nominal defocus was varied between −1.0 µm and −2.5 µm in 0.3-µm steps. The gross numbers of micrographs collected from each sample were: 8,072 (LTag–ATP–DNA), 9,737 (LTag–ADP–DNA), 6,127 (LTag–AMP-PNP-Mg2+–DNA) and 8,460 (LTag–ATP-Mg2+–DNA). Data for the LTag–AMP-PNP-Mg2+–DNA, LTag–AMP-PNP-Mg2+–EP, LTag–AMP-PNP-Mg2+–AT and LTag–AMP-PNP-Mg2+–full-origin structures were collected on a Titan Krios G4 transmission electron microscope (Thermo Fisher Scientific) located at the Imaging and Characterization Core Lab of the King Abdullah University of Science and Technology, Saudi Arabia. Micrographs in EER format were recorded at ×130,000 magnification (calibrated pixel size of 0.93 Å px−1) using a Falcon 4i direct detection camera at the end of a Selectris-X energy filter with a 10 eV slit width, with EPU 2.12 software (Thermo Fisher Scientific). Data for LTag–AMP-PNP-Mg2+–DNA were collected with a defocus range of −2.5 to −1.5 μm and an accumulated dose of 38.7 e− Å−2. Data for LTag–AMP-PNP-Mg2+–EP, LTag–AMP-PNP-Mg2+–AT, and LTag–AMP-PNP-Mg2+–full-origin data were collected with a defocus range of −2.6 to −1.1 µm in steps of 0.3 µm, and a total dose of 43 e− Å−2 was applied to the specimen over an exposure time of 5 s.
Cryo-EM data processing
Movie stacks were corrected for beam-induced motion using RELION’s implementation54 of MotionCor2 (ref. 55) with dose-weighting, and contrast transfer function (CTF) estimation of the integrated micrographs was performed using CTFFIND 4.1 (ref. 56). The micrographs were denoised before picking with Topaz v.0.3.0 (ref. 57), both using default models. The picks were extracted using a box size of 384 and subjected to several iterations of two-dimensional classification and selection using VDAM in RELION 4 (ref. 54) (with decreasing binning factors of 4×, 2× and 1×). In general, particle subsets from above were imported into CryoSPARC (Structura Biotechnology) and re-extracted from Patch Motion Correction and Patch CTF Estimation jobs and initially subjected to ab initio reconstruction with three classes (allowing for DNA-bound, apo and junk categories). If two junk classes were detected, the process was repeated using two classes. The resulting particle selections were further corrected for beam-induced motion with local motion correction. Eventual final reconstructions were derived using non-uniform refinement, with CTF and per-particle scale refinements enabled. Refinement masks were constructed with a soft edge (of approximately 20 pixels at half normalized threshold value) around the ordered protein (core helicase) and associated DNA segments of the complex. Gold-standard Fourier shell correlation resolutions were calculated using independently refined random halves of the particle datasets. The LTag–ATP–DNA reconstruction was initially refined to 2.9 Å in RELION 4 using SIDESPLITTER. The final particle subset following two-dimensional classification was subjected to a three-class three-dimensional (VDAM) initial model job. This produced two junk classes; the non-junk class was subjected to two-class three-dimensional classification. This yielded apo (54%) and DNA-bound (44%) classes. The latter was iteratively auto-refined using CTF refinements and Bayesian polishing. A mask was created by thresholding the 15-Å low-pass filtered reconstruction to exclude non-core helicase domains, followed by dilation and padding with 2 and 10 pixels, respectively. The refined particle stack with its assignments was imported into cryoSPARC and rerefined using non-uniform refinement. This gave a better regularized map despite a slightly lower reported global gold-standard Fourier shell correlation resolution. The 3DVA analysis2 of the LTag–ATP-Mg2+–DNA consensus reconstruction was performed using its refinement mask, with three modes and a filter resolution of 5 Å (the global gold-standard Fourier shell correlation resolution being 3 Å), as well as a high-pass resolution of 150 Å. Latent coordinates exhibited a spherical and/or three-dimensional Gaussian distribution, characteristic of continuous conformational variability. Twenty volumes (frame_000 to frame_019) were sampled along each principal component (0, 1, 2). Data had been exhaustively cleaned by repeat classification without separation of conformations. Validation volumes were generated through rolling window partition of particle embeddings centred on the sampled frames along each component, followed by weighted reconstruction using their gold-standard consensus assignments. The 3DVAs of LTag–ADP–DNA and LTag–ATP–DNA were carried out similarly. The LTag–AMP-PNP-Mg2+–DNA structure was wholly refined in RELION 4. In brief, particles picked using Topaz (2,859,203 particles) were extracted with a box size of 256 pixels and 4x downscaling (3.72 Å px−1), and extensively cleaned by two-dimensional classification. The resulting particles (957,358 particles) were subjected to three-dimensional classification with three classes. Particles from the best class (235,707 particles) were re-extracted to full size (0.93 Å px−1) and subjected to three-dimensional refinement, CTF refinement and polishing, resulting in a 3.1-Å resolution map. The refinement mask was created similarly to that used in the LTag–ATP–DNA reconstruction, although it was inclusive of the OBD tier. This was later tightened to the core helicase and DNA for purposes of resolution estimation (and application of global B factor sharpening). LTag–ATP (apo) reconstruction from the gapped DNA sample was refined in RELION. Steps up to and including two-dimensional classification and subset selection were done in RELION 4. Ab initio reconstruction was obtained from CryoSPARC. Three-dimensional classification and auto-refinement were carried out in RELION 5 using Blush regularization (with CTF refinement). Bayesian polishing was not performed. The total numbers of particles incorporated into the final reconstructions were: 92,330 (apo LTag–ATP), 210,910 (LTag–ATP–DNA), 97,587 (LTag–ADP–DNA), 235,707 (LTag–AMP-PNP-Mg2+–DNA) and 201,416 (LTag–ATP-Mg2+–DNA). Local resolution maps were calculated in Phenix and rendered on the full map (before postprocessing) with adjusted colouring. The 3DFSC58 metrics were obtained from the web server at https://3dfsc.salk.edu/.
Origin DNA datasets were processed using CryoSPARC 4.5.3 (Structura Biotechnology). Recorded movies underwent beam-induced motion correction using the Patch Motion Correction module, and CTF estimation was performed with Patch CTF Estimation59. Particles were picked using the Blob Picker with a particle diameter of 130 Å and extracted in a box size of 320 Å. After two rounds of two-dimensional classification, 198,000, 148,881 and 80,452 particles were retained for the EP, AT and full-origin datasets, respectively. These particles were used to generate ab initio reconstructions and refined using CryoSPARC non-uniform refinement. Subsequent CTF refinement and postprocessing resulted in final maps at a resolution of 3.1 Å for half-origin datasets and 3.7 Å for the full-origin dataset. Finally, particle stacks were re-extracted in RELION 5.0-beta-2 and subjected to further three-dimensional classification to identify classes with stronger DNA density.
Model building and refinement
Resulting volumes were postprocessed with EMReady v.1 and v.2 (ref. 28) (using default parameters and without specifying an input structure or mask) for purposes of interactive model-building in ISOLDE29, alongside masked full and/or globally sharpened maps as per automatically calculated B factors. RELION or CryoSPARC postprocessed maps were also used to derive draft models from ModelAngelo (v.0.1)60 to guide the subsequent process. No form of postprocessed maps was used for iterative map refinement itself. For modelling of the LTag–ATP–DNA complex, initially, the crystal structure of ATP-bound LTag (1SVM)11 was real-space refined into the LTag–ATP–DNA map using PHENIX, and oligo-dT6 from the E1–ADP-Mg2+–ssDNA crystal structure (2GXA)25 was fitted into density in ChimeraX. The map–model association was first globally simulated in ISOLDE, followed by multiple cycles of local rebuilding and Phenix real-space refinement with ISOLDE’s recommended settings. In later rounds, further bases in the tracking strand, and complementary strand were added and remodelled. Several iterations of LocScale v.1.0 (ref. 61) and ISOLDE refinement were used to improve the DNA nexus map and model. For modelling of the LTag–ADP–DNA complex, the crystal structure of ADP-bound LTag (1SVL)11 was flexibly fitted into the LTag–ADP–DNA reconstruction using iMODFIT and real-space refined, with ssDNA incorporated as before. The model was then rebuilt and refined as previously. For modelling of the LTag–AMP-PNP-Mg2+–DNA complex, a refined model of LTag–ATP–DNA (omitting the duplex portion of DNA) was initially rigid-body fitted into the LTag–AMP-PNP-Mg2+–DNA reconstruction in ChimeraX. Adaptive distance restraints were applied to individual chains in ISOLDE29 and simulated against the map. Restraints were progressively released and the model fully rebuilt. Towards the end, ATP was replaced with AMP-PNP and Mg2+, resimulated and rebuilt. For modelling of the LTag–ATP-Mg2+–DNA complex into the 3DVA volumes, the LTag–ATP–DNA model, following rigid-body fitting into the LTag–ATP-Mg2+–DNA consensus map, was first rebuilt into a working consensus model. This model was flexibly fitted into the central-most sampled volume (frame_010) from the first principal component. The top-ranked AlphaFold2 (ref. 62) monomer structure prediction for SV40 LTag was superimposed on to each of the six subunits using MatchMaker63. Residues not pertaining to the core helicase 265–627 were deleted. Adaptive distance restraints were applied to individual chains, followed by a whole map–model simulation in ISOLDE. Restraints were progressively released and the model rebuilt end to end. The derived model was placed into two intermediate volumes (frame_005 and frame_015). Adaptive restraints were reapplied and models entirely rebuilt as above. These models were placed into terminal volumes (frame_000 and frame_019), and rebuilt using the same procedure. The central model from principal component 0 was placed into the central volume of component 1. After remodelling, it was used as basis for modelling the two intermediate and then terminal frames as before. The process was repeated for principal component 2. Each residue in all of the above models was manually inspected and/or rebuilt at least once. Q-scores were calculated using the model–map Q-score33 ChimeraX plugin using the default recommended sigma of 0.6. Side-chain-focused model and map validation was carried out using EMRinger v.1.0.0 (ref. 64), averaging a score of 2.9 (well above the requisite 1.0). The consensus LTag–ATP-Mg2+–DNA structure could not be definitively modelled throughout owing to its representing multiple averaged states and was used chiefly as an interim model to generate initial model drafts for variability volumes. Corroborative modelling was performed using Phenix variability refinement65 and assessed using root mean square deviation against the interactively built models, relative to d99 resolution of the maps. For modelling of the LTag–ATP-Mg2+–DNA complex into 3DVA validation volumes, models of terminal frames from each component were rerefined into the back-projected volumes from their corresponding particle subsets in ISOLDE. For modelling of the LTag–ADP–DNA and LTag–ATP–DNA complexes into 3DVA volumes, respective consensus structures were refined into the terminal frames of each component using bulk flexible fitting with adaptive restraints in ISOLDE. For modelling of the LTag–ATP (apo) complex, the LTag–ATP (1SVM) crystal structure was first Phenix real-space refined into the apo LTag–ATP reconstruction postprocessed with EMReady. AlphaFold2 monomer prediction corresponding to amino acids 266–627 was superposed with each of the six chains using MatchMaker and subjected to molecular dynamics bulk flexible fitting with adaptive distance restraints in ISOLDE. Restraints were released inside a whole map–model simulation, followed by localized rebuilding in ISOLDE. For modelling of the LTag–AMP-PNP–full-origin, LTag–AMP-PNP–EP and LTag–AMP-PNP–AT complexes, the LTag–AMP-PNP-Mg2+–DNA fork structure was interactively remodelled into maps of LTag bound to AT and EP half origins using ISOLDE. The paired strand and further tracking-strand bases were added with Coot before refinement in ISOLDE. All final Phenix real-space refined structures were assigned secondary structure annotations using DSSP66. For structures containing AMP-PNP, Mogul ligand restraints downloaded from the Global Phasing Grade Web Server (https://grade.globalphasing.org/) were incorporated into Phenix real-space refinement.
Map and model visualization
Maps were visualized in UCSF Chimera67 and ChimeraX68, and all model illustrations and morphs were prepared using ChimeraX 1.7 and 1.8 or PyMOL 2.6.0.
LTag helicase unwinding assay
EP half-origin primers with 32-nucleotide extensions (5′-CACTACTTCTGGAATAGCTCAGAGGCCGAGGCGGCCTCGGC CTCTGCATAAATAAAAAAAATTA-3′ and 5′-TAATTTTTTTTATTTATGCAGAGGCCGAGGCCGCCTCG GCCTCTGAGCTATTCCA GAAGTAGTG-3′) were first annealed in equivalent portions in an annealing buffer (40 mM Tris-HCl (pH 7.5), 50 mM NaCl and 10 mM MgCl2). The mixture was heated to 95 °C for 5 min then gradually cooled at room temperature at 1 °C per minute. Subsequently, the mixture was subjected to 10% Tris–borate–EDTA polyacrylamide gel electrophoresis at 100 V for 1 h and 15 min. The product was later purified from the gel using ethanol precipitation. The unwinding assay was conducted in a total reaction of 20 μl. Initially, 5 nM of the substrate was incubated with LTag for 10 min in a reaction buffer (20 mM Tris-HCl (pH 7.5), 50 mM KCl, 0.1 mg ml−1 bovine serum albumin, 10 mM MgCl2 and 1 mM DTT) at 37 °C. Various concentrations of LTag were used (50, 100, 200, 300, 400 and 500 nM). The reaction was initiated by addition of 4 mM ATP. After 45 min, the reaction was quenched with stop buffer (1% sodium dodecyl sulfate, 50 mM EDTA and 30% glycerol). Subsequently, 10 μl of the final reaction was loaded on to a 10% Tris–borate–EDTA polyacrylamide gel and run at 100 V for 1 h. Finally, the products were visualized using a Typhoon 9400 laser imager (GE Healthcare).
Statistics and reproducibility
No statistical methods were used to predetermine sample size. The experiments were not randomized, and investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data supporting the findings of this study are available within the paper and its Supplementary Information. The maps of the LTag–ATP–DNA complex reconstituted without Mg2+ have been deposited in the Electron Microscopy Data Bank (EMDB) with accession codes EMD-50289 and EMD-50287, and the atomic models in the Protein Data Bank (PDB) under accession codes 9FB6 and 9FB4. The 3DVA maps have been deposited in the EMDB with accession codes EMD-52180 and EMD-52176 (frames 1 and 5 of component 1), EMD-52182 and EMD-52181 (frames 1 and 5 of component 2), and EMD-52183 and EMD-52184 (frames 1 and 5 of component 3). The map of the LTag–ADP–DNA complex has been deposited in the EMDB with accession code EMD-50002 and the atomic model in the PDB under accession code 9EVH. The 3DVA maps have been deposited in the EMDB with accession codes EMD-52108 and EMD-52109 (frames 1 and 5 of component 1), EMD-52110 and EMD-52111 (frames 1 and 5 of component 2) and EMD-50288 and EMD-50286 (frames 1 and 5 of component 3). The map of the LTag–AMP-PNP–DNA complex reconstituted with Mg2+ has been deposited in the EMDB with accession code EMD-50010 and the atomic model in the PDB under accession code 9EVP. The consensus map of the LTag–ATP–DNA complex reconstituted with Mg2+ has been deposited in the EMDB with accession code EMD-50290. The 3DVA volumes have been deposited in the EMDB with accession codes EMD-50246, EMD-50245, EMD-50179, EMD-50190 and EMD-50250 (frames 1 to 5 of component 1); EMD-50261, EMD-50257, EMD-50180, EMD-50256 and EMD-50262 (frames 1 to 5 of component 2); and EMD-50288, EMD-50265, EMD-50264, EMD-50244 and EMD-50286 (frames 1 to 5 of component 3); and the atomic models in the PDB under accession codes 9F75, 9F74, 9F3T, 9F5I and 9F7N (frames 0 to 5 of component 1); 9F9W, 9F9O, 9F3U, 9F9N and 9F9X (frames 1 to 5 of component 2); and 9FB5, 9FA2, 9FA1, 9F73 and 9FB0 (frames 1 to 5 of component 3). The maps of LTag–AMP-PNP-Mg2+–EP, LTag–AMP-PNP-Mg2+–AT, and LTag–AMP-PNP-Mg2+–full origin have been deposited in the EMDB with accession codes EMD-62206, EMD-62209 and EMD-62502, respectively. The models of LTag–AMP-PNP-Mg2+–EP and LTag–AMP-PNP-Mg2+–AT have been deposited in the PDB with accession codes 9KAE and 9KAK, respectively. Further atomic models used in this study are as follows: cocrystal structure of LTag and ATP (PDB 1SVM) or ADP (PDB 1SVL), cocrystal structure of LTag and EP origin DNA (PDB 4GDF), cocrystal structure of LTag OBD bound to palindrome DNA (PDB 2ITL), cryo-EM structure of ScCMG helicase nucleating origin DNA melting (PDB 7Z13), cryo-EM structure of ScCMG helicase bound to forked DNA (PDB 6U0M) and cryo-EM structure of MCM double hexamer bound to DNA (PDB 7P30).
References
Fernandez, A. J. & Berger, J. M. Mechanisms of hexameric helicases. Crit. Rev. Biochem. Mol. Biol. 56, 621–639 (2021).
Punjani, A. & Fleet, D. J. 3D variability analysis: resolving continuous flexibility and discrete heterogeneity from single particle cryo-EM. J. Struct. Biol. 213, 107702 (2021).
Gao, Y. & Yang, W. Different mechanisms for translocation by monomeric and hexameric helicases. Curr. Opin. Struct. Biol. 61, 25–32 (2020).
Fanning, E. Simian virus 40 large T antigen: the puzzle, the pieces, and the emerging picture. J. Virol. 66, 1289–1293 (1992).
Stillman, B. Smart machines at the DNA replication fork. Cell 78, 725–728 (1994).
Waga, S. & Stillman, B. The DNA replication fork in eukaryotic cells. Annu. Rev. Biochem. 67, 721–751 (1998).
Douglas, M. E., Ali, F. A., Costa, A. & Diffley, J. F. X. The mechanism of eukaryotic CMG helicase activation. Nature 555, 265–268 (2018).
Ilves, I., Petojevic, T., Pesavento, J. J. & Botchan, M. R. Activation of the MCM2-7 helicase by association with Cdc45 and GINS proteins. Mol. Cell 37, 247–258 (2010).
Moyer, S. E., Lewis, P. W. & Botchan, M. R. Isolation of the Cdc45/Mcm2-7/GINS (CMG) complex, a candidate for the eukaryotic DNA replication fork helicase. Proc. Natl Acad. Sci. USA 103, 10236–10241 (2006).
Chang, Y. P. et al. Mechanism of origin DNA recognition and assembly of an initiator-helicase complex by SV40 large tumor antigen. Cell Rep. 3, 1117–1127 (2013).
Gai, D., Zhao, R., Li, D., Finkielstein, C. V. & Chen, X. S. Mechanisms of conformational change for a replicative hexameric helicase of SV40 large tumor antigen. Cell 119, 47–60 (2004).
Li, D. et al. Structure of the replicative helicase of the oncoprotein SV40 large tumour antigen. Nature 423, 512–518 (2003).
Luo, X., Sanford, D. G., Bullock, P. A. & Bachovchin, W. W. Solution structure of the origin DNA-binding domain of SV40 T-antigen. Nat. Struct. Biol. 3, 1034–1039 (1996).
Cuesta, I. et al. Conformational rearrangements of SV40 large T antigen during early replication events. J. Mol. Biol. 397, 1276–1286 (2010).
Dean, F. B., Borowiec, J. A., Eki, T. & Hurwitz, J. The simian virus 40 T antigen double hexamer assembles around the DNA at the replication origin. J. Biol. Chem. 267, 14129–14137 (1992).
Mastrangelo, I. A. et al. ATP-dependent assembly of double hexamers of SV40 T antigen at the viral origin of DNA replication. Nature 338, 658–662 (1989).
Parsons, R. E. et al. Cooperative assembly of simian virus 40 T-antigen hexamers on functional halves of the replication origin. J. Virol. 65, 2798–2806 (1991).
Tjian, R. The binding site on SV40 DNA for a T antigen-related protein. Cell 13, 165–179 (1978).
Dodson, M., Dean, F. B., Bullock, P., Echols, H. & Hurwitz, J. Unwinding of duplex DNA from the SV40 origin of replication by T antigen. Science 238, 964–967 (1987).
Yardimci, H. et al. Bypass of a protein barrier by a replicative DNA helicase. Nature 492, 205–209 (2012).
Itsathitphaisarn, O., Wing, R. A., Eliason, W. K., Wang, J. & Steitz, T. A. The hexameric helicase DnaB adopts a nonplanar conformation during translocation. Cell 151, 267–277 (2012).
Rzechorzek, N. J., Hardwick, S. W., Jatikusumo, V. A., Chirgadze, D. Y. & Pellegrini, L. CryoEM structures of human CMG-ATPgammaS-DNA and CMG-AND-1 complexes. Nucleic Acids Res. 48, 6980–6995 (2020).
Thomsen, N. D. & Berger, J. M. Running in reverse: the structural basis for translocation polarity in hexameric helicases. Cell 139, 523–534 (2009).
Meagher, M., Epling, L. B. & Enemark, E. J. DNA translocation mechanism of the MCM complex and implications for replication initiation. Nat. Commun. 10, 3117 (2019).
Enemark, E. J. & Joshua-Tor, L. Mechanism of DNA translocation in a replicative hexameric helicase. Nature 442, 270–275 (2006).
Eickhoff, P. et al. Molecular basis for ATP-hydrolysis-driven DNA translocation by the CMG helicase of the eukaryotic replisome. Cell Rep. 28, 2673–2688 e2678 (2019).
Gao, Y. et al. Structures and operating principles of the replisome. Science 363, eaav7003 (2019).
He, J., Li, T. & Huang, S. Y. Improvement of cryo-EM maps by simultaneous local and non-local deep learning. Nat. Commun. 14, 3217 (2023).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D Struct. Biol. 74, 519–530 (2018).
SenGupta, D. J. & Borowiec, J. A. Strand-specific recognition of a synthetic DNA replication fork by the SV40 large tumor antigen. Science 256, 1656–1661 (1992).
Langston, L. D., Yuan, Z., Georgescu, R., Li, H. & O’Donnell, M. E. SV40 T-antigen uses a DNA shearing mechanism to initiate origin unwinding. Proc. Natl Acad. Sci. USA 119, e2216240119 (2022).
Wiekowski, M., Schwarz, M. W. & Stahl, H. Simian virus 40 large T antigen DNA helicase. Characterization of the ATPase-dependent DNA unwinding activity and its substrate requirements. J. Biol. Chem. 263, 436–442 (1988).
Pintilie, G. et al. Measurement of atom resolvability in cryo-EM maps with Q-scores. Nat. Methods 17, 328–334 (2020).
Shein, M. et al. Characterizing ATP processing by the AAA+ protein p97 at the atomic level. Nat. Chem. 16, 363–372 (2024).
Adelman, J. L. et al. Mechanochemistry of transcription termination factor Rho. Mol. Cell 22, 611–621 (2006).
Liao, J. C., Jeong, Y. J., Kim, D. E., Patel, S. S. & Oster, G. Mechanochemistry of t7 DNA helicase. J. Mol. Biol. 350, 452–475 (2005).
Bochkareva, E., Martynowski, D., Seitova, A. & Bochkarev, A. Structure of the origin-binding domain of simian virus 40 large T antigen bound to DNA. EMBO J. 25, 5961–5969 (2006).
Borowiec, J. A. & Hurwitz, J. Localized melting and structural changes in the SV40 origin of replication induced by T-antigen. EMBO J. 7, 3149–3158 (1988).
Valle, M., Chen, X. S., Donate, L. E., Fanning, E. & Carazo, J. M. Structural basis for the cooperative assembly of large T antigen on the origin of replication. J. Mol. Biol. 357, 1295–1305 (2006).
Yuan, Z. et al. Structure of the eukaryotic replicative CMG helicase suggests a pumpjack motion for translocation. Nat. Struct. Mol. Biol. 23, 217–224 (2016).
Xu, Z. et al. Synergism between CMG helicase and leading strand DNA polymerase at replication fork. Nat. Commun. 14, 5849 (2023).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493–500 (2024).
Fei, X., Bell, T. A., Barkow, S. R., Baker, T. A. & Sauer, R. T. Structural basis of ClpXP recognition and unfolding of ssrA-tagged substrates. eLife 9, e61496 (2020).
Ripstein, Z. A., Huang, R., Augustyniak, R., Kay, L. E. & Rubinstein, J. L. Structure of a AAA+ unfoldase in the process of unfolding substrate. eLife 6, e25754 (2017).
Javed, A., Major, B., Stead, J. A., Sanders, C. M. & Orlova, E. V. Unwinding of a DNA replication fork by a hexameric viral helicase. Nat. Commun. 12, 5535 (2021).
Yuan, Z. et al. DNA unwinding mechanism of a eukaryotic replicative CMG helicase. Nat. Commun. 11, 688 (2020).
Baretic, D. et al. Cryo-EM structure of the fork protection complex bound to CMG at a replication fork. Mol. Cell 78, 926–940.e913 (2020).
Jones, M. L., Baris, Y., Taylor, M. R. G. & Yeeles, J. T. P. Structure of a human replisome shows the organisation and interactions of a DNA replication machine. EMBO J. 40, e108819 (2021).
Chaban, Y. et al. Structural basis for DNA strand separation by a hexameric replicative helicase. Nucleic Acids Res. 43, 8551–8563 (2015).
Remus, D. et al. Concerted loading of Mcm2-7 double hexamers around DNA during DNA replication origin licensing. Cell 139, 719–730 (2009).
Greiwe, J. F. et al. Structural mechanism for the selective phosphorylation of DNA-loaded MCM double hexamers by the Dbf4-dependent kinase. Nat. Struct. Mol. Biol. 29, 10–20 (2022).
Lewis, J. S. et al. Mechanism of replication origin melting nucleated by CMG helicase assembly. Nature 606, 1007–1014 (2022).
Bokori-Brown, M. et al. Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein. Nat. Commun. 7, 11293 (2016).
Kimanius, D., Dong, L., Sharov, G., Nakane, T. & Scheres, S. H. W. New tools for automated cryo-EM single-particle analysis in RELION-4.0. Biochem. J. 478, 4169–4185 (2021).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
Tan, Y. Z. et al. Addressing preferred specimen orientation in single-particle cryo-EM through tilting. Nat. Methods 14, 793–796 (2017).
Thorne, R. E. Hypothesis for a mechanism of beam-induced motion in cryo-electron microscopy. IUCrJ 7, 416–421 (2020).
Jamali, K. et al. Automated model building and protein identification in cryo-EM maps. Nature 628, 450–457 (2024).
Jakobi, A. J., Wilmanns, M. & Sachse, C. Model-based local density sharpening of cryo-EM maps. eLife 6, e27131 (2017).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Meng, E. C., Pettersen, E. F., Couch, G. S., Huang, C. C. & Ferrin, T. E. Tools for integrated sequence-structure analysis with UCSF Chimera. BMC Bioinformatics 7, 339 (2006).
Barad, B. A. et al. EMRinger: side chain-directed model and map validation for 3D cryo-electron microscopy. Nat. Methods 12, 943–946 (2015).
Afonine, P. V. et al. Conformational space exploration of cryo-EM structures by variability refinement. Biochim. Biophys. Acta Biomembr. 1865, 184133 (2023).
Kabsch, W. & Sander, C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22, 2577–2637 (1983).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Meng, E. C. et al. UCSF ChimeraX: tools for structure building and analysis. Protein Sci. 32, e4792 (2023).
Acknowledgements
This research was supported by King Abdullah University of Science and Technology (KAUST) through core funding (to S.M.H. and A.D.B.) and Competitive Research Award Grant CRG8 URF/1/4036‐01‐01 (to S.M.H. and A.D.B.). We thank past and present members of the S.M.H. and A.D.B. laboratories for help and discussions; L. Zhao, A. Al-Alamoudi, A. Genovese and R. Sougrat for their assistance at the Imaging and Characterization Core Lab at KAUST; and T. Zhang for her help in revising the text. We acknowledge the Midlands Regional Cryo-EM Facility at the Leicester Institute of Structural and Chemical Biology (LISCB), with major funding from the MRC (MC_PC_17136). We thank T. J. Ragan (LISCB, University of Leicester) for his support with data processing and high-performance computing, and N. Kathiresan and the KAUST IBEX team for access to high-performance computing resources and support with data processing.
Author information
Authors and Affiliations
Contributions
T.S., S.M.H. and A.D.B. conceived the study. M.T. and L.A. purified the protein and performed helicase assays. T.S., A.U.D., A.C., C.G.S. and A.D.B. performed cryo-EM imaging and processing. T.S. implemented the 3DVA analysis. A.C. resolved the structure of LTag bound to fork DNA and AMP-PNP. A.U.D., with the help of A.C., resolved the origin DNA structures. T.S., A.U.D. and A.D.B. performed model-building and validation. S.M.H. and A.D.B. supervised the study. T.S. and A.D.B. wrote the manuscript with contributions from the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Pavel Afonine and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Cryo-EM of the LTag-ATP-DNA complex.
a, Workflow schematics for cryo-EM data collection, processing, and modelling. Flows continue from left hand side of following row unless otherwise indicated. b, Gold-standard Fourier shell correlation and Gunier plots. c, Angular distribution of projections. Resolution is estimated using the 0.143 criterion. d, Map isotropy analysis computed by 3DFSC58. e, Two views of the cryo-EM map colored by local resolution.
Extended Data Fig. 2 Cryo-EM of the LTag−ATP/Mg2+−DNA complex.
a, Workflow schematics for cryo-EM data collection, processing, and modelling. Flows continue from left hand side of following row unless otherwise indicated. b, Gold-standard Fourier shell correlation and Gunier plots for the consensus reconstruction. c, Angular distribution of projections. Resolution is estimated using the 0.143 criterion. d, Map isotropy analysis computed by 3DFSC58. e, Two views of the cryo-EM map colored by local resolution.
Extended Data Fig. 3 Cryo-EM of the LTag−EP origin DNA complex.
a, Schematic workflow outlining the steps for cryo-EM data collection and processing. b, Gold-standard Fourier shell correlation. c, Guinier plots. d, Angular distribution of projections. e, Map isotropy analysis computed by 3DFSC58. e, Two views of the cryo-EM map, colored by local resolution.
Extended Data Fig. 4 Structure of LTag bound to EP- and AT-half origin DNA.
a, Structure surface representation, indicating predicted melted bases of the passive strand (gray cartoon) as they exit through the bottom opening between subunits A and F. Bottom view showcasing the nucleotide (AMPPNP) at inter-subunit interfaces and DNA-binding loops engagement of AT tracking strand (Lower panels). b and c, Analysis of inter-subunit interactions at nucleotide-binding pockets, including Buried Surface Area (BSA) and hydrogen bonds (depicted as black dotted lines).
Extended Data Fig. 5 Cryo-EM of the LTag−AT origin DNA complex.
a, Schematic workflow outlining the steps for cryo-EM data collection and processing. b, Gold-standard Fourier shell correlation. c, Guinier plots. d, Angular distribution of projections. e, Map isotropy analysis computed by 3DFSC58. f, Two views of the cryo-EM map, colored by local resolution.
Extended Data Fig. 6 Cryo-EM of the LTag−full origin DNA complex.
a, Schematic workflow outlining the steps for cryo-EM data collection and processing. b, Gold-standard Fourier shell correlation. c, Guinier plots. d, Angular distribution of projections. e, Map isotropy analysis computed by 3DFSC58. f, Two views of the cryo-EM map, colored by local resolution.
Extended Data Fig. 7 Model of LTag double hexamer assembly starting from dimeric intermediates.
The top right panel shows the alignment of the LTag OBD model (PDB ID: 2ITL37) bound to the SV40 origin PEN sequences with two LTag dimers (PDB ID: 4GDF10). This alignment reveals that OBDs bound to the pseudo-PEN sequences cannot reach PEN2 or PEN4, as their linkers are too short; therefore, the OBDs must detach to allow the hexamerization of either hexamer (middle panel). Consequently, LTag double hexamers assemble with their OBDs floating, which explains the disordered OBD density observed in this study. The assembly of each hexamer triggers local melting of at least five bases (bottom panel), priming the helicase for ATP-coupled translocation.
Extended Data Fig. 8 Conservation of DNA melting mechanisms between LTag and CMG helicases.
This figure highlights the internal DNA separation mechanism within the LTag helicase bound to forked DNA and its resemblance to the yeast CMG double hexamer structure during DNA melting at the origin51 (dCMGE; PDB 7z13). In both cases, the formation of a separation wedge is facilitated by ATP binding, the staircase arrangement of DNA binding loops, and the engagement of these loops with the tracking strand. These elements collectively drive strand separation within the helicase inner chamber, positioning the helicase for ATP-powered translocation. This configuration is mirrored in the translocating CMG structure46 (PDB 6u0m), showcasing the conserved mechanism of action. Conversely, the pre-CMG formation MCM double hexamer50 (dMCM; PDB 7p30) displays planar DNA binding loops and a disengaged tracking strand, indicative of a state not yet primed for ATP-dependent movement.
Supplementary information
Supplementary Information
Supplementary Figs 1–10, Table 1 and references.
Supplementary Video 1
Morphing between five variability volumes across the first principal component trajectory. Volumes are coloured according to the different LTag subunits or DNA.
Supplementary Video 2
Morphing between five variability volumes across the second principal component trajectory. Volumes are coloured according to the specific LTag subunits and DNA.
Supplementary Video 3
Morphing between five variability volumes across the third principal component trajectory. Volumes are coloured according to the specific LTag subunits and DNA
Supplementary Video 4
Morphing between five molecular models along the first variability trajectory. This close-up highlights the net translocation of DNA across the LTag channel and the movement of the DNA-binding loops. LTag subunits are shown as ribbons, and DNA and the side chain of H513 as sticks.
Supplementary Video 5
Molecular dynamics video demonstrating a metastable hydrogen phosphate + ADP in posthydrolysis configuration inside an ‘ADP-type’ nucleotide pocket. The hydrolysed phosphate is stabilized by surrounding positive/polar residues. The nucleotide pocket and relative cryo-EM map corresponds to the C subunit in the last frame of the first 3DVA component. The simulation was carried out in ISOLDE.4
Supplementary Video 6
Morphing between five molecular models along the second variability trajectory. This close-up highlights the net translocation of DNA across the LTag channel and the upward movement of the DNA-binding loop of the F subunit (coloured light blue), signifying a preparatory step for translocation restart. LTag subunits are shown as ribbons, and DNA and the side chain of H513 as sticks.
Supplementary Video 7
Morphing between five molecular models along the third variability trajectory. This close-up highlights that there is no net translocation of DNA (see text for details). LTag subunits are shown as ribbons, and DNA and the side chain of H513 as sticks.
Supplementary Video 8
Animation illustrating DNA unwinding by LTag through a full translocation cycle, based on three structures. The first and second structures represent initial and final frames of the second variability component. The third structure, based on the initial frame, has the LTag subunits rotated one step around the six-fold symmetry axis of the N tier. ATP hydrolysis at the constrained interface of the hexamer (BC subunits) triggers coordinated C-tier domain rotations and DNA-binding loop adjustments, driving DNA through the helicase chamber (tracking strand in magenta, passive strand in blue). ADP/ATP exchange by the F subunit resets the staircase configuration, melting one DNA base pair. The tracking strand advances without rotation, while the duplex DNA rotates.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shahid, T., Danazumi, A.U., Tehseen, M. et al. Structural dynamics of DNA unwinding by a replicative helicase. Nature 641, 240–249 (2025). https://doi.org/10.1038/s41586-025-08766-w
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-08766-w